Genome-Wide Identification and Expression Analysis of the High-Mobility Group B (HMGB) Gene Family in Plant Response to Abiotic Stress in Tomato
Abstract
:1. Introduction
2. Results
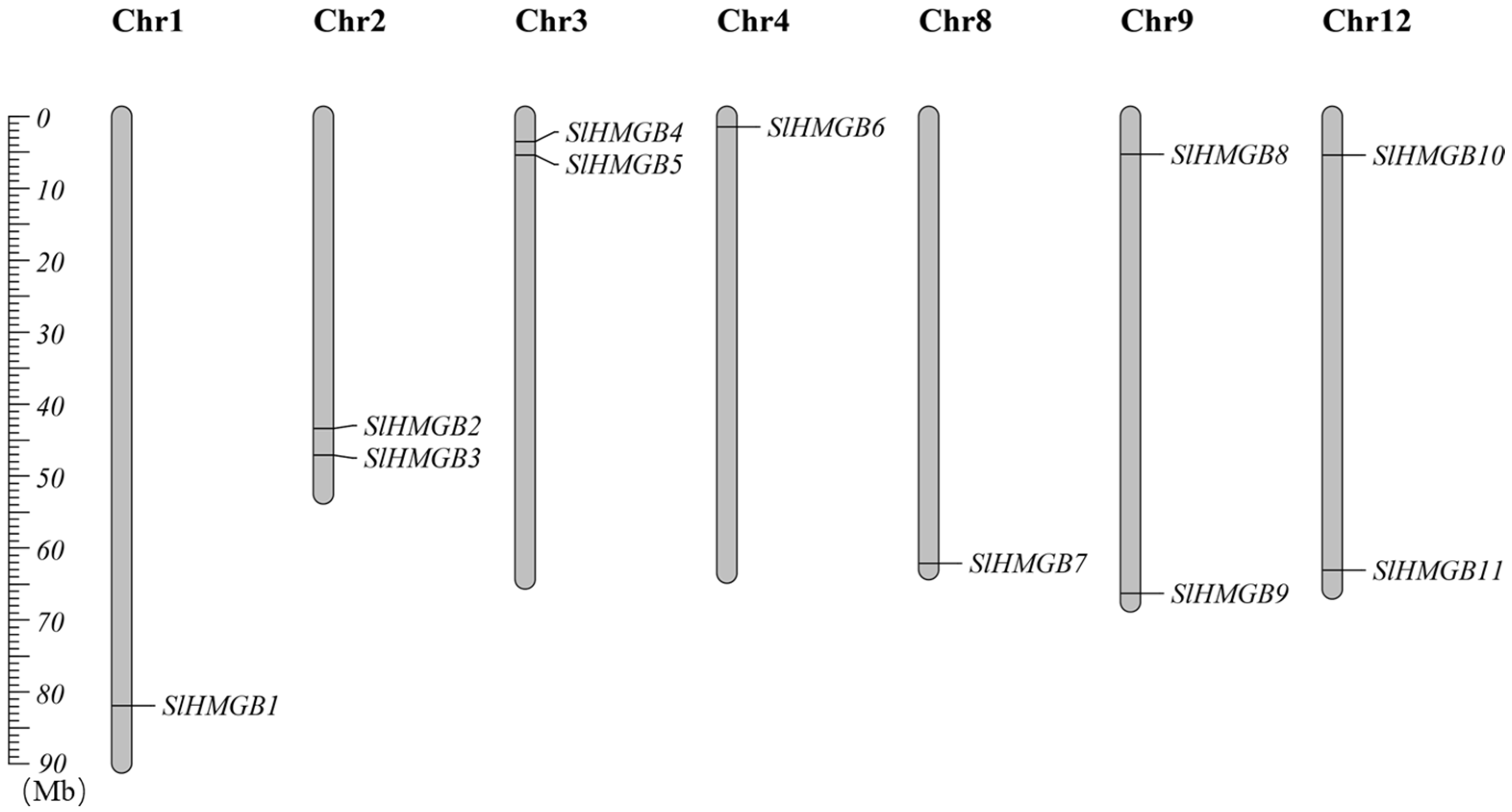
2.1. Identification and Chromosome Localization of the HMGB Genes in Tomato
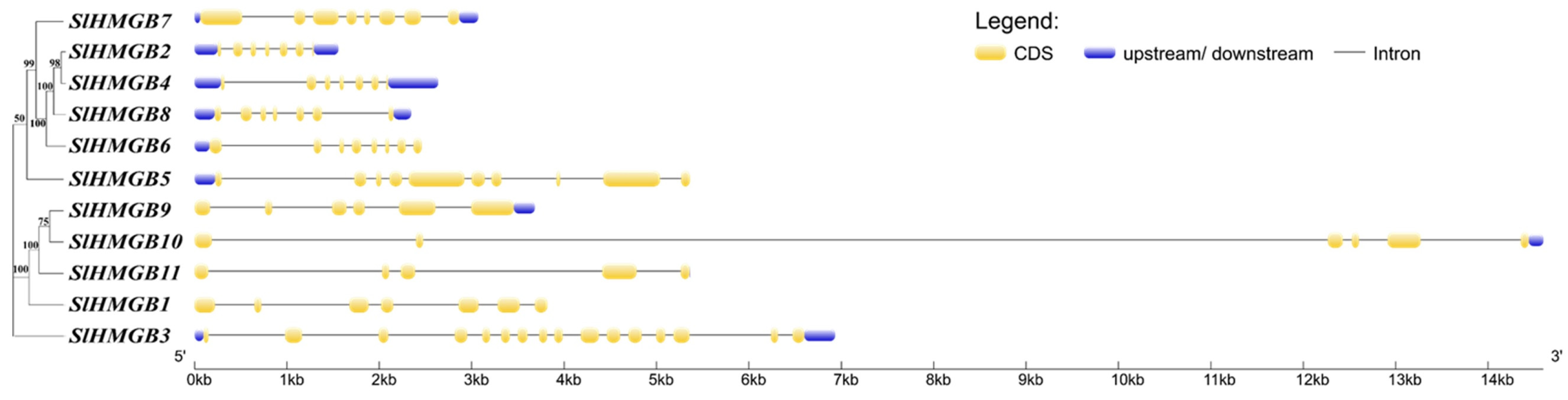
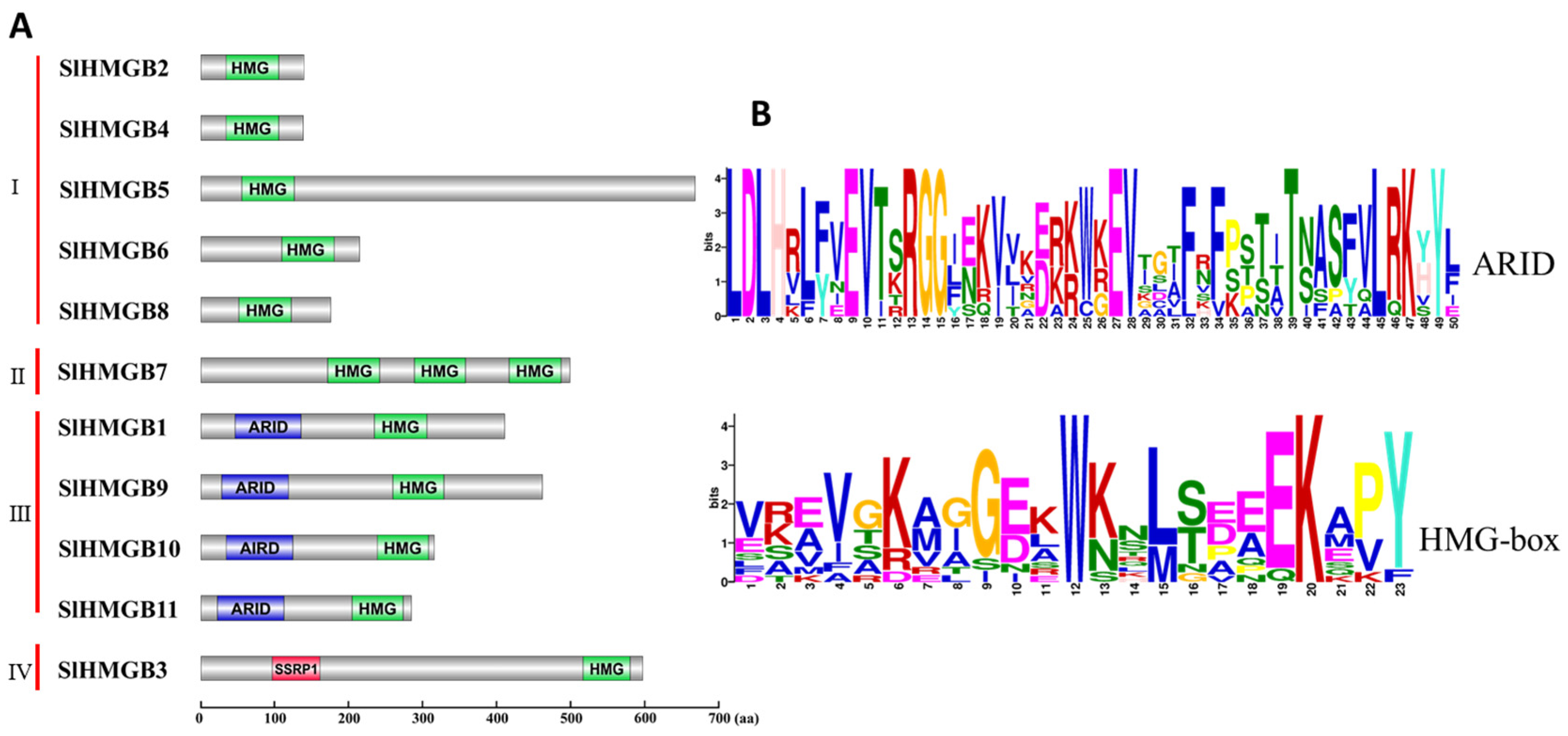
2.2. Gene Structure and Conserved Domains of SlHMGBs
2.3. Phylogenetic Analysis of HMGB Members in Tomato
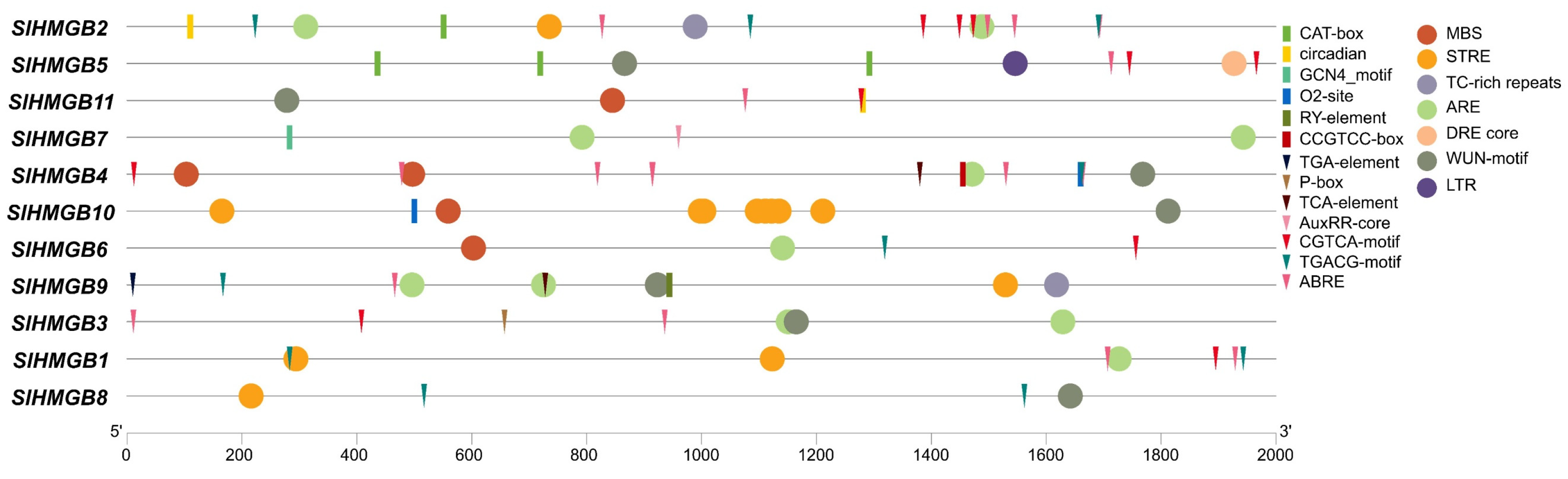
2.4. Cis-Acting Element Analysis of SlHMGB Promoters
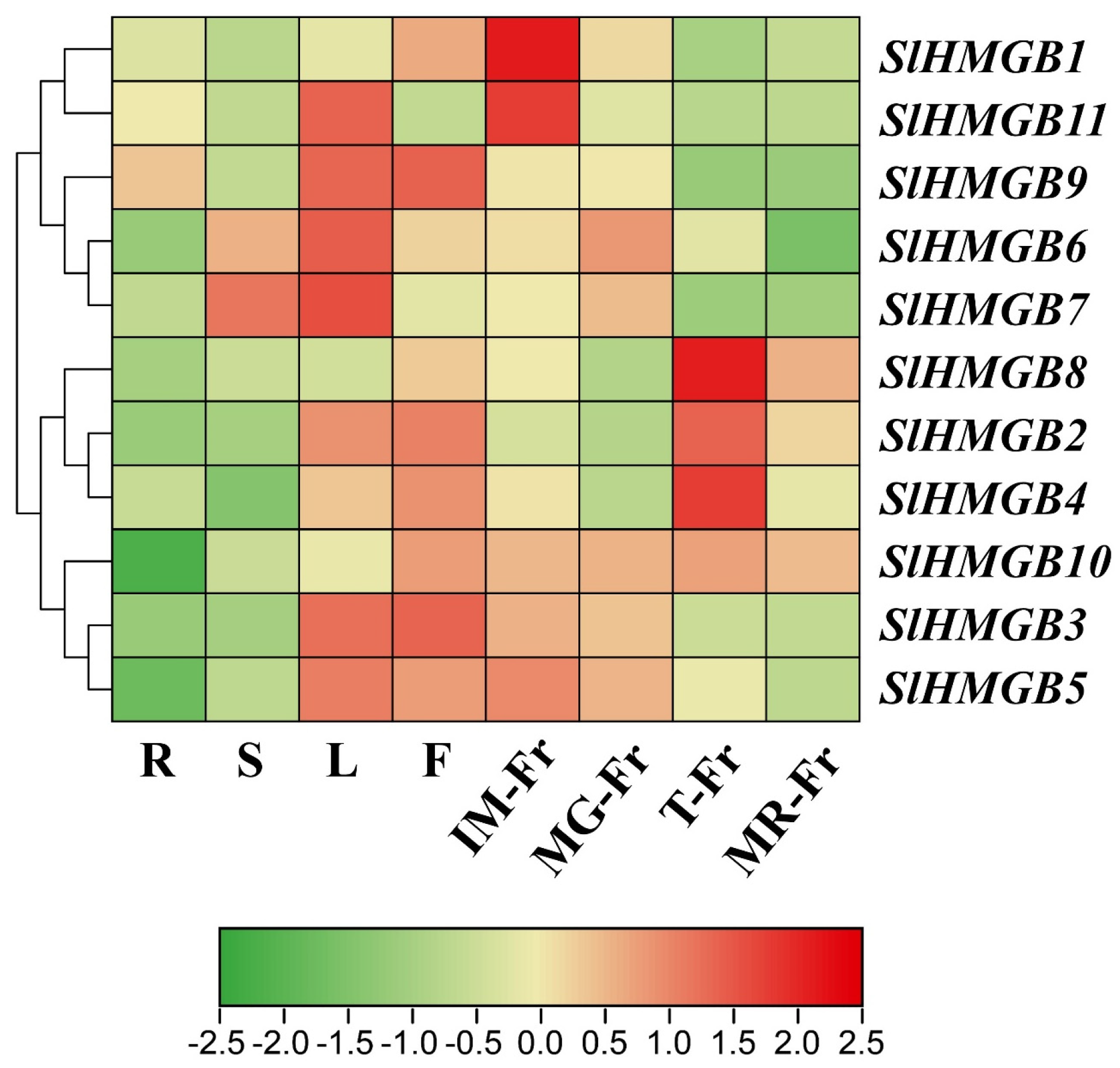
2.5. Analysis of Tissue-Specific Expression Patterns of SlHMGB Genes
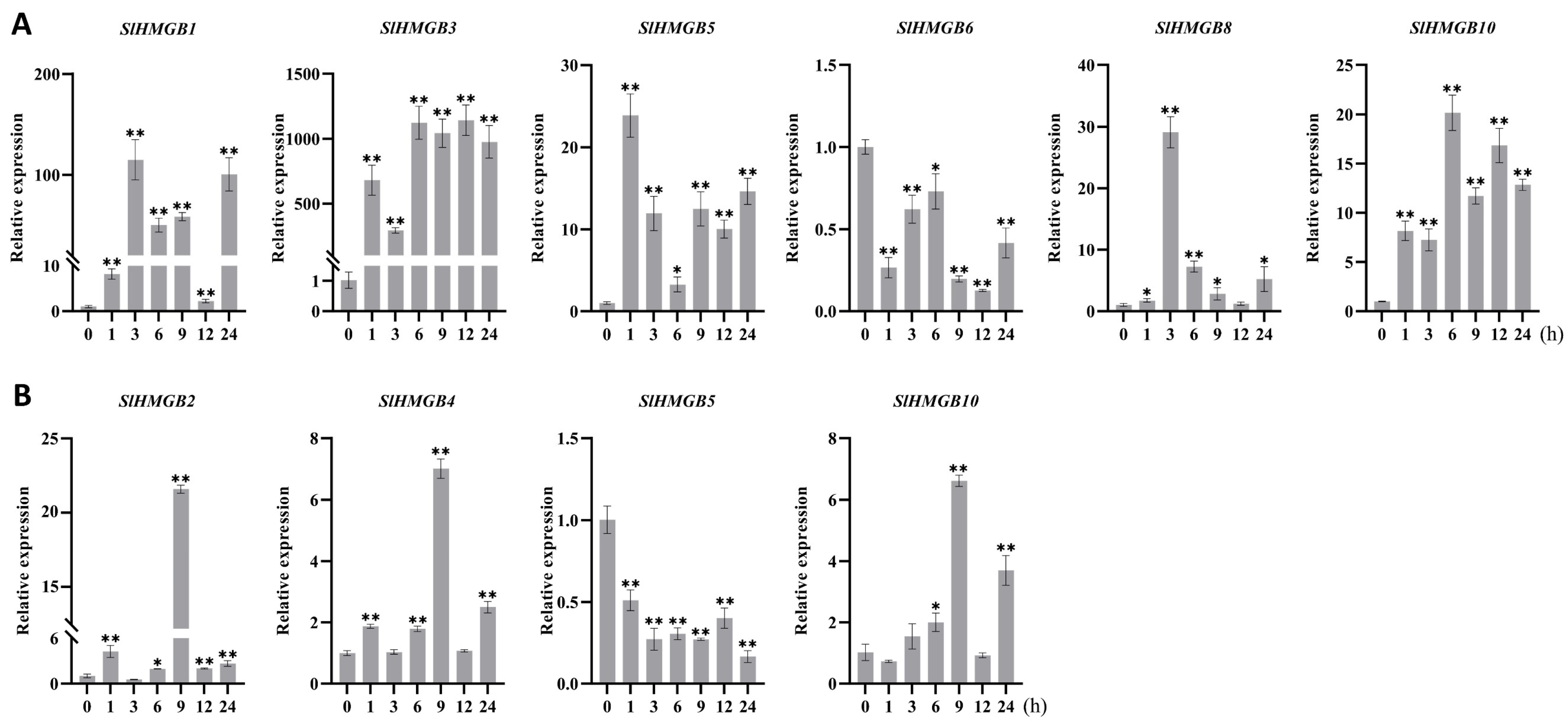
2.6. Expression Analysis of SlHMGB Genes under Abiotic Stress
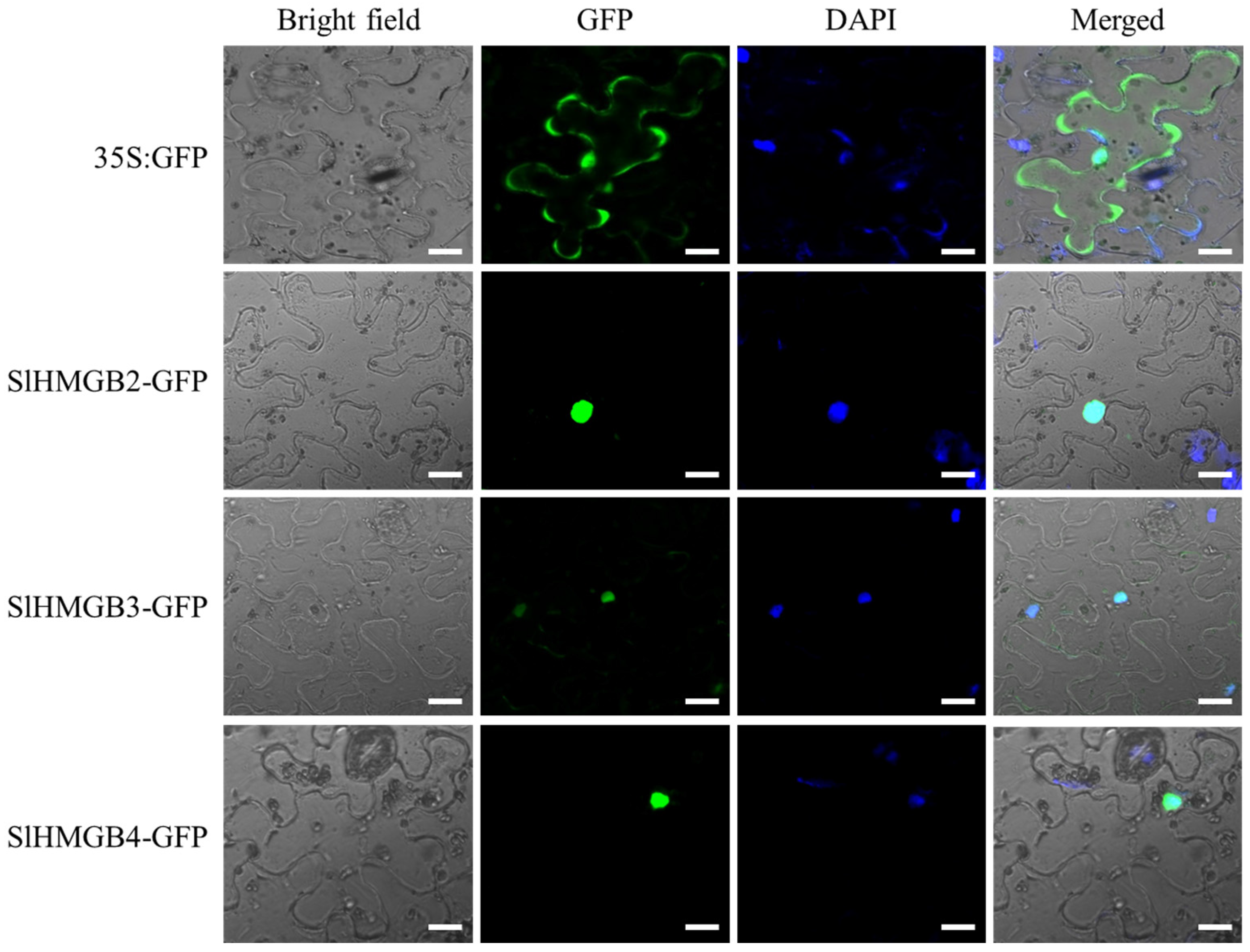
2.7. Subcellular Localization of SlHMGB Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Identification of HMGB Genes in Tomato
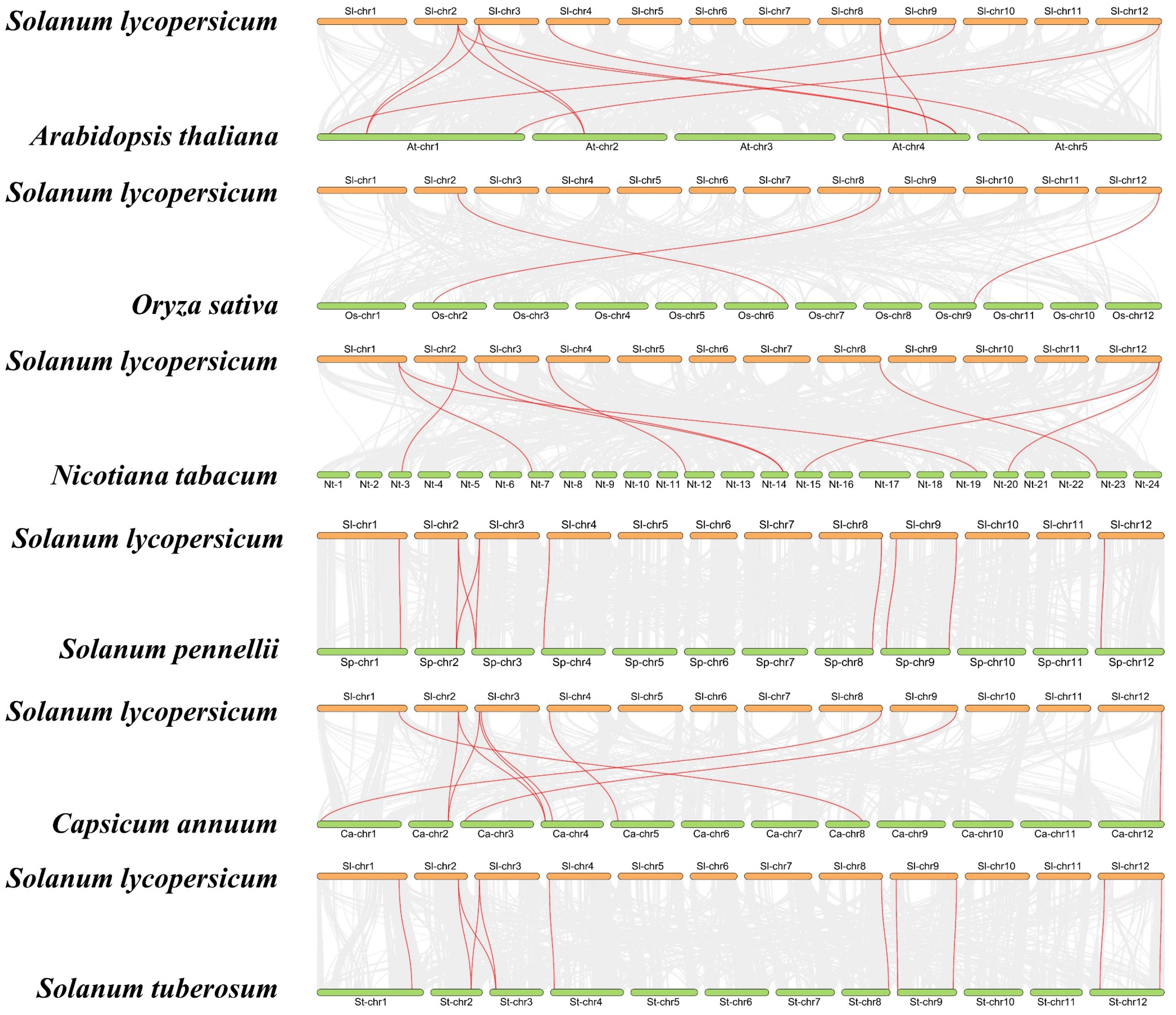
4.3. Gene Structure, Chromosome Mapping, and Syntenic Analysis
4.4. Protein Structural Domain and Motif Analysis
4.5. Phylogenetic Analysis
4.6. Cis-Acting Elements Prediction
4.7. Expression Analyses by Quantitative Real-Time PCR (qRT-PCR)
4.8. Subcellular Localization
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bustin, M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem. Sci. 2001, 26, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Grasser, K.D.; Grill, S.; Duroux, M.; Launholt, D.; Thomsen, M.S.; Nielsen, B.V.; Nielsen, H.K.; Merkle, T. HMGB6 from Arabidopsis thaliana