Antitumor Effects of an Anthocyanin-Rich Grain Diet in a Mouse Model of Lewis Lung Carcinoma
Abstract
1. Introduction
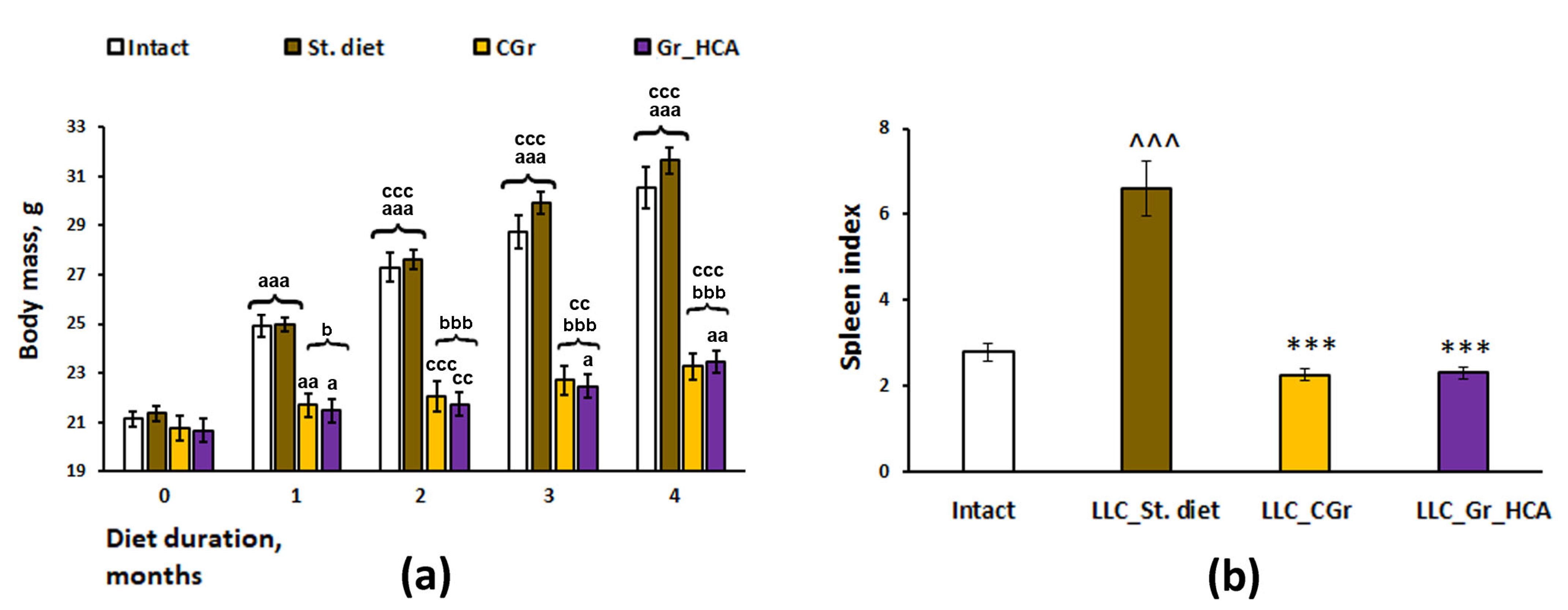
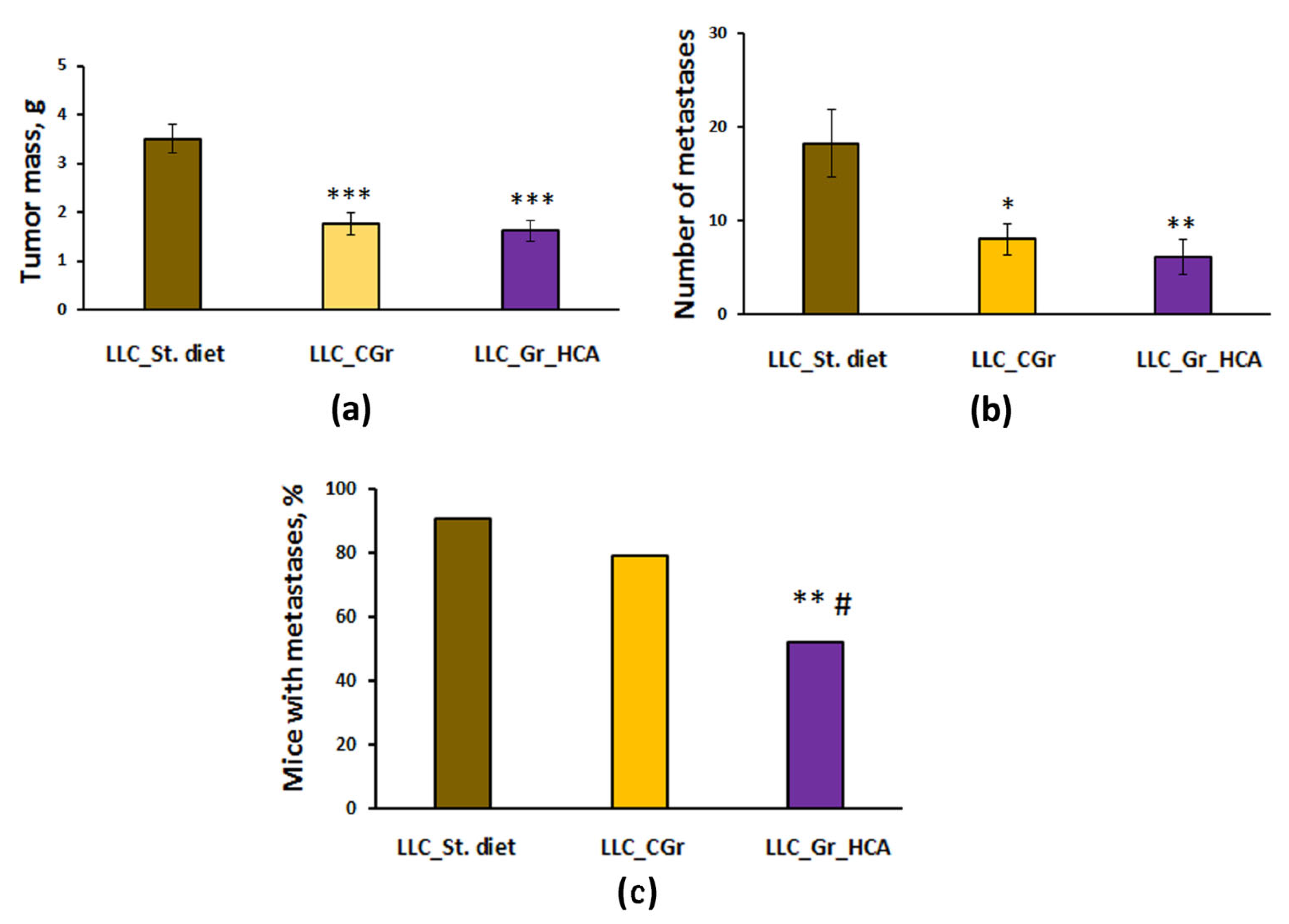
