N-Acetylgalactosamine-4-sulfatase (Arylsulfatase B) Regulates PD-L1 Expression in Melanoma by an HDAC3-Mediated Epigenetic Mechanism
Abstract
:1. Introduction
2. Results
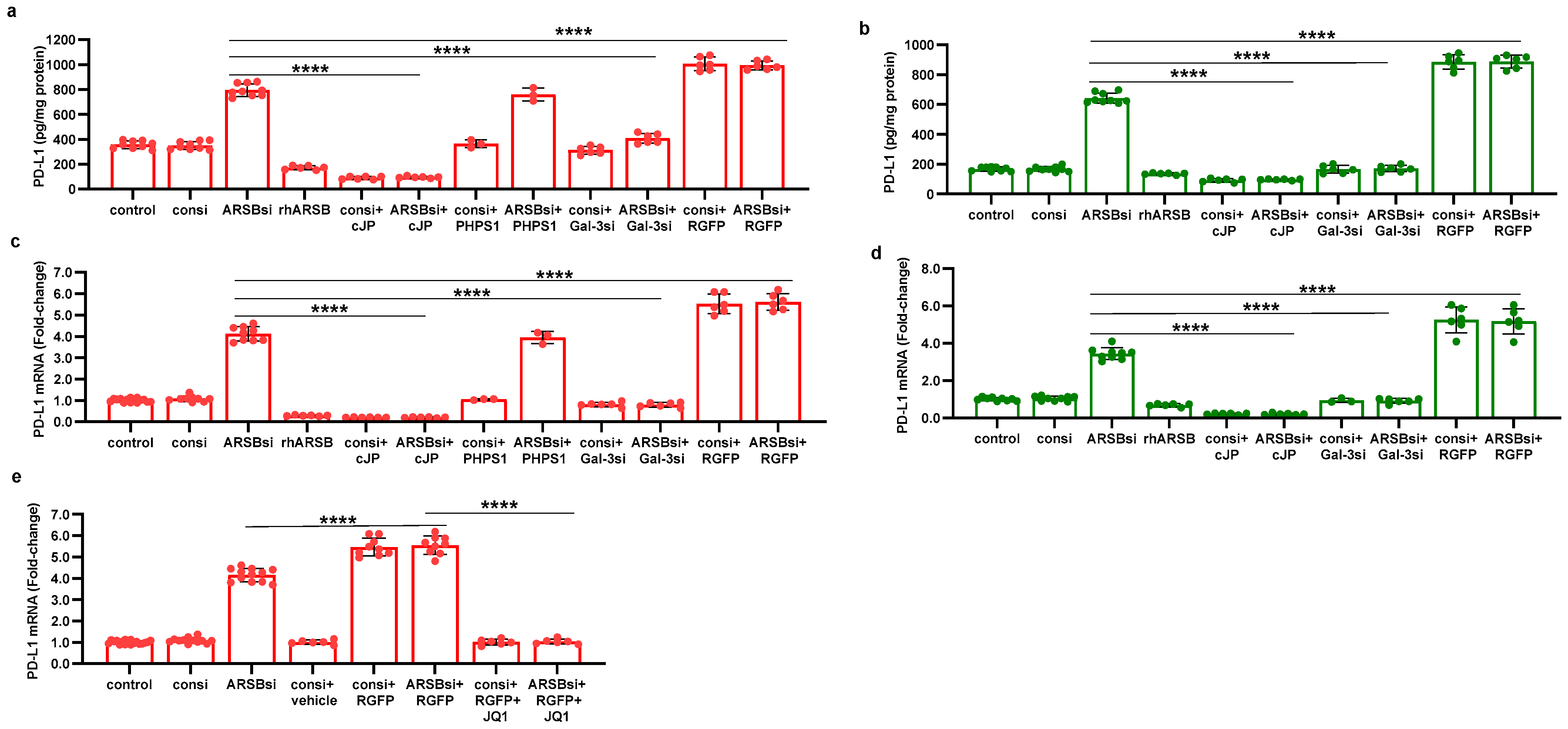
2.1. PD-L1 Expression Is Reduced by Exogenous ARSB and Increased by ARSB Knockdown
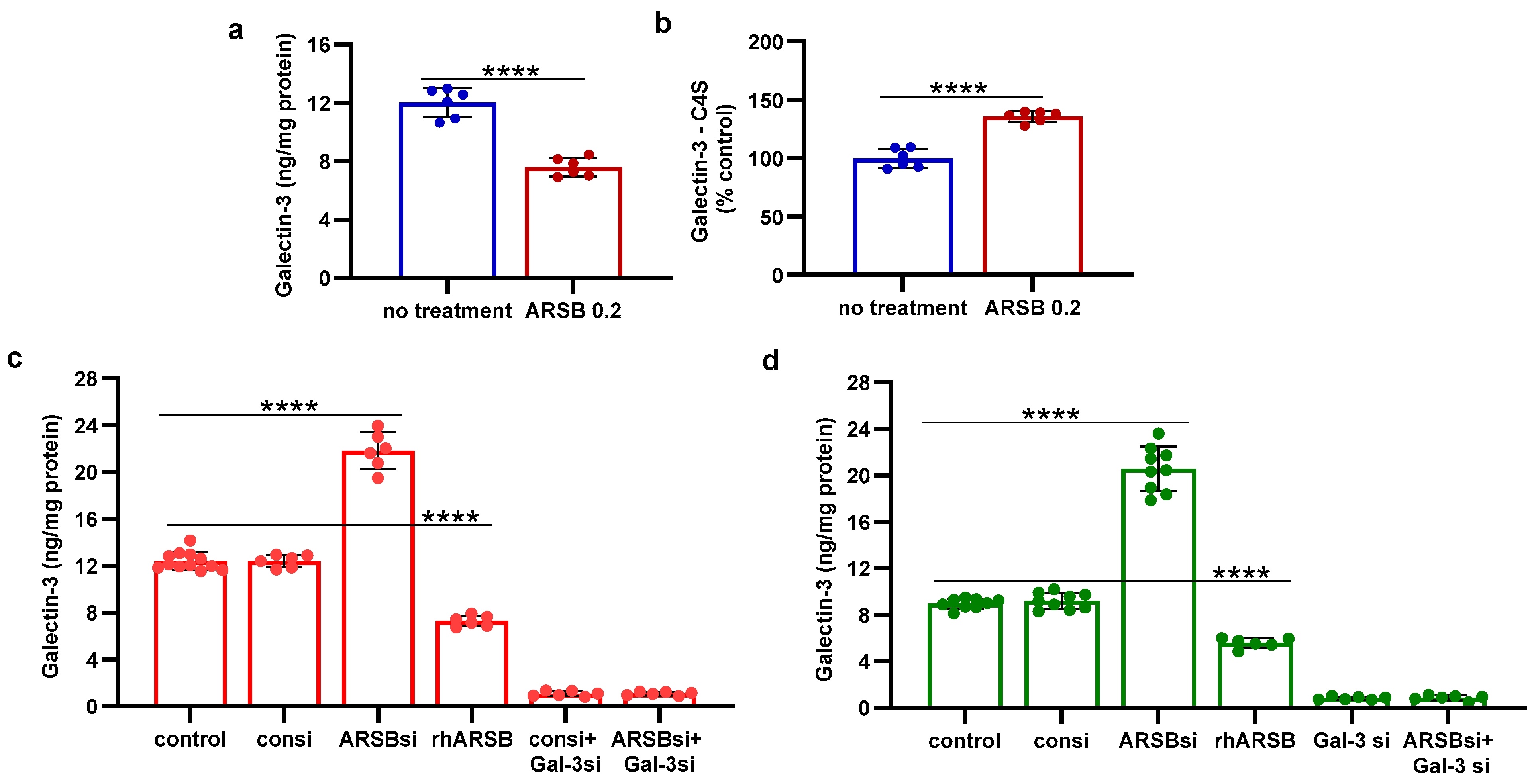
2.2. Exogenous ARSB Reduces Free Galectin-3 Due to Increased Galectin-3 Binding with Chondroitin 4-Sulfate
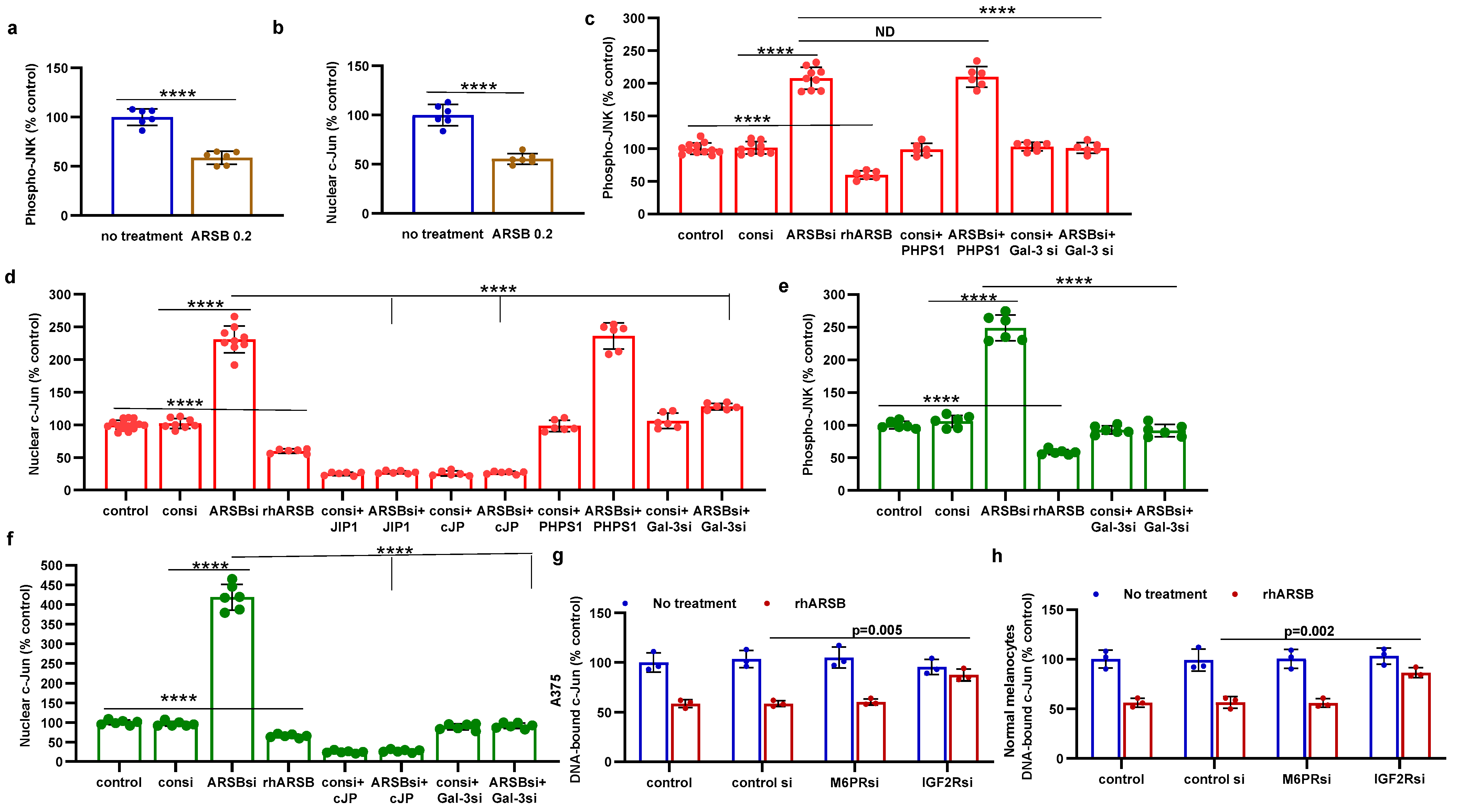
2.3. Impact of Galectin-3 on Phospho-(Thr183/Tyr185)-JNK and Nuclear c-Jun
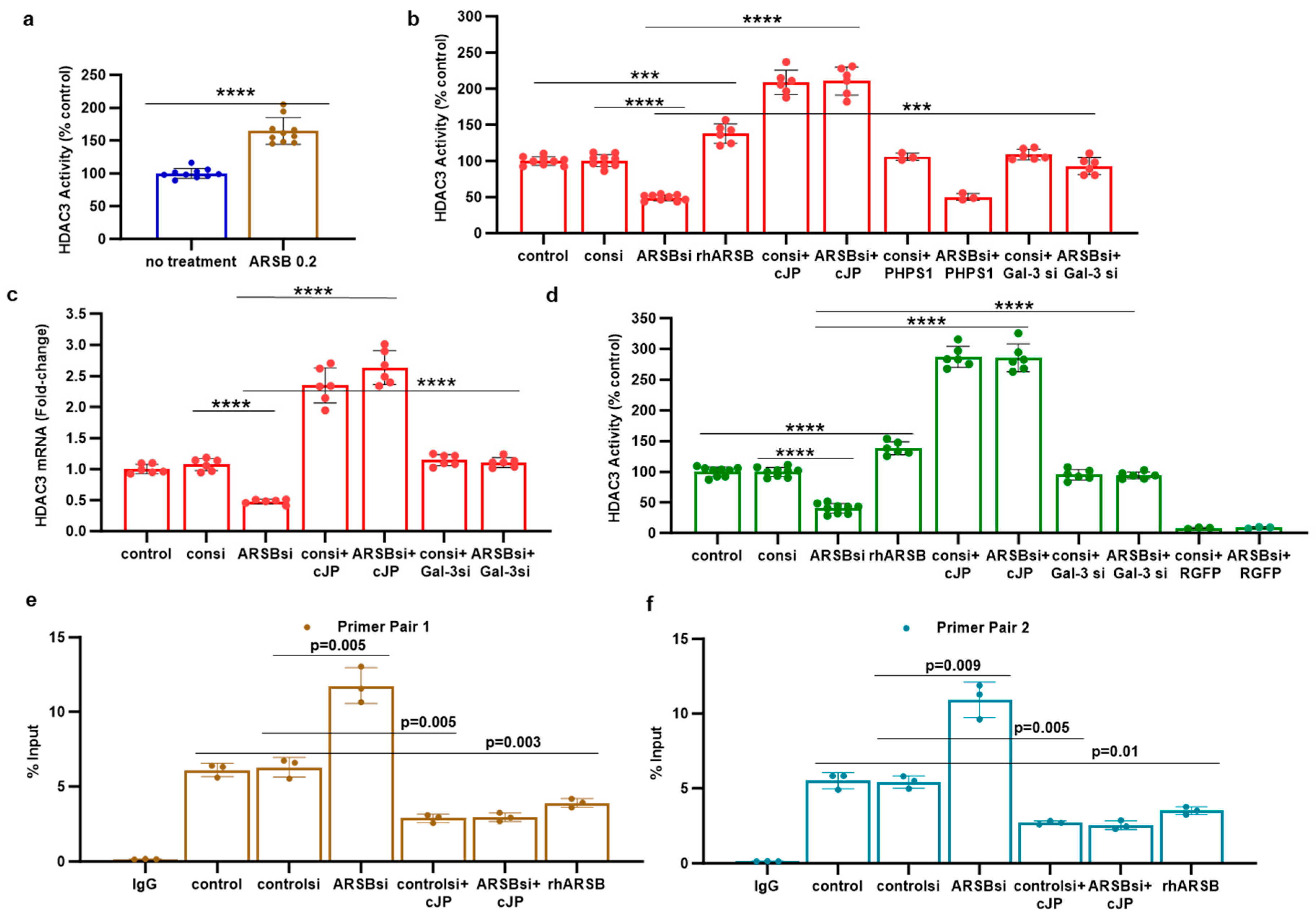
2.4. HDAC3 Activity and Expression Is Modulated by ARSB and Galectin-3
2.5. Regulation of PD-L1 Promoter by H3 Acetylation
2.6. Impact of β-D-Xyloside on Mediators of PD-L1 Expression
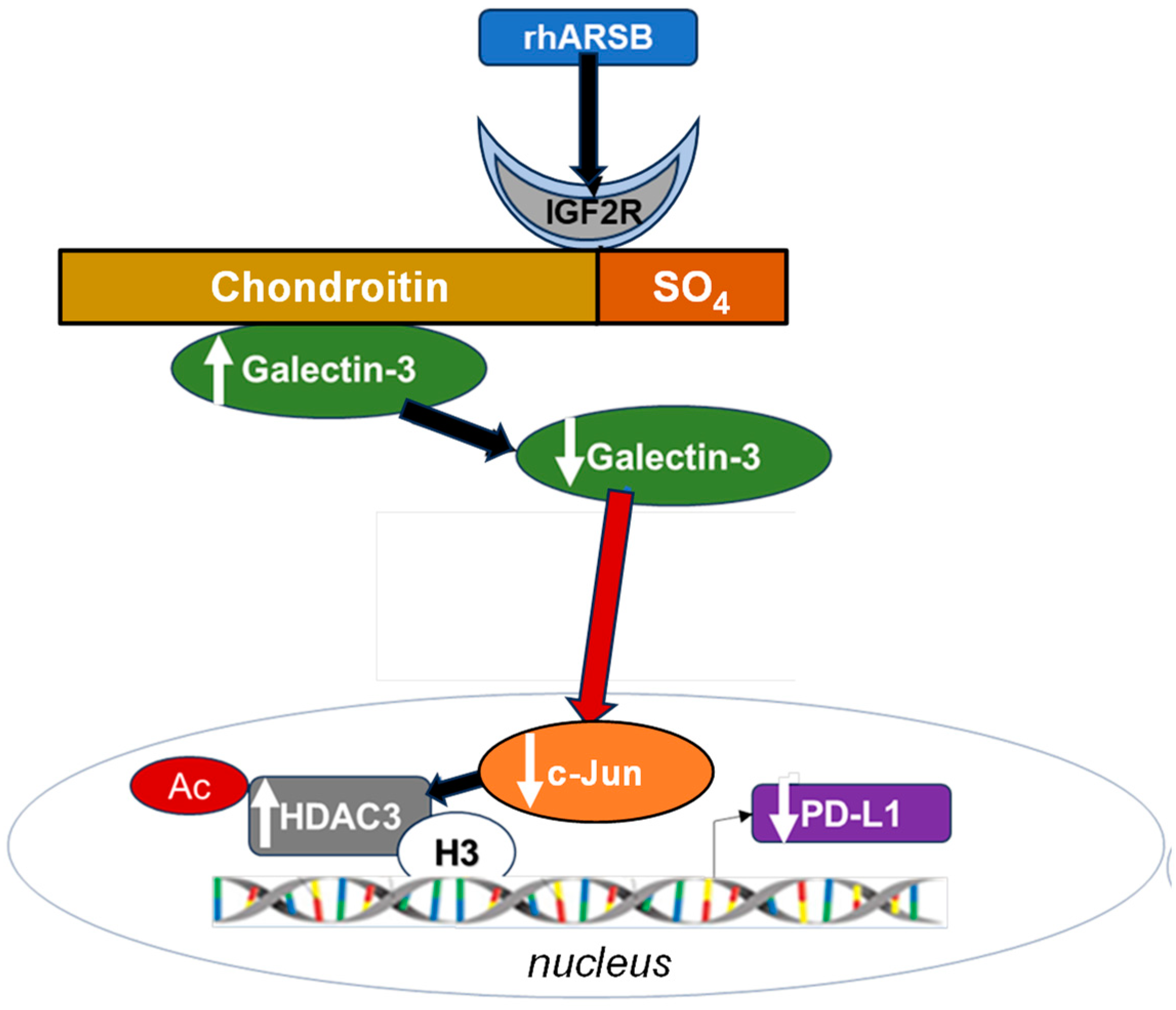
2.7. Overall Pathway of PD-L1 Expression by ARSB
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animal Procedures
4.3. Treatment of A375 Human Melanoma Cells by Exogenous ARSB, siRNAs, and Other Agents
4.4. Treated and Control Cells Were Harvested and Frozen at −80 °C for Subsequent Analysis
4.5. Immunohistochemistry of PD-L1 in A375 Cells
4.6. Arylsulfatase B (ARSB) Activity Assay
4.7. Measurement of Total Sulfated Glycosaminoglycans (sGAGs) and Chondroitin-4-Sulfate
4.8. Total Sulfotransferase Activity
4.9. ELISAs for Galectin-3, Phospho-(Thr183/Tyr185)-JNK, PD-L1
4.10. Oligonucleotide-Based ELISA to Detect Nuclear c-Jun
4.11. HDAC3 Activity and Expression
4.12. mRNA Expression of HDAC3 and PDL1
4.13. Chromatin Immunoprecipitation (ChIP) Assay for Histone 3-Acetylation
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Pilon-Thomas, S.; Mackay, A.; Vohra, N.; Mulé, J.J. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J. Immunol. 2010, 184, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Pizzimenti, C.; Franchina, M.; Pepe, L.; Russotto, F.; Tralongo, P.; Micali, M.G.; Militi, G.B.; Lentini, M. Programmed cell death ligand 1 immunohistochemical expression cutaneous melanoma: A controversial relationship. Int. J. Mol. Sci. 2024, 25, 676. [Google Scholar] [CrossRef]
