Surface Glycans of Microvesicles Derived from Endothelial Cells, as Probed Using Plant Lectins
Abstract
:1. Introduction
2. Results
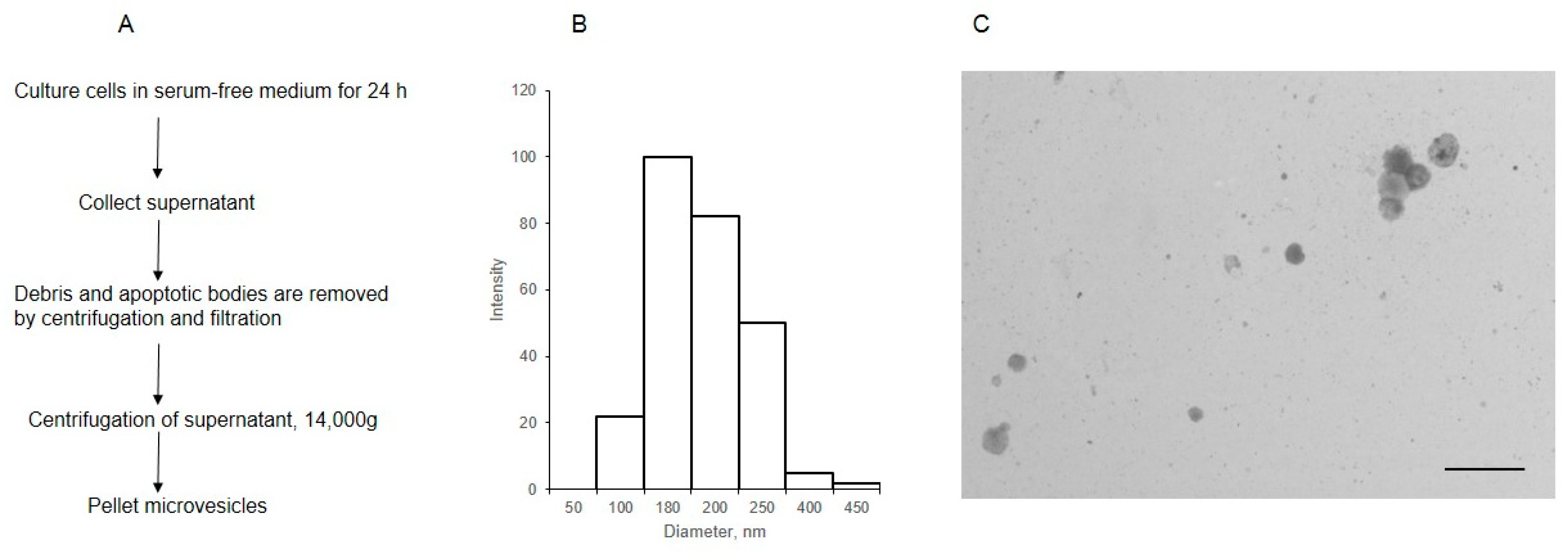
2.1. Isolation and Imaging of MVs
2.2. Test System for Glycan Profiling
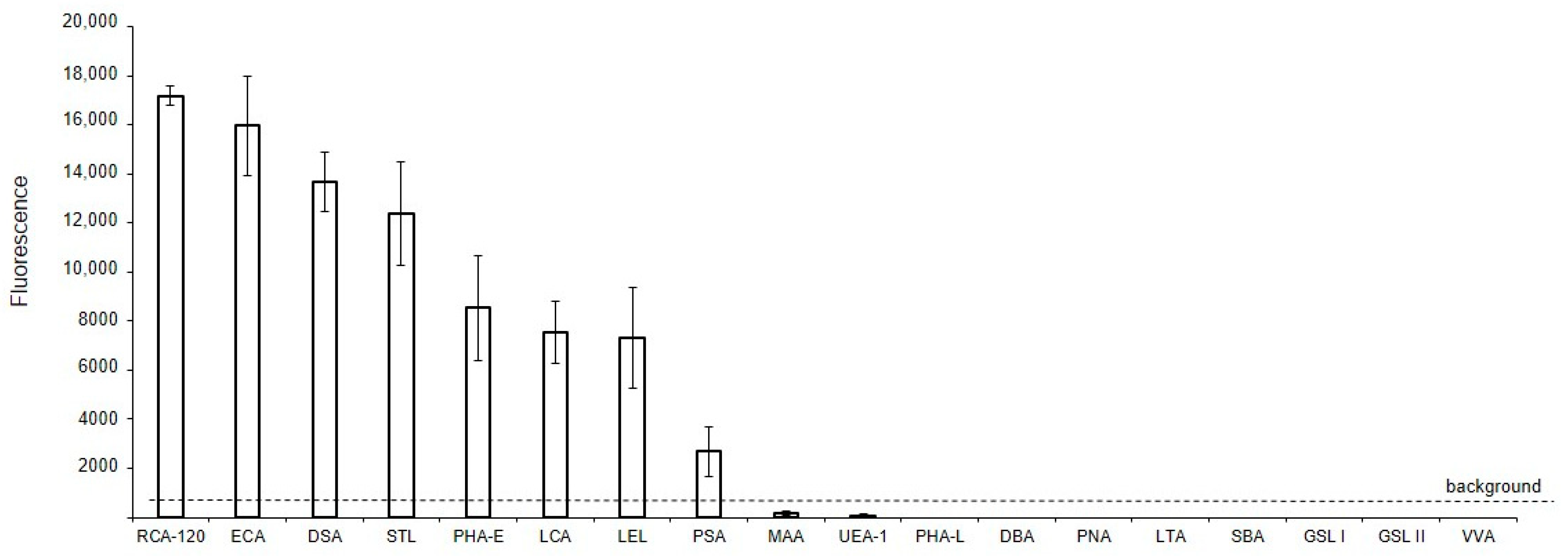
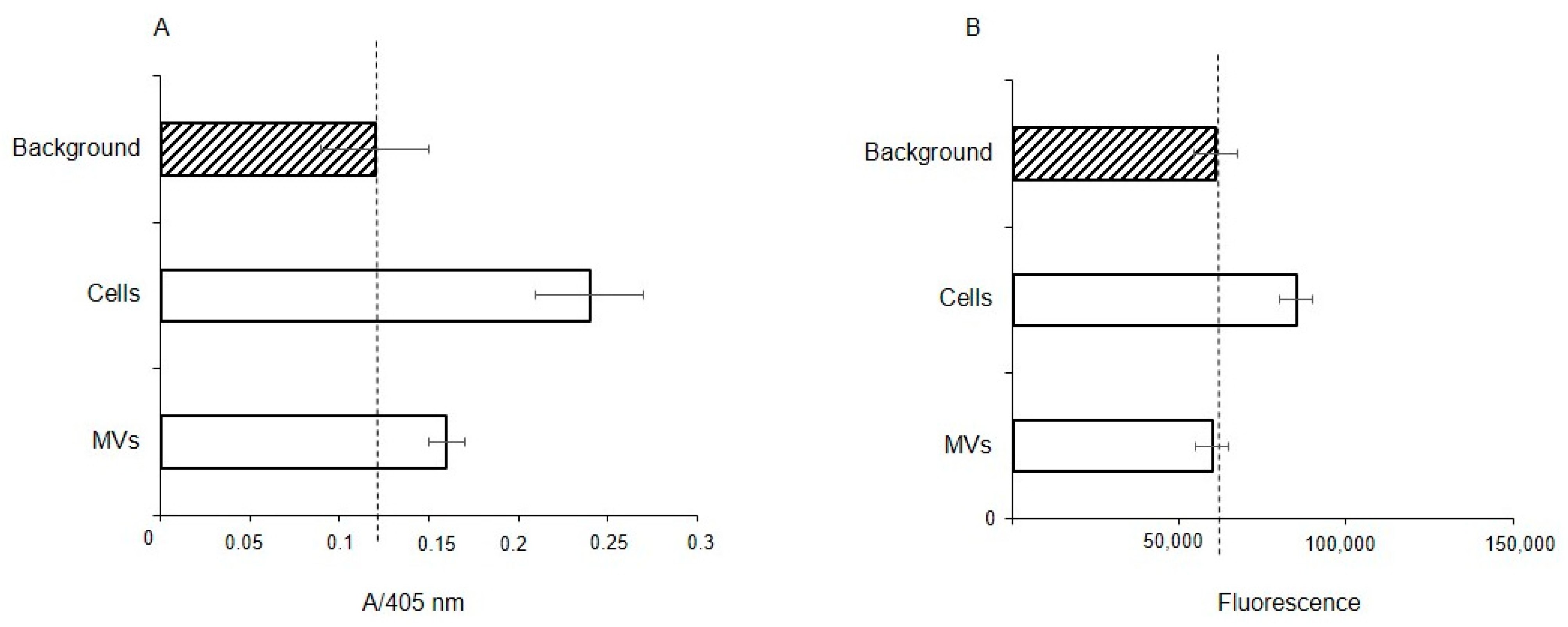
2.3. Solid-Phase Assay MV Glycan Profile
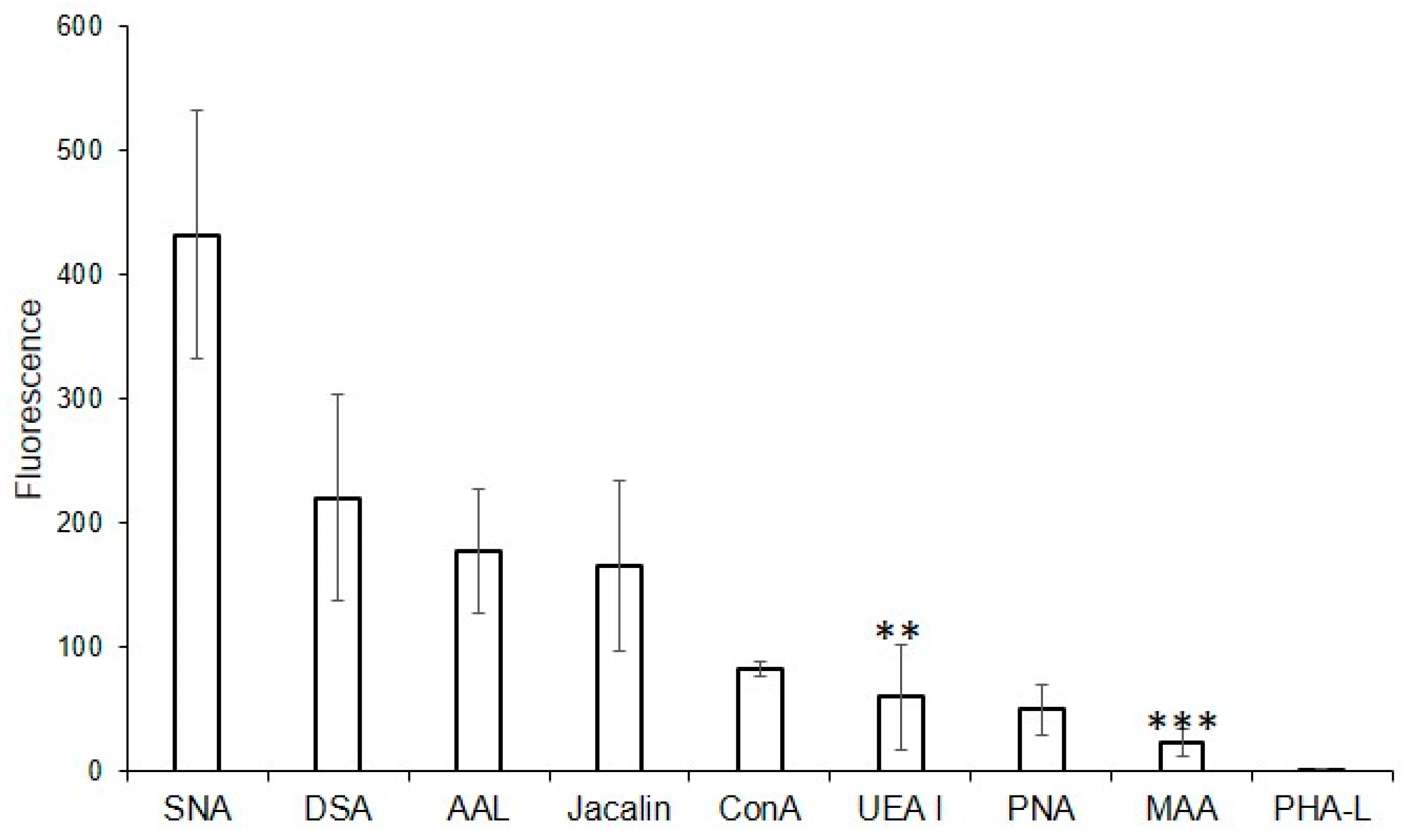
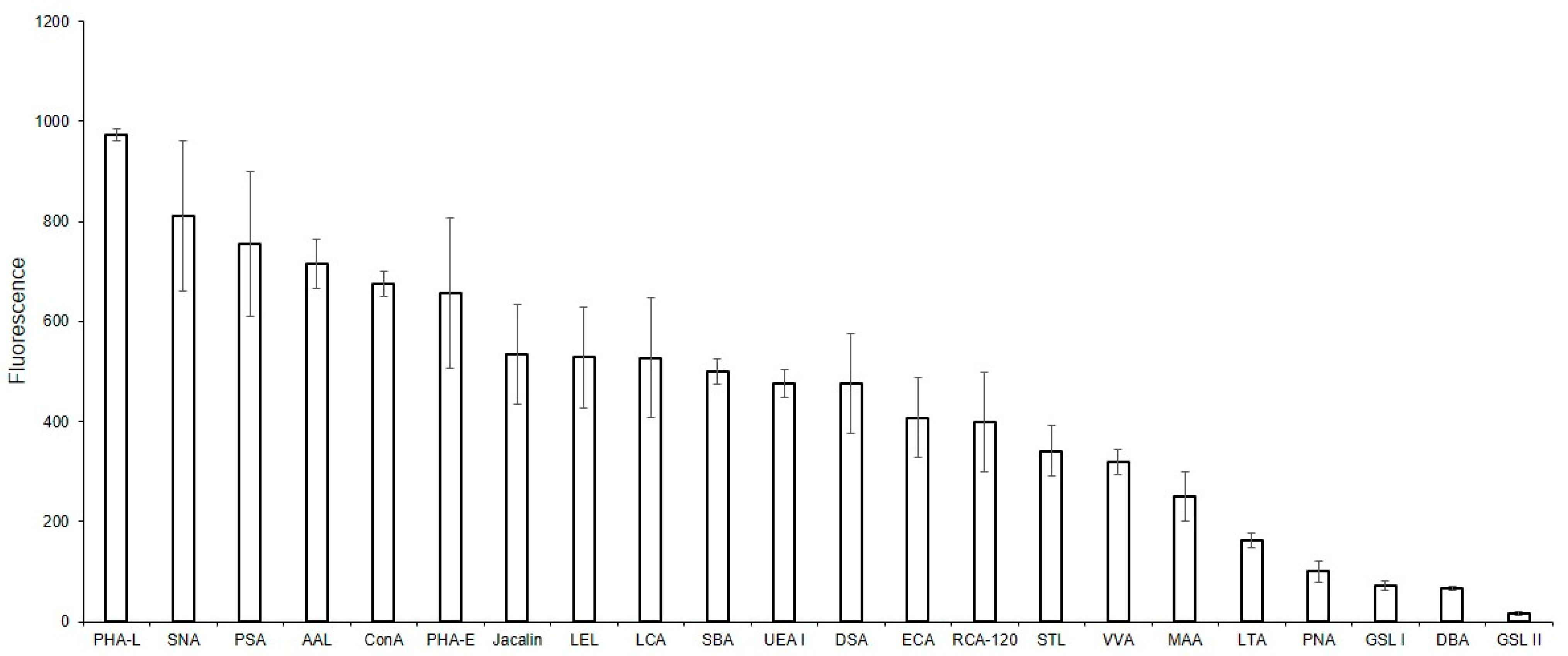
2.4. Glycan Profile of MVs According to Flow Cytometry
2.5. Binding of Lectins to Parent EA.hy 926 Cells
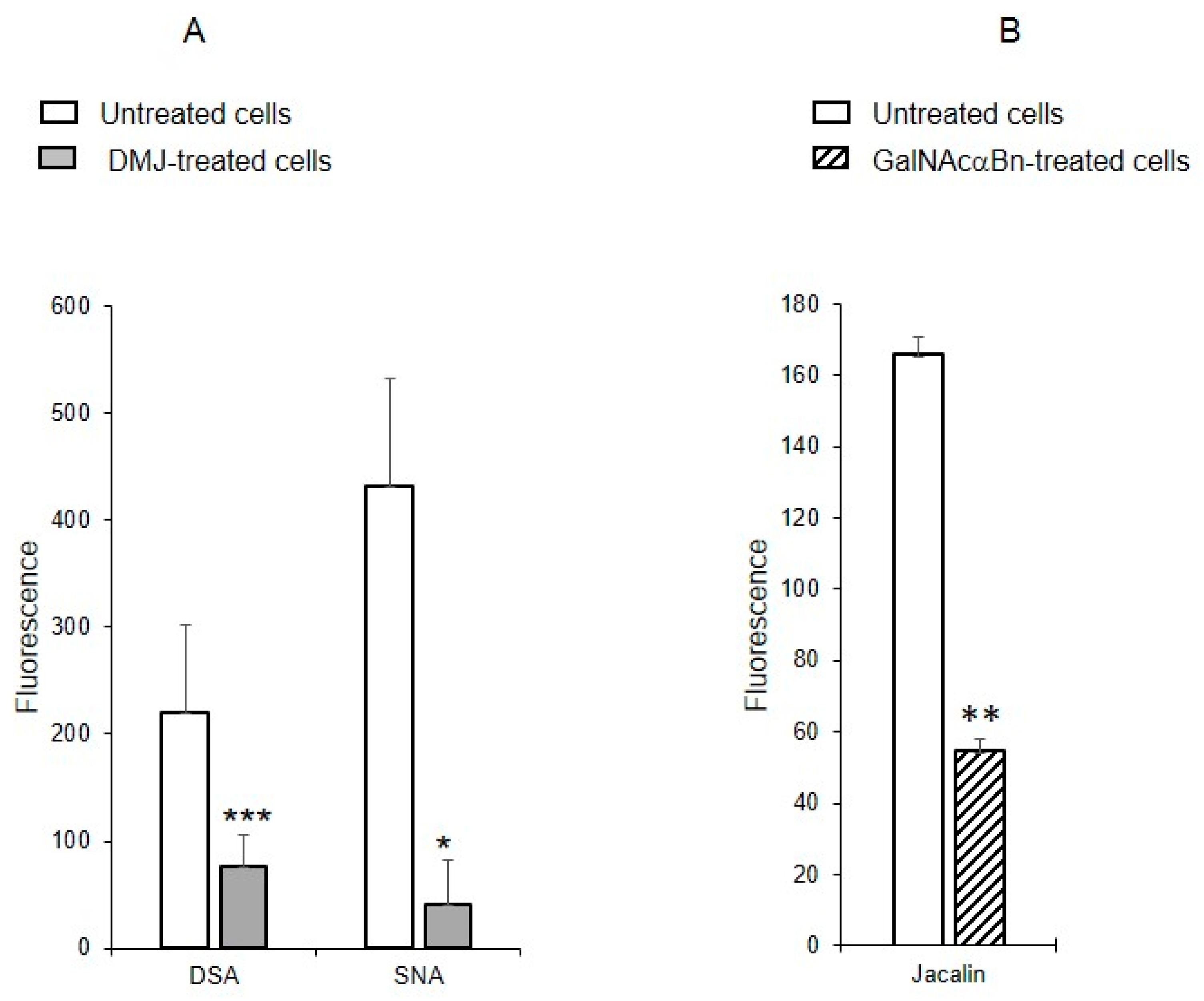
2.6. Binding of Lectins to MVs Derived from Cells Treated with Glycosylation Inhibitors
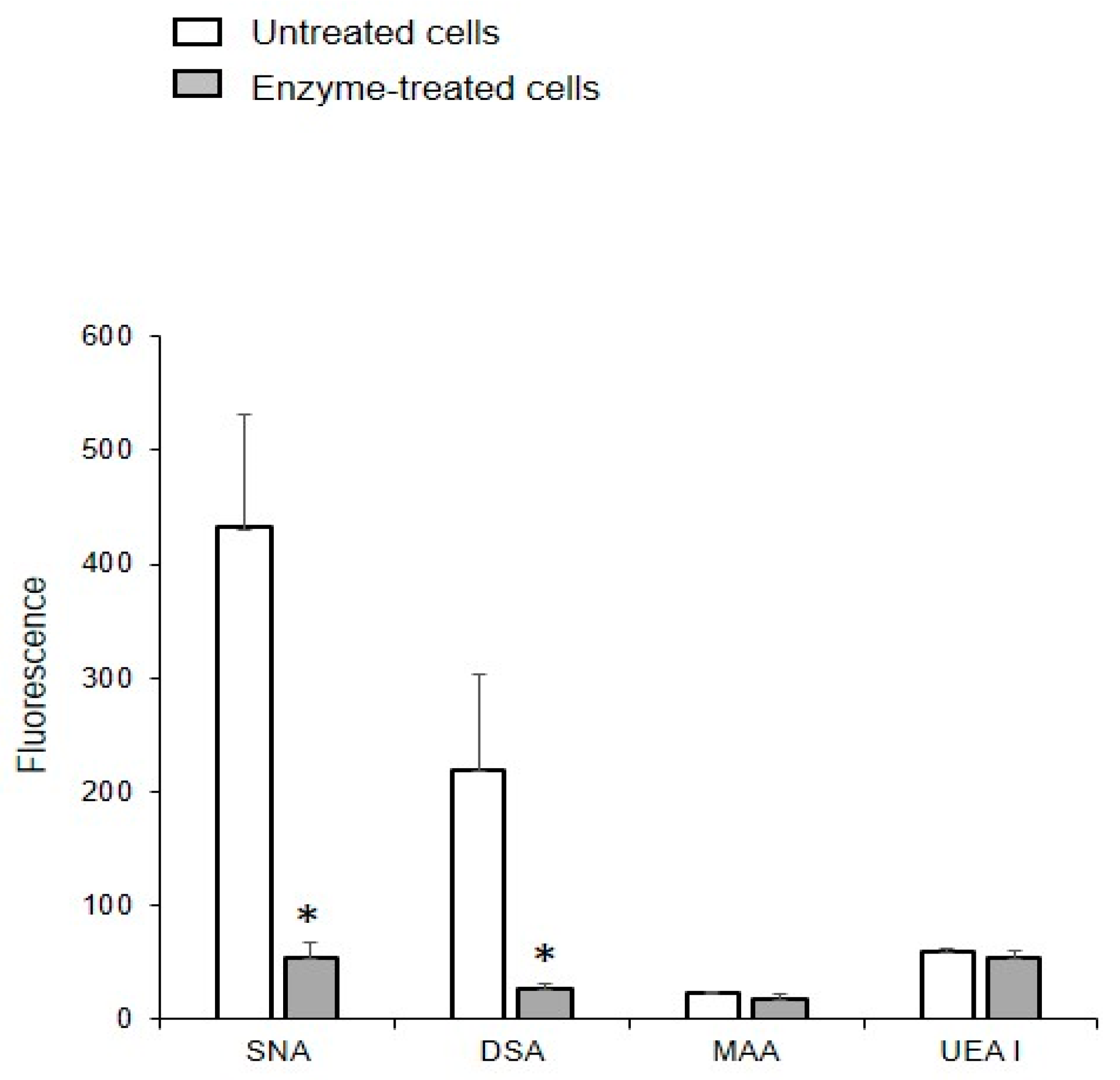
2.7. Binding of Lectins to MVs Derived from Cells after Depletion of Glycocalyx
2.8. Glycosidase Activity of EA. hy926 Cells and MVs