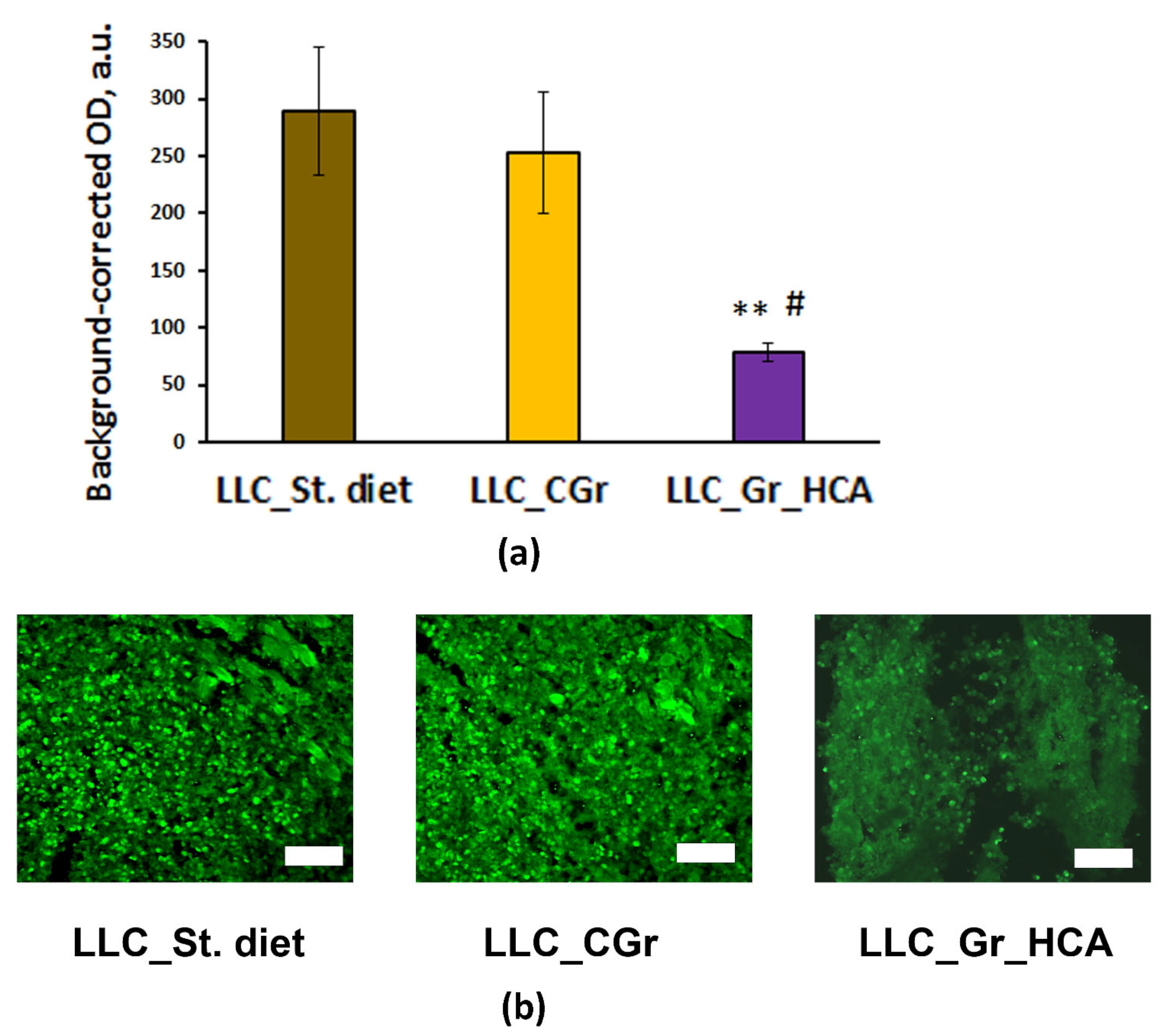
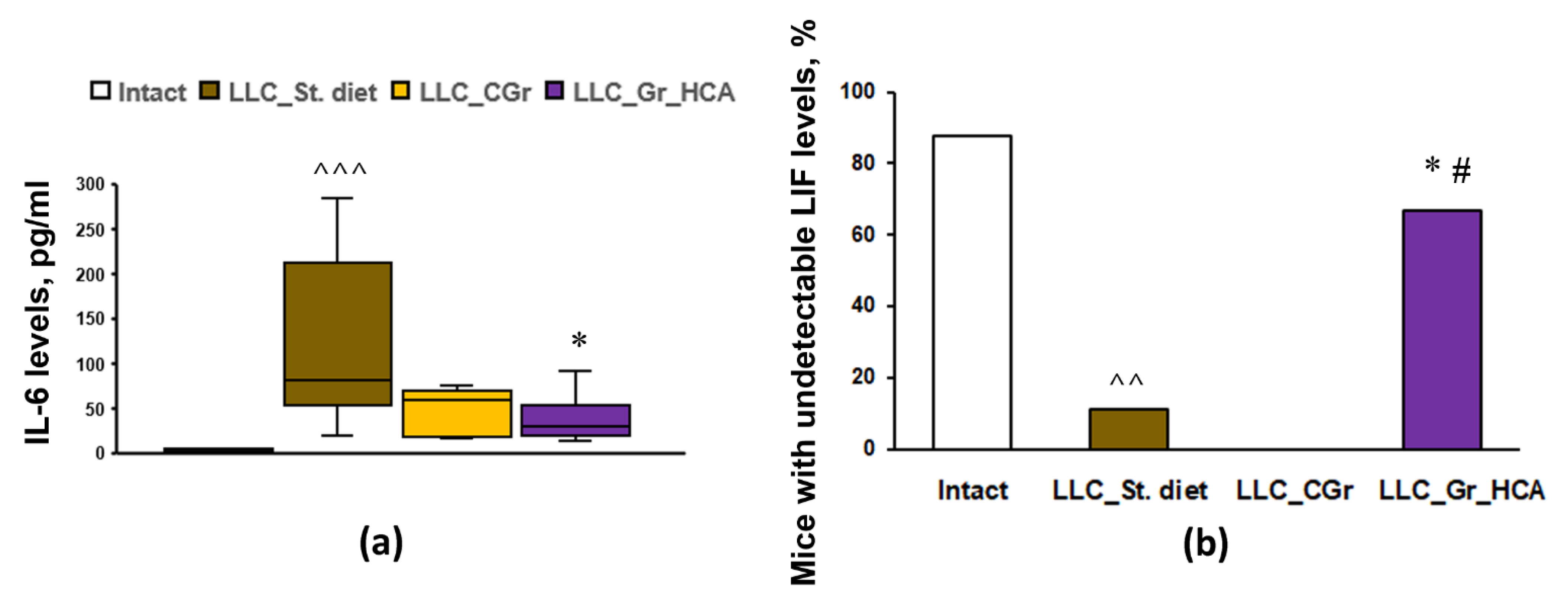
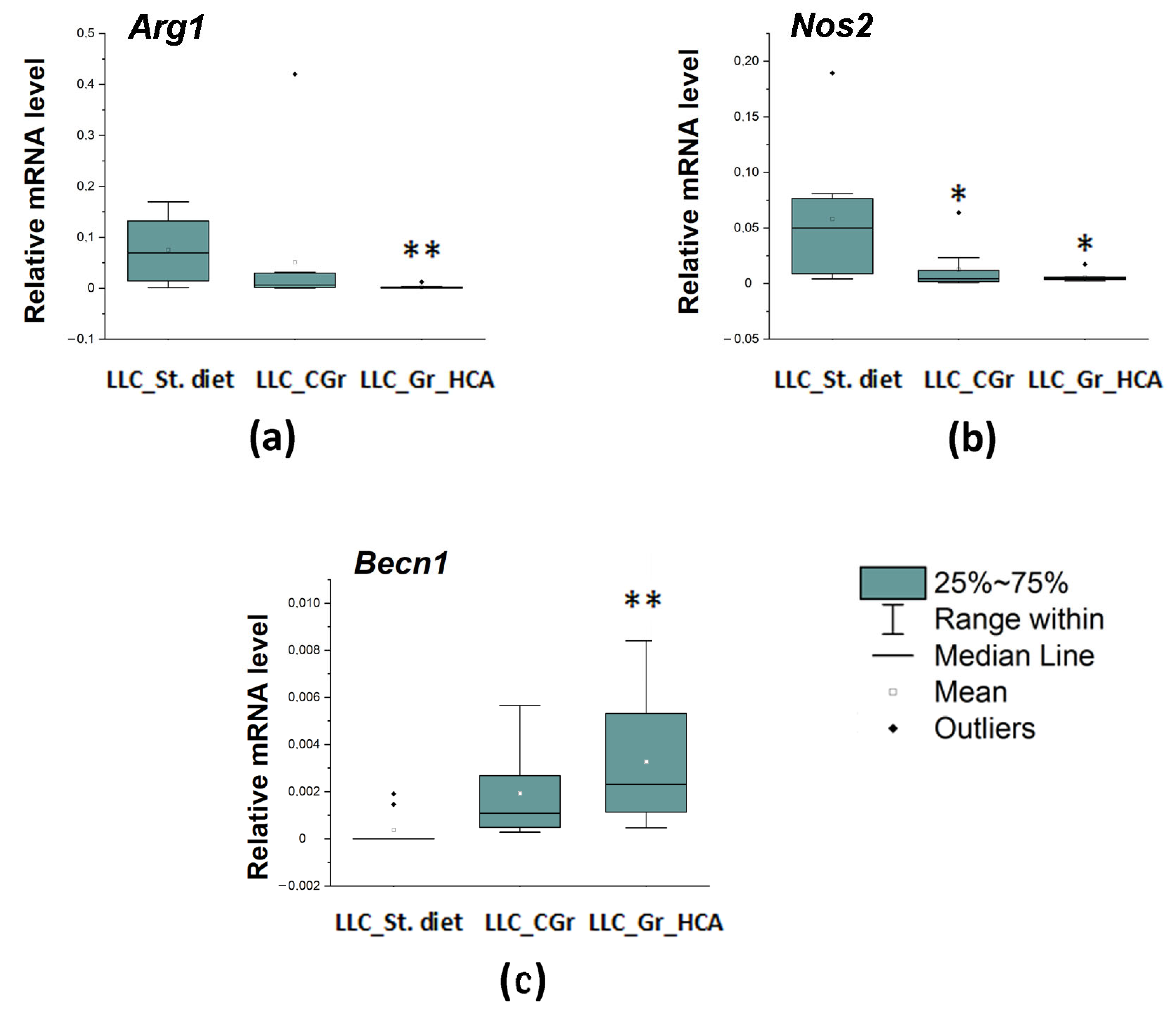
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Procedures Involving Animals
4.2. Immunological Multiplex Assay
4.3. Immunohistochemical (IHC) Analysis
4.4. Gene Expression Analysis
- Tubb5_F: TGAAGCCACAGGTGGCAAGTAT,
- Tubb5_R: CCAGACTGACCGAAAACGAAGT;
- Arg1_F: AAGAGCTGGCTGGTGTGGTG,
- Arg1_R: ACACAGGTTGCCCATGCAGA;
- Nos2_F: ATCGACCCGTCCACAGTATGT,
- Nos2_R: CATGATGGACCCCAAGCAAGA;
- Becn1_F: GAACTCACAGCTCCATTACTTA,
- Becn1_R: ATCTTCGAGAGACACCATCC.