specifies a novel type of plant chromosomal HMGB protein. Biochemistry 2004, 43, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Grasser, K.D.; Launholt, D.; Grasser, M. High mobility group proteins of the plant HMGB family: Dynamic chromatin modulators. Biochim. Biophys. Acta Gene Struct. Expr. 2007, 1769, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Štros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Štros, M.; Launholt, D.; Grasser, K.D. The HMG-box: A versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 2007, 64, 2590–2606. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.S.; Coppens, F.; Ma, L.; Antosch, M.; Marktl, B.; Merkle, T.; Beemster, G.T.; Houben, A.; Grasser, K.D. The plant-specific family of DNA-binding proteins containing three HMG-box domains interacts with mitotic and meiotic chromosomes. New Phytol. 2011, 192, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.K.; Williams, L.A.; Brady-Passerini, K.; Brown, R.H.; Gasser, C.S. Positive- and negative-acting regulatory elements contribute to the tissue-specific expression of INNER NO OUTER, a YABBY-type transcription factor gene in Arabidopsis. BMC Plant Biol. 2012, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Pfab, A.; Grønlund, J.T.; Holzinger, P.; Längst, G.; Grasser, K.D. The Arabidopsis histone chaperone FACT: Role of the HMG-Box domain of SSRP1. J. Mol. Biol. 2018, 430, 2747–2759. [Google Scholar] [CrossRef]
- Grasser, K.D.; Wurz, A.; Feix, G. Isolation and characterization of high-mobility-group proteins from maize. Planta 1991, 185, 350–355. [Google Scholar] [CrossRef]
