Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease
Abstract
1. Introduction
2. Cellular Mechanism behind Adenosine Effects
2.1. Adenosine Production and Metabolism
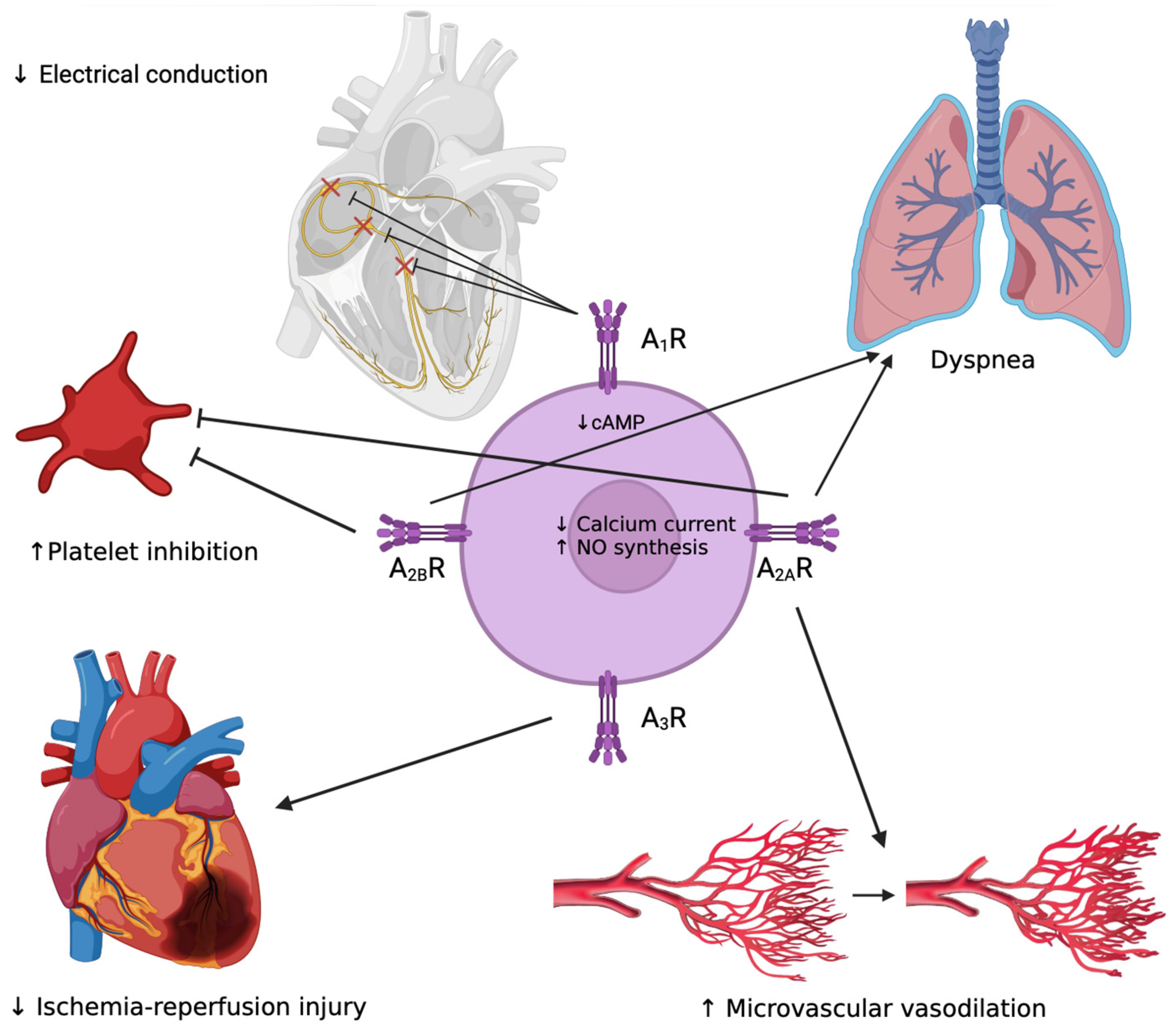
2.2. Adenosine Receptor and Effects on the Cardiovascular System
2.3. Anti-Inflammatory Effects of Adenosine
2.4. Adenosine in Pre-Conditioning in Myocardial Ischemia
2.5. Anti-Platelet Effects of Adenosine
3. The Role of Adenosine in Anti-Platelet Therapy Side Effects
3.1. Ticagrelor
3.2. Cangrelor
4. The Role of Adenosine in Treatment of the No-Reflow Phenomenon
5. Diagnosis and Treatment of Supraventricular Arrythmias
6. Adenosine in Diagnosis of Coronary Artery Disease
6.1. Adenosine Analogues
6.2. Cardiac Stress Imaging
6.3. Intracoronary Physiological Assesment
7. Adenosine Side Effects
8. Conclusions
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Faigle, M.; Knapp, S.; Karhausen, J.; Ibla, J.; Rosenberger, P.; Odegard, K.C.; Laussen, P.C.; Thompson, L.F.; Colgan, S.P. Endothelial catabolism of extracellular adenosine during hypoxia: The role of surface adenosine deaminase and CD26. Blood 2006, 108, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef]
- Hussain, T.; Mustafa, S.J. Binding of A1 Adenosine Receptor Ligand [3H]8-Cyclopentyl-1,3-Dipropylxanthine in Coronary Smooth Muscle. Circ. Res. 1995, 77, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Ashton, K.J.; Rose’Meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Musser, B.; Morgan, M.E.; Leid, M.; Murray, T.F.; Linden, J.; Vestal, R.E. Species comparison of adenosine and β-adrenoceptors in mammalian atrial and ventricular myocardium. Eur. J. Pharmacol. Mol. Pharmacol. 1993, 246, 105–111. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Pelleg, A.; Hurt, C.; Miyagawa, A.; Michelson, E.L.; Dreifus, L.S. Differential sensitivity of cardiac pacemakers to exogenous adenosine in vivo. Am. J. Physiol.-Heart Circ. Physiol. 1990, 258, H1815–H1822. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 161–188. [Google Scholar]
- Morrison, R.R.; Talukder, M.H.; Ledent, C.; Mustafa, S.J. Cardiac effects of adenosine in A2A receptor knockout hearts: Uncovering A2B receptors. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H437–H444. [Google Scholar] [CrossRef] [PubMed]
- Sanjani, M.S.; Teng, B.; Krahn, T.; Tilley, S.; Ledent, C.; Mustafa, S.J. Contributions of A 2A and A 2B adenosine receptors in coronary flow responses in relation to the K ATP channel using A2B and A2A/2B double-knockout mice. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H2322–H2333. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M.; Mozzicato, S.; Joshi, B.V.; Jacobson, K.A.; Liang, B.T.; Dougherty, C.; et al. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K.; Mullane, K.; Flameng, W. Role of adenosine in the heart and circulation. Cardiovasc. Res. 1996, 32, 797–813. [Google Scholar] [CrossRef]
- Berne, R.M. Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am. J. Physiol.-Leg. Content 1963, 204, 317–322. [Google Scholar] [CrossRef]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Francis, C.E.; Ravid, K. An A3-Subtype Adenosine Receptor Is Highly Expressed in Rat Vascular Smooth Muscle Cells: Its Role in Attenuating Adenosine-Induced Increase in cAMP. Microvasc. Res. 1997, 54, 243–252. [Google Scholar] [CrossRef]