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/today (accessed on 10 April 2024).
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Polyphenols by Generating H(2)O(2), Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Nunes, A.R.; Falcao, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Koh, A.H.S.; Zhou, W. Enhancing health benefits of bakery products using phytochemicals. Adv. Food Nutr. Res. 2022, 99, 239–281. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Khlestkina, E.K. Wheat, Barley, and Oat Breeding for Health Benefit Components in Grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R.; Rhein, S.O.; Meisel, L.A.; Fuentes, J.; Speisky, H.; Schwember, A.R.; De Camargo, A.C. Wheat and Rice beyond Phenolic Acids: Genetics, Identification Database, Antioxidant Properties, and Potential Health Effects. Plants 2022, 11, 3283. [Google Scholar] [CrossRef]
- Gamel, T.H.; Saeed, S.M.G.; Ali, R.; Abdel-Aal, E.M. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods 2023, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Kaur, S.; Sharma, A.; Kumari, A.; Tiwari, V.; Sharma, S.; Kapoor, P.; Sheoran, B.; Goyal, A.; Krishania, M. Rising Demand for Healthy Foods-Anthocyanin Biofortified Colored Wheat Is a New Research Trend. Front. Nutr. 2022, 9, 878221. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, D.V.; Shevchenko, O.G.; Golubev, D.A.; Platonova, E.Y.; Zemskaya, N.V.; Shoeva, O.Y.; Gordeeva, E.I.; Patov, S.A.; Shaposhnikov, M.V.; Khlestkina, E.K.; et al. Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants 2023, 12, 2010. [Google Scholar] [CrossRef]
- Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients 2020, 12, 3877. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-Biofortified Colored Wheat Prevents High Fat Diet-Induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, e1900999. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; De Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Esselen, M.; Fritz, J.; Hutter, M.; Teller, N.; Baechler, S.; Boettler, U.; Marczylo, T.H.; Gescher, A.J.; Marko, D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S143–S153. [Google Scholar] [CrossRef] [PubMed]
- Karthi, N.; Kalaiyarasu, T.; Kandakumar, S.; Mariyappana, P.; Manju, V. Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv. 2016, 6, 45064–45076. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Geng, B.; Yi, Z. Pelargonidin induces antitumor effects in human osteosarcoma cells via autophagy induction, loss of mitochondrial membrane potential, G2/M cell cycle arrest and downregulation of PI3K/AKT signalling pathway. J. BUON 2018, 23, 735–740. [Google Scholar] [PubMed]
- Liu, X.; Zhang, D.; Hao, Y.; Liu, Q.; Wu, Y.; Liu, X.; Luo, J.; Zhou, T.; Sun, B.; Luo, X.; et al. Cyanidin Curtails Renal Cell Carcinoma Tumorigenesis. Cell Physiol. Biochem. 2018, 46, 2517–2531. [Google Scholar] [CrossRef]
- Morgounov, A.; Karaduman, Y.; Akin, B.; Aydogan, S.; Baenziger, P.S.; Bhatta, M.; Chudinov, V.; Dreisigacker, S.; Govindan, V.; Güler, S.; et al. Yield and quality in purple-grained wheat isogenic lines. Agronomy 2020, 10, 86. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant Capacity and Profiles of Phenolic Acids in Various Genotypes of Purple Wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Khlestkina, E.K.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilovskii Zhurnal Genet. I Sel. (Vavilov J. Genet. Breed.) 