- Grasser, K.D.; Krech, A.B.; Feix, G. The maize chromosomal HMGa protein recognizes structural features of DNA and increases DNA flexibility. Plant J. 1994, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Goldberg, R.B. A plant DNA binding protein shares highly conserved sequence motifs with HMG-box proteins. Nucleic Acids Res. 1991, 19, 4769. [Google Scholar] [CrossRef] [PubMed]
- Spiker, S. High-mobility group chromosomal proteins of wheat. J. Biol. Chem. 1984, 259, 12007–12013. [Google Scholar] [CrossRef]
- Webster, C.I.; Packman, L.C.; Pwee, K.H.; Gray, J.C. High mobility group proteins HMG-1 and HMG-I/Y bind to a positive regulatory region of the pea plastocyanin gene promoter. Plant J. 1997, 11, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, W.; Pwee, K.-H.; Kumar, P.P. Cloning and characterization of rice HMGB1 gene. Gene 2003, 312, 103–109. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, W.; Pwee, K.-H.; Kumar, P.P. Rice HMGB1 protein recognizes DNA structures and bends DNA efficiently. Arch. Biochem. Biophys. 2003, 411, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, Y.J.; Liang, Y.; Niu, Q.K.; Tan, X.Y.; Chu, L.C.; Chen, L.Q.; Zhang, X.Q.; Ye, D. The ARID-HMG DNA-binding protein AtHMGB15 is required for pollen tube growth in Arabidopsis thaliana. Plant J. 2014, 79, 741–756. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A.A. Dangers in and out. Science 2009, 323, 1683–1684. [Google Scholar] [CrossRef]
- Dinesh-Kumar, S.P.; Choi, H.W.; Manohar, M.; Manosalva, P.; Tian, M.; Moreau, M.; Klessig, D.F. Activation of plant innate immunity by extracellular high mobility group box 3 and its inhibition by salicylic acid. PLoS Pathog. 2016, 12, e1005518. [Google Scholar]