2.9. Binding of CTB to MVs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Isolation of EA.hy 926 Cell-Derived MVs
4.4. Scanning Transmission Electron Microscopy
4.5. Glass Slides Preparation
4.6. Study of Interaction between MVs and Lectins on Glass Slide
4.7. Insertion of FSL into MVs
4.8. Binding of Plant Lectins to Cells and MVs, Flow Cytometry
4.9. Measurement of Fucosidase or Sialidase Activity in EA.hy 926 Cell-Derived MVs
4.10. Isolation of MVs Derived from EA.hy 926 Cells, Treated with Inhibitors of Glycosylation
4.11. Isolation of MVs Derived from EA.hy 926 Cells with Depleted Glycocalyx
4.12. Binding of Cholera Toxin B to MVs
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Sneider, A.; Witwer, K.W.; Bergese, P.; Bhattacharyya, S.N.; Cocks, A.; Cocucci, E.; Erdbrugger, U.; Falcon-Perez, J.M.; Freeman, D.W.; et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: An ISEV position paper arising from the ISEV membranes and EVs workshop. J. Extracell. Vesicles 2020, 8, 1684862. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Ohkawa, Y.; Maeda, K.; Kizuka, Y.; Taniguchi, N. Extracellular vesicles and glycosylation. In The Role of Glycosylation in Health and Disease, Advances in Experimental Medicine and Biology, 1st ed.; Lauc, G.I., Trbojević-Akmačić, Eds.; Springer Nature Switzerland: Cham, Switzerland, 2021; pp. 137–150. [Google Scholar] [CrossRef]
- Zheng, W.; He, R.; Liang, X.; Roudi, S.; Bost, J.; Coly, P.-M.; van Niel, G.; Andaloussi, S. Cell-specific targeting of extracellular vesicles though engineering the glycocalyx. J. Extracell. Vesicles 2020, 11, 12290. [Google Scholar] [CrossRef] [PubMed]