- Karmouty-Quintana, H.; Xia, Y.; Blackburn, M.R. Adenosine signaling during acute and chronic disease states. J. Mol. Med. 2013, 91, 173–181. [Google Scholar] [CrossRef]
- Haskó, G. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Ceruti, S. P1 receptors and cytokine secretion. Purinergic Signal. 2007, 3, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, D. Purinergic Regulation of Neutrophil Function. Front. Immunol. 2018, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of Neutrophil Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. Regulation of Macrophage Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Carsons, S.; Littlefield, M.J.; Rieger, J.M.; Figler, R.; Reiss, A.B. Adenosine A2A receptor activation supports an atheroprotective cholesterol balance in human macrophages and endothelial cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Liu, G.S.; Richards, S.C.; Olsson, R.A.; Mullane, K.; Walsh, R.S.; Downey, J.M. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc. Res. 1994, 28, 1057–1061. [Google Scholar] [CrossRef]
- Liu, G.S.; Thornton, J.; Van Winkle, D.M.; Stanley, A.W.; Olsson, R.A.; Downey, J.M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 1991, 84, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.; Alkhulaifi, A.; Pugsley, W. Preconditioning the human myocardium. Lancet 1993, 342, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.; Berger, M.; Kussmaul, W.G.; Hirshfeld, J.W., Jr.; Herrmann, H.C.; Laskey, W.K. Adaptation to ischemia during percutaneous transluminal coronary angioplasty: Clinical, hemodynamic, and metabolic features. Circulation 1990, 82, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Leesar, M.A.; Stoddard, M.; Ahmed, M.; Broadbent, J.; Bolli, R. Preconditioning of Human Myocardium With Adenosine During Coronary Angioplasty. Circulation 1997, 95, 2500–2507. [Google Scholar] [CrossRef]
- De Luca, G.; Iorio, S.; Venegoni, L.; Marino, P. Evaluation of Intracoronary Adenosine to Prevent Periprocedural Myonecrosis in Elective Percutaneous Coronary Intervention (From the PREVENT-ICARUS Trial). Am. J. Cardiol. 2012, 109, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Wzorek, J.; Wolska, N.; Polak, D.; Watala, C.; Rozalski, M. Adenosine receptor agonists deepen the inhibition of platelet aggregation by P2Y12 antagonists. Vasc. Pharmacol. 2019, 113, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 78, e187–e285. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Brown, R.A.; Spina, D.; Page, C.P. Adenosine receptors and asthma. Br. J. Pharmacol. 2008, 153, S446–S456. [Google Scholar] [CrossRef]