- Lichota, J.; Ritt, C.; Grasser, K.D. Ectopic expression of the maize chromosomal HMGB1 protein causes defects in root development of tobacco seedlings. Biochem. Biophys. Res. Commun. 2004, 318, 317–322. [Google Scholar] [CrossRef]
- Lildballe, D.L.; Pedersen, D.S.; Kalamajka, R.; Emmersen, J.; Houben, A.; Grasser, K.D. The expression level of the chromatin-associated HMGB1 protein influences growth, stress tolerance, and transcriptome in Arabidopsis. J. Mol. Biol. 2008, 384, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kwak, K.J.; Kang, H. Expression of a high mobility group protein isolated from Cucumis sativus affects the germination of Arabidopsis thaliana under abiotic stress conditions. J. Integr. Plant Biol. 2008, 50, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.J.; Kim, J.Y.; Kim, Y.O.; Kang, H. Characterization of transgenic Arabidopsis plants overexpressing high mobility group B proteins under high salinity, drought or cold stress. Plant Cell Physiol. 2007, 48, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chinpongpanich, A.; Phean-O-Pas, S.; Thongchuang, M.; Qu, L.-J.; Buaboocha, T. C-terminal extension of calmodulin-like 3 protein from Oryza sativa L.: Interaction with a high mobility group target protein. Acta Biochim. Biophys. Sin. 2015, 47, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, S.; Li, T.; Yu, S.; Zhao, H.; Liu, H.; Luo, L. Overexpression of OsHMGB707, a high mobility group protein, enhances rice drought tolerance by promoting stress-related gene expression. Front. Plant Sci. 2021, 12, 711271. [Google Scholar] [CrossRef]