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, J.; Tsao, R.; Wang, Z.; Sun, B.; Wang, J. Whole Grain Consumption for the Prevention and Treatment of Breast Cancer. Nutrients 2019, 11, 1769. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrugger, S.; Maerkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frokiaer, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Shabkhizan, R.; Haiaty, S.; Moslehian, M.S.; Bazmani, A.; Sadeghsoltani, F.; Saghaei Bagheri, H.; Rahbarghazi, R.; Sakhinia, E. The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Adv. Nutr. 2023, 14, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef]
- Schiavano, G.F.; De Santi, M.; Brandi, G.; Fanelli, M.; Bucchini, A.; Giamperi, L.; Giomaro, G. Inhibition of Breast Cancer Cell Proliferation and In Vitro Tumorigenesis by a New Red Apple Cultivar. PLoS ONE 2015, 10, e0135840. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuki, S.; Fukui, S.; Watanabe, A.; Akune, S.; Tanabe, M.; Yoshida, K. Delphinidin induces autolysosome as well as autophagosome formation and delphinidin-induced autophagy exerts a cell protective role. J. Biochem. Mol. Toxicol. 2012, 26, 445–453. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef]
- Bonelli, S.; Geeraerts, X.; Bolli, E.; Keirsse, J.; Kiss, M.; Pombo Antunes, A.R.; Van Damme, H.; De Vlaminck, K.; Movahedi, K.; Laoui, D.; et al. Beyond the M-CSF receptor—Novel therapeutic targets in tumor-associated macrophages. FEBS J. 2018, 285, 777–787. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, H.; Lin, W.W.; Descargues, P.; Grivennikov, S.; Kim, Y.; Luo, J.L.; Karin, M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Yao, G.; Gao, J.; Yang, B.; Zhao, Y.; Rao, Z.; Gao, J. M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics 2012, 67, 901–906. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-kappaB/SOX4 signaling. Cell Death Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, S.S.; Kim, W.J.; Moon, S.K. IL-5-induced migration via ERK1/2-mediated MMP-9 expression by inducing activation of NF-kappaB in HT1376 cells. Oncol. Rep. 2012, 28, 1084–1090. [Google Scholar] [CrossRef]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, A.; Hashemi, V.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M.; Jadidi-Niaragh, F. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed. Pharmacother. 2018, 108, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Koyanagi-Aoi, M.; Otani, K.; Zen, Y.; Maniwa, Y.; Aoi, T. Interleukin-6 blockade attenuates lung cancer tissue construction integrated by cancer stem cells. Sci. Rep. 2017, 7, 12317. [Google Scholar] [CrossRef]
- Shang, G.S.; Liu, L.; Qin, Y.W. IL-6 and TNF-alpha promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol. Lett. 2017, 13, 4657–4660. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.; Tan, L.; Bolland, D.E.; Coffman, L.G.; Peterson, L.F.; Talpaz, M.; Neamati, N.; Buckanovich, R.J. Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene 2019, 38, 1576–1584. [Google Scholar] [CrossRef]
- Pascual-Garcia, M.; Bonfill-Teixidor, E.; Planas-Rigol, E.; Rubio-Perez, C.; Iurlaro, R.; Arias, A.; Cuartas, I.; Sala-Hojman, A.; Escudero, L.; Martinez-Ricarte, F.; et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. Nat. Commun. 2019, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- Campanella, G.S.; Medoff, B.D.; Manice, L.A.; Colvin, R.A.; Luster, A.D. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J. Immunol. Methods 2008, 331, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, L.; Kang, H.; Wan, Z.; Zhang, M.; Gang, B.; Liu, J.; Liu, G.; Gu, W. LAMP1 controls CXCL10-CXCR3 axis mediated inflammatory regulation of macrophage polarization during inflammatory stimulation. Int. Immunopharmacol. 