- Rubio, R. Relationship between coronary flow and adenosine production and release. J. Mol. Cell. Cardiol. 1974, 6, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Cao, D.; Sartori, S.; Baber, U.; Dangas, G.; Zhang, Z.; Vogel, B.; Kunadian, V.; Briguori, C.; Cohen, D.J.; et al. Dyspnea-Related Ticagrelor Discontinuation after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2023, 16, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Angiolillo, D.J. Personalised antiplatelet therapies for coronary artery disease: What the future holds. Eur. Heart J. 2023, 44, 3059–3072. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Cannon, C.P.; Emanuelsson, H.; Michelson, E.L.; Harrington, R.A.; Husted, S.; James, S.; Katus, H.; Pais, P.; Raev, D.; et al. The Incidence of Bradyarrhythmias and Clinical Bradyarrhythmic Events in Patients With Acute Coronary Syndromes Treated With Ticagrelor or Clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) Trial. J. Am. Coll. Cardiol. 2011, 57, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, D.; Muraca, I.; Berteotti, M.; Gori, A.M.; Sorrentino, A.; Bertelli, A.; Marcucci, R.; Valenti, R. Pathophysiological and Molecular Basis of the Side Effects of Ticagrelor: Lessons from a Case Report. Int. J. Mol. Sci. 2023, 24, 10844. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Caniato, F.; Cappelli, F.; Mattesini, A.; Meucci, F.; Sori, A.; Stolcova, M.; Agostini, C.; Bernardo, P.; Di Mario, C. Discontinuation of both cangrelor and ticagrelor because of severe dyspnea during primary angioplasty. J. Cardiovasc. Med. 2021, 22, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Pugliese, N.R.; Giannoni, A. Reversal of Ticagrelor-Induced Arrhythmias and Cheyne–Stokes Respiration with Aminophylline Infusion. J. Cardiovasc. Pharmacol. 2017, 70, 290–292. [Google Scholar] [CrossRef]
- Minner, S.A.; Simone, P.; Chung, B.B.; Shah, A.P. Successful Reversal of Bradycardia and Dyspnea With Aminophylline After Ticagrelor Load. J. Pharm. Pract. 2018, 31, 112–114. [Google Scholar] [CrossRef]
- Di Mario, C.; Mugelli, A.; Filardi, P.P.; Rosano, G.; Rossi, F. Long-term dual antiplatelet therapy: Pharmacological and clinical implications. J. Cardiovasc. Med. 2018, 19, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Firstenberg, M.S.; Price, M.J.; Tummala, P.E.; Hutyra, M.; Welsby, I.J.; Voeltz, M.D.; Chandna, H.; Ramaiah, C.; Brtko, M.; et al. Bridging Antiplatelet Therapy with Cangrelor in Patients Undergoing Cardiac Surgery: A Randomized Controlled Trial. JAMA 2012, 307, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Muraca, I.; Marcucci, R.; Ciatti, F.; Berteotti, M.; Gori, A.M.; Carrabba, N.; Migliorini, A.; Marchionni, N.; Valgimigli, M. “Tailored” antiplatelet bridging therapy with cangrelor: Moving toward personalized medicine. Platelets 2022, 33, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.A.; Bhatt, D.L.; Prats, J.; Day, J.R.S.; Steg, P.G.; Stone, G.W.; Hamm, C.W.; Mahaffey, K.W.; Price, M.J.; Gibson, C.M.; et al. Characteristics of dyspnoea and associated clinical outcomes in the CHAMPION PHOENIX study. Thromb. Haemost. 2017, 117, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.H.; Kloner, R.A. No-Reflow Phenomenon. Circulation 2002, 105, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Chesebro, J.H.; Knatterud, G.; Roberts, R.; Borer, J.; Cohen, L.S.; Dalen, J.; Dodge, H.T.; Francis, C.K.; Hillis, D.; Ludbrook, P. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987, 76, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Van ‘t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.; de Boer, M.J.; Zijlstra, F. Angiographic Assessment of Myocardial Reperfusion in Patients Treated With Primary Angioplasty for Acute Myocardial Infarction: Myocardial Blush Grade. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef]