- Li, S.; Xin, M.; Luan, J.; Liu, D.; Wang, C.; Liu, C.; Zhang, W.; Zhou, X.; Qin, Z. Overexpression of CsHMGB alleviates phytotoxicity and propamocarb residues in cucumber. Front. Plant Sci. 2020, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Liu, Z.; Li, X.; Tan, B.; Wu, J.; Gao, C. Screening and functional identification of salt tolerance HMG genes in Betula platyphylla. Environ. Exp. Bot. 2021, 181, 104235. [Google Scholar] [CrossRef]
- Grasser, K.D.; Wohlfarth, T.; Bäumlein, H.; Feix, G. Comparative analysis of chromosomal HMG proteins from monocotyledons and dicotyledons. Plant Mol. Biol. 1993, 23, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Minamikawa, T. Isolation and characterization of a cDNA encoding a high mobility group protein HMG-1 from Canavalia gladiata D.C. Biochim. Biophys. Acta. 1998, 1396, 47–50. [Google Scholar] [CrossRef]
- Zheng, C.C.; Bui, A.Q.; O’Neill, S.D. Abundance of an mRNA encoding a high mobility group DNA-binding protein is regulated by light and an endogenous rhythm. Plant Mol. Biol. 1993, 23, 813–823. [Google Scholar] [CrossRef]
- Van Lijsebettens, M.; Grasser, K.D. The role of the transcript elongation factors FACT and HUB1 in leaf growth and the induction of flowering. Plant Signal. Behav. 2010, 5, 715–717. [Google Scholar] [CrossRef]