2024, 132, 111929. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC chemokines in angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotesmall es, Cyrillican on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, T.E.; Chepanova, A.A.; Zakharenko, A.L.; Filimonov, A.S.; Luzina, O.A.; Dyrkheeva, N.S.; Nikolin, V.P.; Popova, N.A.; Salakhutdinov, N.F.; Lavrik, O.I. Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors-New Usnic Acid Derivatives. Int. J. Mol. Sci. 2024, 25, 1210. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Shvaikovskaya, A.A.; Zhanaeva, S.Y.; Moysak, G.I.; Akopyan, A.A.; Rzaev, J.A.; Danilenko, K.V.; Aftanas, L.I. Concordance between the In Vivo Content of Neurospecific Proteins (BDNF, NSE, VILIP-1, S100B) in the Hippocampus and Blood in Patients with Epilepsy. Int. J. Mol. Sci. 2023, 25, 502. [Google Scholar] [CrossRef] [PubMed]
- Feofanova, N.A.; Bets, V.D.; Borisova, M.A.; Litvinova, E.A. L-fucose reduces gut inflammation due to T-regulatory response in Muc2 null mice. PLoS ONE 2022, 17, e0278714. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Belichenko, V.M.; Bashirzade, A.A.; Tenditnik, M.V.; Dubrovina, N.I.; Akopyan, A.A.; Ovsyukova, M.V.; Fedoseeva, L.A.; Pupyshev, A.B.; Aftanas, L.I.; Amstislavskaya, T.G.; et al. Comparative analysis of early neurodegeneration signs in a mouse model of Alzheimer’s disease-like pathology induced by two types of the central (Intracerebroventricular vs. Intrahippocampal) administration of Abeta(25–35) oligomers. Behav. Brain Res. 2023, 454, 114651. [Google Scholar] [CrossRef]
| Analyte | Group | F, p/ H, p (LLC_St. Diet, LLC_CGr, LLC_Gr_HCA) | |||
|---|---|---|---|---|---|
| Intact | LLC_St. Diet | LLC_CGr | LLC_Gr_HCA | ||
| TNFα | 4.6 (2.3; 5.4) | 16.6 (13.5; 23.7) ^^ | 13.5 (12.4; 18.6) | 15.3 (11.2; 37.9) | H(2, N = 20) < 1 |
| IFNγ | 0.89 (0.0; 1.29) | 2.47(0.89; 5.44) | 10.4 (3.2; 13.9) | 0.86 (0.0; 2.47) | H(2, N = 20) = 5.23, p > 0.05 |
| LIF | 0.0 (0.0; 0.0) | 1.6 (1.04; 3.11) ^^^ | 0.49 (0.13; 0.49) | 0.0 (0.0; 1.6) | H(2, N = 20) = 3.37, p > 0.05 |
| IP-10 | 121.5 (105.7; 150.5) | 629.4 (435.5; 656.4) ^^^ | 847.7 (657.2; 1007.4) | 858.4 (676.6; 1031.7) * | H(2, N = 20) = 7.14, p < 0.05 |
| KC | 75.9 (55.3; 109.2) | 422.7 (190.9; 1119.5) ^ | 348.2 (95.7; 374.1) | 238.6 (132.5; 554.1) | H(2, N = 20) < 1 |
| IL-1α | 111.7 (59.1; 146.5) | 109.0 (81.6; 106.8) | 114.6 (0.0; 178.4) | 106.9 (78.3; 118.7) | F(2, 17) < 1 |
| IL-1β | 0.0 (0.0; 0.0) | 0.0 (0.0; 1.28) | 1.28 (0.0; 1.28) | 0.0 (0.0; 0.43) | H(2, N = 20) < 1 |
| IL-2 | 2.0 (0.0; 6.08) | 3.18 (0.81; 3.18) | 0.0 (0.0; 0.0) | 3.18 (3.18; 3.18) | H(2, N = 20) = 2.43, p > 0.05 |
| IL-3 | 0.07 (0.0; 0.34) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | H(2, N = 20) = 1.08, p > 0.05 |
| IL-4 | 0.0 (0.0; 0.17) | 0.0 (0.0; 0.0) | 0.0 (0.0; 1.62) | 0.0 (0.0; 0.0) | H(2, N = 20) = 6.32, p < 0.05 |
| IL-5 | 2.96 (0.21; 6.69) | 8.4 (6.8; 13.0) | 11.6 (8.4; 30.9) | 8.0 (0.4; 15.9) | F(2, 17) = 2.54, p > 0.05 |
| IL-6 | 0.75 (0.28; 3.0) | 80.3 (53.3; 194.6) ^^^ | 59.3 (20.5; 62.5) | 29.9(21.9; 39.9) * | F(2, 17) = 3.33, p = 0.06 |
| IL-7 | 0.07 (0.0; 0.3) | 2.12 (1.04; 3.83) ^ | 0.53 (0.53; 0.53) | 3.26 (0.07; 5.61) | H(2, N = 20) = 1.26, p > 0.05 |
| IL-9 | 114.2 (34.7; 255.4) | 145.1 (59.0; 190.5) | 523.2 (339.6; 686.5) * | 225.1 (100.3; 285.4) | H(2, N = 18) = 6.63, p < 0.05 |