- Caiazzo, G.; Musci, R.L.; Frediani, L.; Uminska, J.; Wanha, W.; Filipiak, K.J.; Kubica, J.; Navarese, E.P. State of the Art: No-Reflow Phenomenon. Cardiol. Clin. 2020, 38, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular Obstruction and the No-Reflow Phenomenon after Percutaneous Coronary Intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Schiavo, P.L.; et al. Open-Label, Randomized, Placebo-Controlled Evaluation of Intracoronary Adenosine or Nitroprusside After Thrombus Aspiration During Primary Percutaneous Coronary Intervention for the Prevention of Microvascular Obstruction in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [CrossRef]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Adenosine as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Kloner, R.A.; Forman, M.B.; Gibbons, R.J.; Ross, A.M.; Alexander, R.W.; Stone, G.W. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: The AMISTAD-2 trial. Eur. Heart J. 2006, 27, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef]
- Navarese, E.P.; Buffon, A.; Andreotti, F.; Gurbel, P.A.; Kozinski, M.; Kubica, A.; Musumeci, G.; Cremonesi, A.; Tavazzi, L.; Kubica, J.; et al. Adenosine improves post-procedural coronary flow but not clinical outcomes in patients with acute coronary syndrome: A meta-analysis of randomized trials. Atherosclerosis 2012, 222, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, B.; Guo, Y.; Zheng, F. Efficacy of Adenosine in Patients With Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A PRISMA-Compliant Meta-Analysis. Medicine 2015, 94, e1279. [Google Scholar] [CrossRef]
- Polimeni, A.; De Rosa, S.; Sabatino, J.; Sorrentino, S.; Indolfi, C. Impact of intracoronary adenosine administration during primary PCI: A meta-analysis. Int. J. Cardiol. 2016, 203, 1032–1041. [Google Scholar] [CrossRef]
- Rankin, A.C.; Oldroyd, K.G.; Chong, E.; Rae, A.P.; Cobbe, S.M. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Heart 1989, 62, 195. [Google Scholar] [CrossRef] [PubMed]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef]
- Shaw, L.J.; Berman, D.S.; Picard, M.H.; Friedrich, M.G.; Kwong, R.Y.; Stone, G.W.; Senior, R.; Min, J.K.; Hachamovitch, R.; Scherrer-Crosbie, M.; et al. Comparative Definitions for Moderate-Severe Ischemia in Stress Nuclear, Echocardiography, and Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2014, 7, 593–604. [Google Scholar] [CrossRef]
- Beller, G.A. New directions in myocardial perfusion imaging. Clin. Cardiol. 1993, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; van Son, J.A.; Kirkeeide, R.L.; De Bruyne, B.; Gould, K.L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993, 87, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Campo, G.; DI Serafino, L.; Zanon, S.; Rubino, F.; Monizzi, G.; Biscaglia, S.; Ancona, M.; Polimeni, A.; Niccoli, G.; et al. #FullPhysiology: A systematic step-by-step guide to implement intracoronary physiology in daily practice. Minerva Cardiol. Angiol. 2023, 71, 504–514. [Google Scholar] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [PubMed]