- Horrevorts, S.K.; Stolk, D.; van de Ven, R.; Hulst, M.; van Het Hof, B.; Duinkerken, S.; Heineke, M.; Ma, W.; Dusoswa, S.; Nieuwland, R.; et al. Glycan-mjdified apoptotic melanoma-derived extracellular vesicles as antigen source for anti-tumor vaccination. Cancers 2019, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Dusoswa, S.; Horrevorts, S.; Kalay, H.; Paauw, N.; Nieuwland, R.; Pegtel, M.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, J.; Jankowski, H.; Bond, D.; McCague, S.; Munro, B.; Predebon, M.; Scarlett, C.; Skelding, K.; Weidenhofer, J. Lipidomic profiling of extracellular vesicles derived from prostate and prostate cancer cell lines. Lipids Health Dis. 2018, 17, 211. [Google Scholar] [CrossRef]
- Scruggs, A.; Cioffi, E.; Cioffi, D.; King, J.; Bauer, N. Lectin-based characterization of vascular cell microparticle glycocalyx. PLoS ONE 2015, 10, e0135533. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Hoja-Łukowicz, D.; Szweda, S.; Drożdż, A.; Stępień, E.; Przybyło, M. Human melanoma-derived ectosomes are enriched with specific glycan epitopes. Life Sci. 2018, 207, 395–411. [Google Scholar] [CrossRef]
- Surman, M.; Wilczak, M.; Przybyło, M. Lectin-based study reveals the presence of disease-relevant glycoepitopes in bladder cancer cells and ectosomes. Int. J. Mol. Sci. 2022, 23, 14368. [Google Scholar] [CrossRef]
- Shimoda, A.; Miura, R.; Tateno, H.; Seo, N.; Shiku, H.; Sawada, S.-I.; Sasaki, Y.; Akiyoshi, K. Assessment of surface glycan diversity on extracellular vesicles by lectin microarray and glycoengineering strategies for drug delivery applications. Small Meth. 2022, 6, 2100785. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.; Eng, W.; Pilobello, K.; Hendricks-Muñoz, K.; Mahal, L. Identification of a conserved glycan signature for microvesicles. J. Proteome Res. 2011, 10, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, Y.; Li, Y.; Tao, J.; Ding, L.; Wu, J.; Ju, H. Lectin-mediated in situ rolling circle amplification on exosomes for probing cancer-related glycan pattern. Anal. Chim. Acta. 2018, 1039, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Pazos, R.; Royo, F.; González, E.; Roura-Ferrer, M.; Martinez, A.; Gamiz, J.; Reichardt, N.-C.; Falcón-Pérez, J. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci. Rep. 2019, 9, 11920. [Google Scholar] [CrossRef] [PubMed]
- Markova, K.; Kozyreva, A.; Gorshkova, A.; Aleksandrova, E.; Berezkina, M.; Mikhailova, V. Methodological approaches to assessing the size and morphology of microvesicles of cell lines. Bull. Exp. Biol. Med. 2020, 2, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Data of Consortium for Functional Glycomics. Available online: http://www.functionalglycomics.org (accessed on 11 July 2012).