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010, 116, 1757–1766. [Google Scholar] [CrossRef]
- Massi, D.; Brusa, D.; Merelli, B.; Ciano, M.; Audrito, V.; Serra, S.; Buonincontri, R.; Baroni, G.; Nassini, R.; Minocci, D.; et al. PD-L1 marks a subset of melanomas with a shorter overall survival distinct genetic morphological characteristics. Ann. Oncol. 2014, 25, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; O-Sullivan, I.; Tu, J.; Chen, Z.; Tobacman, J.K. Exogenous recombinant N-acetylgalactosamine-4-sulfatase (Arylsulfatase B.; ARSB) inhibits progression of B16F10 cutaneous melanomas and modulates cell signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166913. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Feferman, L.; Terai, K.; Dudek, A.Z.; Tobacman, J.K. Decline in arylsulfatase B leads to increased invasiveness of melanoma cells. Oncotarget 2017, 17, 4169–4180. [Google Scholar] [CrossRef]
- Tobacman, J.K.; Bhattacharyya, S. Profound impact of decline in N-acetylgalactosamine-4-sulfatase (Arylsulfatase B) on molecular pathophysiology and human diseases. Int. J. Mol. Sci. 2022, 23, 13146. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Han, X.; Ouyang, Y.; Zhang, F.; Linhardt, R.J.; Tobacman, J.K. Decline in arylsulfatase B expression increases EGFR expression by inhibiting the protein-tyrosine phosphatase SHP2 and activating JNK in prostate cells. J. Biol. Chem. 2018, 293, 11076–11087. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Arylsulfatase B regulates versican expression by galectin-3 and AP-1 mediated transcriptional effects. Oncogene 2014, 33, 5467–5476. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Chondroitin sulfatases differentially regulate Wnt signaling in prostate stem cells through effects on SHP2, phospho-ERK1/2, and Dickkopf Wnt signaling pathway inhibitor (DKK3). Oncotarget 2017, 8, 100242–100260. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Increased expression of colonic Wnt9A through Sp1-mediated transcriptional effects involving arylsulfatase B, chondroitin 4-sulfate, and galectin-3. J. Biol. Chem. 2014, 289, 17564–17575. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Tobacman, J.K. Hypoxia reduces arylsulfatase B activity and silencing arylsulfatase B replicates and mediates the effects of hypoxia. PLoS ONE 2012, 7, e33250. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.M.; Sodré, A.L.; Villagra, A.; Sarnaik, A.; Sotomayor, E.M.; Weber, J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol. Res. 2015, 3, 1375–1385. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, F.; Sherwin, S.; Farrelly, M.; Yan, X.G.; Croft, A.; Liu, T.; Jin, L.; Zhang, X.D.; Jiang, C.C. Cooperativity of HOXA5 and STAT3 is critical for HDAC8 inhibition-mediated transcriptional activation of PD-L1 in human melanoma cells. J. Investig. Dermatol. 2018, 138, 922–932. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.L.; Poklepovic, A.; Kirkwood, J.; Dent, P. HDAC inhibitors enhance the immunotherapy response of melanoma cells. Oncotarget 2017, 8, 83155–83170. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Ahn, A.; Stockwell, P.A.; Parry, M.; Motwani, J.; Gallagher, S.J.; Shklovskaya, E.; Tiffen, J.; Eccles, M.R.; et al. Marked Global DNA hypomethylation Is associated with constitutive PD-L1 expression in melanoma. iScience 2018, 4, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.J.; Tiffen, J.C.; Hersey, P. Histone modifications, modifiers and readers in melanoma resistance to targeted and immune therapy. Cancers 2015, 7, 1959–1982. [Google Scholar] [CrossRef]