- Michl-Holzinger, P.; Mortensen, S.A.; Grasser, K.D. The SSRP1 subunit of the histone chaperone FACT is required for seed dormancy in Arabidopsis. J. Plant Physiol. 2019, 236, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Pfab, A.; Breindl, M.; Grasser, K.D. The Arabidopsis histone chaperone FACT is required for stress-induced expression of anthocyanin biosynthetic genes. Plant Mol. Biol. 2018, 96, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.J.; Arwood, L.J.; Spiker, S.; Guiltinan, M.J.; Thompson, W.F. High mobility group chromosomal proteins bind to AT-rich tracts flanking plant genes. Plant Mol. Biol. 1991, 16, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ritt, C.; Grimm, R.; Fernandez, S.; Alonso, J.C.; Grasser, K.D. Basic and acidic regions flanking the HMG domain of maize HMGa modulate the interactions with DNA and the self-association of the protein. Biochemistry 1998, 37, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Q.; Pwee, K.H.; Manjunatha Kini, R. Interaction of wheat high-mobility-group proteins with four-way-junction DNA and characterization of the structure and expression of HMGA gene. Arch. Biochem. Biophys. 2003, 409, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Antosch, M.; Mortensen, S.A.; Grasser, K.D. Plant Proteins Containing High Mobility Group Box DNA-Binding Domains Modulate Different Nuclear Processes. Plant Physiol. 2012, 159, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Niu, S.; Yan, S.; Zhang, Y. Identification and characterization of the CONSTANS (CO)/CONSTANS-like (COL) genes related to photoperiodic signaling and flowering in tomato. Plant Sci. 2020, 301, 110653. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef]