| IL-10 | 0.0 (0.0; 4.07) | 30.5 (11.3; 129.3) ^^^ | 34.5 (4.07; 58.4) | 33.4 (8.2; 131.2) | H(2, N = 20) < 1 |
| IL-12p40 | 1.31 (1.31; 7.62) | 4.7 (1.31; 18.9) | 1.31 (0.0; 1.31) | 0.0 (0.0; 0.0) | H(2, N = 20) = 4.92, p = 0.086 |
| IL-12p70 | 3.8 (0.0; 10.7) | 13.9 (7.5; 30.6) | 13.9 (0.0; 55.3) | 3.8 (0.0; 7.5) | H(2, N = 20) < 1 |
| IL-13 | 80.5 (56.9; 91.6) | 91.6 (60.3; 100.5) | 87.2 (73.8; 91.6) | 87.2 (73.8; 91.6) | H(2, N = 20) < 1 |
| IL-15 | 16.5 (3.0; 37.7) | 37.7 (27.1; 48.4) | 16.5 (5.9; 27.1) | 3.0 (0.0; 27.1) | H(2, N = 20) = 2.87, p > 0.05 |
| IL-17 | 1.21 (0.16; 4.09) | 0.93 (0.0; 2.58) | 0.0 (0.0; 2.05) | 0.0 (0.0; 0.0) | H(2, N = 20) = 3.79, p > 0.05 |
| LIX | 1584.2 (780.9; 2531.5) | 1330.6 (227.4; 1711.1) | 3155.6 (561.5; 3710.4) | 1347.4 (184.5; 2807.1) | F(2, 17) = 1.56, p > 0.05 |
| MCP-1 | 17.7 (0.0; 23.4) | 420.7 (172.0; 3448.9) ^^^ | 356.3 (252.9; 1367.6) | 759.6 (189.1; 2959.2) | H(2, N = 20) < 1 |
| MIG | 35.8 (30.6; 45.6) | 166.4 (115.7; 227.3) ^^^ | 327.2 (68.6; 369.6) | 202.3 (147.1; 263.8) | H(2, N = 20) < 1 |
| MIP-1α | 18.7 (0.0; 37.5) | 82.4 (82.4; 129.6) ^^ | 82.4 (82.4; 119.7) | 73.4 (0.0; 138.6) | H(2, N = 20) < 1 |
| MIP-1β | 55.7 (35.6; 62.7) | 78.7 (74.1; 111.1) | 66.6 (52.8; 74.1) | 81.4 (58.6; 121.6) | H(2, N = 20) = 1.72, p > 0.05 |
| MIP-2 | 18.7 (9.3; 167.7) | 18.7 (0.0; 146.8) | 146.8 (104.8; 218.8) | 0.0 (0.0; 146.8) | H(2, N = 20) = 3.78, p > 0.05 |
| RANTES | 21.7 (9.4; 25.4) | 17.9 (9.6; 22.9) | 17.6 (8.1; 17.6) | 24.8 (15.7; 26.8) | F(2, 17) = 1.95, p > 0.05 |
| Eotaxin | 1403.1 (865.3; 2269.9) | 839.2 (525.5; 1166.6) ^ | 1365.4 (1211.6; 1608.4) * | 1252.9 (804.7; 1378.2) * | F(2, 17) = 3.68, p < 0.05 |
| VEGF | 0.58 (0.19; 0.91) | 1.43 (0.87; 2.17) ^ | 0.87 (0.48; 1.8) | 0.91 (0.68; 1.34) | H(2, N = 20) < 1 |
| M-CSF | 8.2 (4.6; 11.7) | 8.2 (5.8; 1122.1) | 8.2 (8.2; 10.5) | 3.4 (0.96; 3.4) | H(2, N = 20) = 6.22, p < 0.05 |
| G-CSF | 217.4 (192.3; 260.3) | 2711.5 (1898.8; 4267.6) ^^^ | 788.2 (626.5; 819.2) ** | 1210.2 (703.9; 2598.6) * | F(2, 17) = 5.28, p < 0.05 |
| GM-CSF | 27.1 (20.6; 29.9) | 27.1 (20.6; 32.8) | 32.8 (0.0; 37.9) | 10.3 (0.0; 27.1) | H(2, N = 20) = 1.2, p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Akopyan, A.A.; Litvinova, E.A.; Popova, N.A.; Amstislavskaya, T.G.; Khlestkina, E.K. Antitumor Effects of an Anthocyanin-Rich Grain Diet in a Mouse Model of Lewis Lung Carcinoma. Int. J. Mol. Sci. 2024, 25, 5727. https://doi.org/10.3390/ijms25115727
Tikhonova MA, Shoeva OY, Tenditnik MV, Akopyan AA, Litvinova EA, Popova NA, Amstislavskaya TG, Khlestkina EK. Antitumor Effects of an Anthocyanin-Rich Grain Diet in a Mouse Model of Lewis Lung Carcinoma. International Journal of Molecular Sciences. 2024; 25(11):5727. https://doi.org/10.3390/ijms25115727
Chicago/Turabian StyleTikhonova, Maria A., Olesya Y. Shoeva, Michael V. Tenditnik, Anna A. Akopyan, Ekaterina A. Litvinova, Nelly A. Popova, Tamara G. Amstislavskaya, and Elena K. Khlestkina. 2024. "Antitumor Effects of an Anthocyanin-Rich Grain Diet in a Mouse Model of Lewis Lung Carcinoma" International Journal of Molecular Sciences 25, no. 11: 5727. https://doi.org/10.3390/ijms25115727
APA StyleTikhonova, M. A., Shoeva, O. Y., Tenditnik, M. V., Akopyan, A. A., Litvinova, E. A., Popova, N. A., Amstislavskaya, T. G., & Khlestkina, E. K. (2024). Antitumor Effects of an Anthocyanin-Rich Grain Diet in a Mouse Model of Lewis Lung Carcinoma. International Journal of Molecular Sciences, 25(11), 5727. https://doi.org/10.3390/ijms25115727