- Olausson, J.; Tibell, L.; Jonsson, B.-H.; Pahlsson, P. Detection of a high affinity binding site in recombinant Aleuria aurantia lectin. Glycoconj. J. 2008, 25, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Yamada, S.; Yamada, K. Analysis of the role of glycosylation of the human fibronectin receptor. J. Biol. Chem. 1989, 264, 18011–18018. [Google Scholar] [CrossRef] [PubMed]
- Kuan, S.-F.; Byrd, J.; Basbaum, C.; Kim, Y. Inhibition of mucin glycosylation by aryl-N-acetyl-agalactosaminides in human colon cancer cells. J. Biol. Chem. 1989, 264, 19271–19277. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Reina, J.; Potenza, D.; Heggelund, J.; Mackenzie, A.; Krengel, U.; Bernardi, A. Comprehensive analysis of blood group antigen binding to classical and El Tor cholera toxin B-pentamers by NMR. Glycobiology. 2014, 24, 766–778. [Google Scholar] [CrossRef]
- Heim, J.; Hodnik, V.; Heggelund, J.; Anderluh, G.; Krengel, U. Crystal structures of cholera toxin in complex with fucosylated receptors point to importance of secondary binding site. Sci. Rep. 2019, 9, 12243. [Google Scholar] [CrossRef]
- Kenworthy, A.; Schmieder, S.; Raghunathan, K.; Tiwari, A.; Wang, T.; Kelly, C.; Lencer, W. Cholera toxin as a probe for membrane biology. Toxins 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.; Momosaki, K.; Shimeno, H.; Nacamatsu, A.; Radin, N. Effects of D-Threo-PDMP, an inhibitor of glucosylceramide synthetase, on expression of cell surface glycolipid antigen and binding to adhesive proteins by B16 melanoma cells. J. Cell. Physiol. 1989, 141, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 2013, 1831, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Wands, A.; Cervin, J.; Huang, H.; Zhang, Y.; Youn, G.; Brautigam, C.; Dzebo, M.; Björklund, P.; Wallenius, V.; Bright, D.; et al. Fucosylated molecules competitively interfere with cholera toxin binding to host cells. ACS Infect Dis. 2018, 4, 758–770. [Google Scholar] [CrossRef]
- Lingwood, C.; Manis, A.; Mahfoud, R.; Khan, F.; Binnington, B.; Mylvaganam, M. New aspects of the regulation of glycosphingolipid receptor function. Chem. Phys. Lipid. 2010, 163, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yahi, N.; Aulas, A.; Fantini, J. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer’s β amyloid peptide (Ab1-40). PLoS ONE 2010, 5, e9079. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Paulson, J. Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.; Rockwell, H.; Gao, F.; Narain, N.; DiFiglia, M.; Kiebish, M.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S.-i. The glycosynapse. Proc. Natl. Acad. Sci. USA 2002, 9, 225–232. [Google Scholar] [CrossRef]
- Evans, S.; MacKenzie, C. Characterization of protein–glycolipid recognition at the membrane bilayer. J. Mol. Recognit. 1999, 12, 155–168. [Google Scholar] [CrossRef]
- Baranska, P.; Jerczynska, H.; Pawlowska, Z.; Koziokiewicz, W.; Cierniewski, C.S. Expression of integrins and adhesive properties of human endothelial cell line EA.hy 926. Cancer Gen. Prot. 2005, 2, 265–270. [Google Scholar]
- Kitazume, S.; Imamaki, R.; Ogawa, K.; Komi, Y.; Futakawa, S.; Kojima, S.; Hashimoto, Y.; Marth, J.; Paulson, J.; Taniguchi, N. 2,6-Sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J. Biol. Chem. 2010, 285, 6515–6521. [Google Scholar] [CrossRef] [PubMed]