- Hicks, K.C.; Fantini, M.; Donahue, R.N.; Schwab, A.; Knudson, K.M.; Tritsch, S.R.; Jochems, C.; Clavijo, P.E.; Allen, C.T.; Hodge, J.W.; et al. Epigenetic priming of both tumor and NK cells augments antibody-dependent cellular cytotoxicity elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. Oncoimmunology 2018, 7, e1466018. [Google Scholar] [CrossRef]
- Darvin, P.; Sasidharan Nair, V.; Elkord, E. PD-L1 expression in human breast cancer stem cells is epigenetically regulated through posttranslational histone modifications. J. Oncol. 2019, 2019, 3958908. [Google Scholar] [CrossRef]
- Lienlaf, M.; Perez-Villarroel, P.; Knox, T.; Pabon, M.; Sahakian, E.; Powers, J.; Woan, K.V.; Lee, C.; Cheng, F.; Deng, S.; et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol. Oncol. 2016, 10, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, C.; Du, J.; Wang, H.; He, R.; Yin, X.; Li, H.; Li, X.; Wang, H.; Li, K.; et al. Enhanced histone H3 acetylation of the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for PD-L1 expression in drug-resistant cancer cells. J. Exp. Clin. Cancer Res. 2020, 39, 29. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Hu, Q.; Zhang, H.; Yang, F.; Peng, C.; Huang, C. HDAC3 inhibition upregulates PD-L1 expression in B-cell lymphomas and augments the efficacy of anti-PD-L1 therapy. Mol. Cancer Ther. 2019, 18, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, X.; Liu, R.; Pan, Y.; Fang, J.; Cao, L.; Feng, C.; Shang, Q.; Chen, Y.; Shao, C.; et al. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene 2021, 40, 1836–1850. [Google Scholar] [CrossRef] [PubMed]
- Mondello, P.; Tadros, S.; Teater, M.; Fontan, L.; Chang, A.Y.; Jain, N.; Yang, H.; Singh, S.; Ying, H.Y.; Chu, C.S.; et al. Selective inhibition of HDAC3 targets synthetic vulnerabilities and activates immune surveillance in lymphoma. Cancer Discov. 2020, 10, 440–459. [Google Scholar] [CrossRef] [PubMed]