- Han, W.; Zhang, Q.; Suo, Y.; Li, H.; Diao, S.; Sun, P.; Huang, L.; Fu, J. Identification and Expression Analysis of the bHLH Gene Family Members in Diospyros kaki. Horticulturae 2023, 9, 380. [Google Scholar] [CrossRef]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Yun, L.; Ji, L.; Li, G.; Ji, M.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J. Mol. Sci. 2022, 23, 542. [Google Scholar] [CrossRef]
- He, Y.; Yang, T.; Yan, S.; Niu, S.; Zhang, Y. Identification and characterization of the BEL1-like genes reveal their potential roles in plant growth and abiotic stress response in tomato. Int. J. Biol. Macromol. 2022, 200, 193–205. [Google Scholar] [CrossRef]
- Yin, L.; Sun, Y.; Chen, X.; Liu, J.; Feng, K.; Luo, D.; Sun, M.; Wang, L.; Xu, W.; Liu, L.; et al. Genome-wide analysis of the hd-zip gene family in Chinese cabbage (Brassica rapa subsp. pekinensis) and the expression pattern at high temperatures and in carotenoids regulation. Agronomy 2023, 13, 1324. [Google Scholar]
- Chu, Z.; Wang, X.; Li, Y.; Yu, H.; Li, J.; Lu, Y.; Li, H.; Ouyang, B. Genomic organization, phylogenetic and expression analysis of the B-box gene family in tomato. Front. Plant Sci. 2016, 7, 1552. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Gene ID | Genomic Sequence (bp) | CDS Sequence (bp) | Protein | Subcellular Localization Prediction | ||
|---|---|---|---|---|---|---|---|
| Length (aa) | MW (kDa) | pI | |||||
| SlHMGB1 | Solyc01g100620 | 3819 | 1236 | 411 | 45.93 | 8.77 | Nuclear |
| SlHMGB2 | Solyc02g082700 | 1556 | 423 | 140 | 15.66 | 7.76 | Nuclear |
| SlHMGB3 | Solyc02g087710 | 6929 | 1920 | 639 | 71.09 | 5.56 | Nuclear |