- Cordero, O.; Merino, A.; Paez de la Cadena, M.; Bugia, B.; Nogueira, M.; Viñuela, J.; Vicenta, S.; Martinez-Zorzano, V.; de Carlos, A.; Rodriguez-Berrocal, F. Cell surface human α-L-fucosidase. Eur. J. Biochem. 2001, 268, 3321–3331. [Google Scholar] [CrossRef]
- Smith, B.; Bertozzi, C. The clinical impact of glycobiology: Targeting selectins, siglecs and mammalian glycans. Nat Rev. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Tuzikov, A.; Bovin, N. Kode Technology Illustrated Technical Manual, 1st ed.; Kode Biotech: Auckland, New Zealand, 2023. [Google Scholar]



| № | Source | Short Name | Typical Glycans Bound by This Lectin (CFG Data) |
|---|---|---|---|
| 1 | Ricinus communis seeds | RCA-120 | 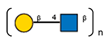 |
| 2 | Erythrina cristagalli seeds | ECA | 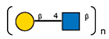 |
| 3 | Datura stramonium seeds | DSA | 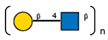 |
| 4 | Solanum tubersolum potato | STL | 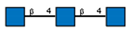 |
| 5 | Phaseolus vulgaris seeds | PHA-E |  |
| 6 | Lens culinaris seeds | LCA | 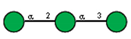 |
| 7 | Lycopersicon esculentum tomato | LEL | 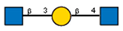 |
| 8 | Pisum sativum peas | PSA | 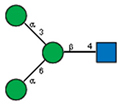 |
| 9 | Phaseolus vulgaris seeds | PHA-L | 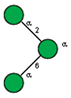 |
| 10 | Arachis hypogaea peanut | PNA |  |
| 11 | Maackia amurensis seeds | MAA | 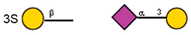 |
| 12 | Ulex europaeus seeds | UEA I |  |
| 13 | Lotus tetragonolobus seeds | LTA |  |
| 14 | Vicia villosa seeds | VVA | 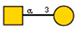 |
| 15 | Griffonia simplicifolia seeds | GSL I | 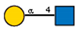 |
| 16 | Lectin II from seeds of Griffonia simplicifolia | GSL II |  |
| 17 | Glycine max soybean | SBA | 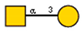 |
| 18 | Dolichos biflorus seeds | DBA | 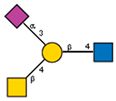 |
| 19 | Sambucus nigra elderberry bark | SNA * |  |
| 20 | Artocarpus integrifolia seeds | Jacalin * |  |
| 21 | Canavalia ensiformis jack-bean | Con-A * | 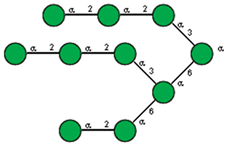 and other mannose-rich N-glycans |
| 22 | Aleuria aurantia orange peel fungus | AAL # |  |
 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slivka, E.V.; Shilova, N.V.; Obraztsova, E.A.; Kapustkina, D.S.; Khaidukov, S.V.; Nokel, A.Y.; Ryzhov, I.M.; Henry, S.M.; Bovin, N.V.; Rapoport, E.M. Surface Glycans of Microvesicles Derived from Endothelial Cells, as Probed Using Plant Lectins. Int. J. Mol. Sci. 2024, 25, 5725. https://doi.org/10.3390/ijms25115725
Slivka EV, Shilova NV, Obraztsova EA, Kapustkina DS, Khaidukov SV, Nokel AY, Ryzhov IM, Henry SM, Bovin NV, Rapoport EM. Surface Glycans of Microvesicles Derived from Endothelial Cells, as Probed Using Plant Lectins. International Journal of Molecular Sciences. 2024; 25(11):5725. https://doi.org/10.3390/ijms25115725
Chicago/Turabian StyleSlivka, Ekaterina V., Nadezhda V. Shilova, Ekaterina A. Obraztsova, Daria S. Kapustkina, Sergey V. Khaidukov, Alexey Yu. Nokel, Ivan M. Ryzhov, Stephen M. Henry, Nicolai V. Bovin, and Eugenia M. Rapoport. 2024. "Surface Glycans of Microvesicles Derived from Endothelial Cells, as Probed Using Plant Lectins" International Journal of Molecular Sciences 25, no. 11: 5725. https://doi.org/10.3390/ijms25115725