- Elleback, E.; Khan, S.; Bastholt, L.; Schmidt, H.; Haslund, C.A.; Donis, M.; Svane, I.M. PD-L1 is a biomarker of real-world clinical outcomes for anti-CTLA-4 plus anti-PD-1 or anti-PD-1 monotherapy in metastatic melanoma. Eur. J. Cancer 2023, 198, 113476. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Chen, J.; Ren, Y.; Liu, J.; Yu, Z.; Cao, H.; Chen, J. Discovery of novel histone deacetylase 6 (HDAC6) inhibitors with enhanced antitumor immunity of anti-PD-L1 immunotherapy in melanoma. J. Med. Chem. 2022, 65, 2434–2457. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, Z.; Surineni, G.; Deng, B.; Zhang, M.; Li, C.; Sun, Z.; Pan, W.; Liu, Y.; Liu, S.; et al. Discovery of novel benzohydroxamate-based histone deacetylase 6 (HDAC6) inhibitors with the ability to potentiate anti-PD-L1 immunotherapy in melanoma. J. Enzyme Inhib. Med. Chem. 2023, 38, 2201408. [Google Scholar] [CrossRef] [PubMed]
- Yeon, M.; Kim, Y.; Jung, H.S.; Jeoung, D. Histone deacetylase inhibitors to overcome resistance to targeted and immuno therapy in metastatic melanoma. Front. Cell Dev. Biol. 2020, 8, 486. [Google Scholar] [CrossRef]
- Knox, T.; Sahakian, E.; Banik, D.; Hadley, M.; Palmer, E.; Noonepalle, S.; Kim, J.; Powers, J.; Gracia-Hernandez, M.; Oliveira, V.; et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of im-munosuppressive proteins in tumor cells. Sci. Rep. 2019, 9, 6136. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Liu, Z.; Li, Z.; Wu, Y.; Zuo, D. Recent advances of molecular mechanisms of regulating PD-L1 expression in melanoma. Int. Immunopharmacol. 2020, 88, 106971. [Google Scholar] [CrossRef] [PubMed]