- Cerqueira, M.D.; Verani, M.S.; Schwaiger, M.; Heo, J.; Iskandrian, A.S. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry. J. Am. Coll. Cardiol. 1994, 23, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.; Steffens, M.; Kleinertz, K.; Morell, R.; Budde, R.; Leischik, R.; Krämer, A.; Overhoff, U.; Strohm, O. Safety of Adenosine Stress Magnetic Resonance Imaging Using a Mobile Cardiac Magnetic Resonance System. J. Cardiovasc. Magn. Reson. 2006, 8, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Price, A.; Vidovich, M.I. Adenosine-induced atrial fibrillation during fractional flow reserve measurement. Cardiol. J. 2012, 19, 650–651. [Google Scholar] [CrossRef]
- Vecchio, E.A.; White, P.J.; May, L.T. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 2017, 8, 243. [Google Scholar] [CrossRef]
| TIMI Flow Grade | |
|---|---|
| TFG 0 | Absence of antegrade flow beyond the point of occlusion. |
| TFG 1 | The contrast passes beyond the occlusion but fails to opacify the entire coronary bed distal to the obstruction. |
| TFG 2 | The contrast passes beyond the occlusion but fails to opacify the entire coronary bed distal to the obstruction, but the rate of its entry or clearance is significantly slower than comparable areas not perfused by the previously occluded vessel (e.g., another coronary artery). |
| TFG 3 | Antegrade flow into the bed distal to the obstruction occurs as promptly as antegrade flow into the bed proximal to the obstruction, and clearance of contrast material from the involved bed is as rapid as clearance from an uninvolved bed in the same vessel or in the opposite artery. |
| Myocardial Blush Grade | |
|---|---|
| MBG 0 | No myocardial blush |
| MBG 1 | Minimal myocardial blush |
| MBG 2 | Moderate myocardial blush but less than that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery |
| MBG 3 | Normal myocardial blush, comparable with that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, E.; Muraca, I.; Berteotti, M.; Gori, A.M.; Valenti, R.; Marcucci, R. Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 5852. https://doi.org/10.3390/ijms25115852
Marchi E, Muraca I, Berteotti M, Gori AM, Valenti R, Marcucci R. Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease. International Journal of Molecular Sciences. 2024; 25(11):5852. https://doi.org/10.3390/ijms25115852
Chicago/Turabian StyleMarchi, Enrico, Iacopo Muraca, Martina Berteotti, Anna Maria Gori, Renato Valenti, and Rossella Marcucci. 2024. "Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease" International Journal of Molecular Sciences 25, no. 11: 5852. https://doi.org/10.3390/ijms25115852
APA StyleMarchi, E., Muraca, I., Berteotti, M., Gori, A. M., Valenti, R., & Marcucci, R. (2024). Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease. International Journal of Molecular Sciences, 25(11), 5852. https://doi.org/10.3390/ijms25115852