| SlHMGB4 | Solyc03g032130 | 2637 | 420 | 139 | 15.52 | 6.21 | Nuclear |
| SlHMGB5 | Solyc03g043600 | 5360 | 2007 | 668 | 74.46 | 7.46 | Nuclear |
| SlHMGB6 | Solyc04g008820 | 2457 | 648 | 215 | 23.74 | 5.46 | Nuclear |
| SlHMGB7 | Solyc08g082070 | 3069 | 1500 | 499 | 58.69 | 9.27 | Nuclear |
| SlHMGB8 | Solyc09g014620 | 2346 | 531 | 176 | 19.96 | 5.62 | Nuclear |
| SlHMGB9 | Solyc09g091960 | 3680 | 1389 | 462 | 52.89 | 6.22 | Nuclear |
| SlHMGB10 | Solyc12g016190 | 14,596 | 951 | 316 | 35.84 | 9.3 | Nuclear |
| SlHMGB11 | Solyc12g094440 | 5362 | 858 | 285 | 32.89 | 9.44 | Nuclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Tang, H.; Wang, J.; Liu, Y.; Ge, L.; Liu, G.; Shi, Q.; Zhang, Y. Genome-Wide Identification and Expression Analysis of the High-Mobility Group B (HMGB) Gene Family in Plant Response to Abiotic Stress in Tomato. Int. J. Mol. Sci. 2024, 25, 5850. https://doi.org/10.3390/ijms25115850
Zheng J, Tang H, Wang J, Liu Y, Ge L, Liu G, Shi Q, Zhang Y. Genome-Wide Identification and Expression Analysis of the High-Mobility Group B (HMGB) Gene Family in Plant Response to Abiotic Stress in Tomato. International Journal of Molecular Sciences. 2024; 25(11):5850. https://doi.org/10.3390/ijms25115850
Chicago/Turabian StyleZheng, Jinhui, Huimeng Tang, Jianquan Wang, Yue Liu, Lianjing Ge, Guobiao Liu, Qinghua Shi, and Yan Zhang. 2024. "Genome-Wide Identification and Expression Analysis of the High-Mobility Group B (HMGB) Gene Family in Plant Response to Abiotic Stress in Tomato" International Journal of Molecular Sciences 25, no. 11: 5850. https://doi.org/10.3390/ijms25115850