- Dahms, N.M.; Lobel, P.; Kornfeld, S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Biol. Chem. 1989, 264, 12115–12118. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, J.; Minamisawa, T.; Tateno, H.; Kominami, J.; Suzuki, K.; Nishi, N.; Nakamura, T.; Hirabayashi, J. Desulfated galactosaminoglycans are potential ligands for galectins: Evidence from frontal affinity chromatography. Biochem. Biophys. Res. Commun. 2008, 373, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wolter, S.; Doerrie, A.; Weber, A.; Schneider, H.; Hoffmann, E.; von der Ohe, J.; Bakiri, L.; Wagner, E.F.; Resch, K.; Kracht, M. c-Jun controls histone modifications, NF-kappaB recruitment, and RNA polymerase II function to activate the ccl2 gene. Mol. Cell Biol. 2008, 28, 4407–4423. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Schneider, S.; Wagner, E.F.; Zhang, X.; Seto, E.; Bohmann, D. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J. 2003, 22, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.B. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J. Biol. Chem. 1977, 252, 6316–6321. [Google Scholar] [CrossRef] [PubMed]
- Feferman, L.; Bhattacharyya, S.; Oates, E.; Haggerty, N.; Wang, T.; Varady, K.; Tobacman, J.K. Carrageenan-free diet shows improved glucose tolerance and insulin signaling in prediabetes: A randomized, pilot clinical trial. J. Diabetes Res. 2020, 2020, 8267980. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The yin and yang relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Inhibition of phosphatase activity follows decline in sulfatase activity and leads to transcriptional effects through sustained phosphorylation of transcription factor MITF. PLoS ONE 2016, 14, e0153463. [Google Scholar] [CrossRef]
- Lim, D.; Lee, K.J.; Kim, Y.; Kim, M.; Ju, H.M.; Kim, M.J.; Choi, D.H.; Choi, J.; Kim, S.; Kang, D.; et al. A basic domain-derived tripeptide inhibits MITF activity by reducing its binding to the promoter of target genes. J. Investig. Dermatol. 2021, 141, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, F.; Zanetti, A.; De Filippis, C.; Tomanin, R. Mucopolysaccharidosis Type VI, an updated overview of the disease. Int. J. Mol. Sci. 2021, 22, 13456. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shen, R.; Yang, K.; Wang, Y.; Song, H.; Liu, X.; Cheng, X.; Wu, R.; Song, Y.; Wang, D. RNF8 enhances the sensitivity of PD-L1 inhibitor against melanoma through ubiquitination of galectin-3 in stroma. Cell Death Discov. 2023, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef] [PubMed]
- Mabbitt, J.; Holyer, I.D.; Roper, J.A.; Nilsson, U.J.; Zetterberg, F.R.; Vuong, L.; Mackinnon, A.C.; Pedersen, A.; Slack, R.J. Resistance to anti-PD-1/anti-PD-L1: Galectin-3 inhibition with GB1211 reverses galectin-3-induced blockade of pembrolizumab and atezolizumab binding to PD-1/PD-L1. Front. Immunol. 2023, 14, 1250559. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef] [PubMed]
- Derosiers, N.; Aguilar, W.; DeGaramo, D.A.; Posey, A.D., Jr. Sweet immune checkpoint targets to enhance T cell therapy. J. Immunol. 2022, 208, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lugemwa, F.N.; Sarkar, A.K.; Esko, J.D. Unusual beta-D-xylosides that prime glycosaminoglycans in animal cells. J. Biol. Chem. 1996, 271, 19159–19165. [Google Scholar] [CrossRef]
- Mencio, C.; Balagurunathan, K.; Koketsu, M. Xyloside derivatives as molecular tools to selectively inhibit heparan sulfate and chondroitin sulfate proteoglycan biosynthesis. Methods Mol. Biol. 2022, 2303, 753–764. [Google Scholar]
- Siegbahn, A.; Thorsheim, K.; Ståhle, J.; Manner, S.; Hamark, C.; Persson, A.; Tykesson, E.; Mani, K.; Westergren-Thorsson, G.; Widmalm, G.; et al. Exploration of the active site of β4GalT7: Modifications of the aglycon of aromatic xylosides. Org. Biomol. Chem. 2015, 13, 3351–3362. [Google Scholar] [CrossRef]
- Chua, J.S.; Kuberan, B. Synthetic xylosides: Probing the glycosaminoglycan biosynthetic machinery for biomedical applications. Acc. Chem. Res. 2017, 50, 2693–2705. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Ellervik, U.; Mani, K. Fine-tuning the structure of glycosaminoglycans in living cells using xylosides. Glycobiology 2018, 28, 499–511. [Google Scholar] [CrossRef]
- Leusmann, S.; Ménová, P.; Shanin, E.; Titz, A.; Rademacher, C. Glycomimetics for the inhibition and modulation of lectins. Chem. Soc. Rev. 2023, 52, 3663–3740. [Google Scholar] [CrossRef] [PubMed]
- Holzberg, D.; Knight, C.G.; Dittrich-Breiholz, O.; Schneider, H.; Dörrie, A.; Hoffmann, E.; Resch, K.; Kracht, M. Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN δ domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 2003, 278, 40213–40223. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.K.; Kendrick, T.S.; Bogoyevitch, M.A. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 2002, 277, 10987–10997. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Xie, J.; Zhong, Z.; Deng, C. Enzyme-responsive micellar JQ1 induces enhanced BET protein inhibition and immunotherapy of malignant tumors. Biomater. Sci. 2021, 9, 6915–6926. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, S.; O-Sullivan, I.; Tobacman, J.K. N-Acetylgalactosamine-4-sulfatase (Arylsulfatase B) Regulates PD-L1 Expression in Melanoma by an HDAC3-Mediated Epigenetic Mechanism. Int. J. Mol. Sci. 2024, 25, 5851. https://doi.org/10.3390/ijms25115851
Bhattacharyya S, O-Sullivan I, Tobacman JK. N-Acetylgalactosamine-4-sulfatase (Arylsulfatase B) Regulates PD-L1 Expression in Melanoma by an HDAC3-Mediated Epigenetic Mechanism. International Journal of Molecular Sciences. 2024; 25(11):5851. https://doi.org/10.3390/ijms25115851
Chicago/Turabian StyleBhattacharyya, Sumit, InSug O-Sullivan, and Joanne K. Tobacman. 2024. "N-Acetylgalactosamine-4-sulfatase (Arylsulfatase B) Regulates PD-L1 Expression in Melanoma by an HDAC3-Mediated Epigenetic Mechanism" International Journal of Molecular Sciences 25, no. 11: 5851. https://doi.org/10.3390/ijms25115851




