Multimodal Biosensing of Foodborne Pathogens
Abstract
1. Introduction
2. Multiple Signal Channels
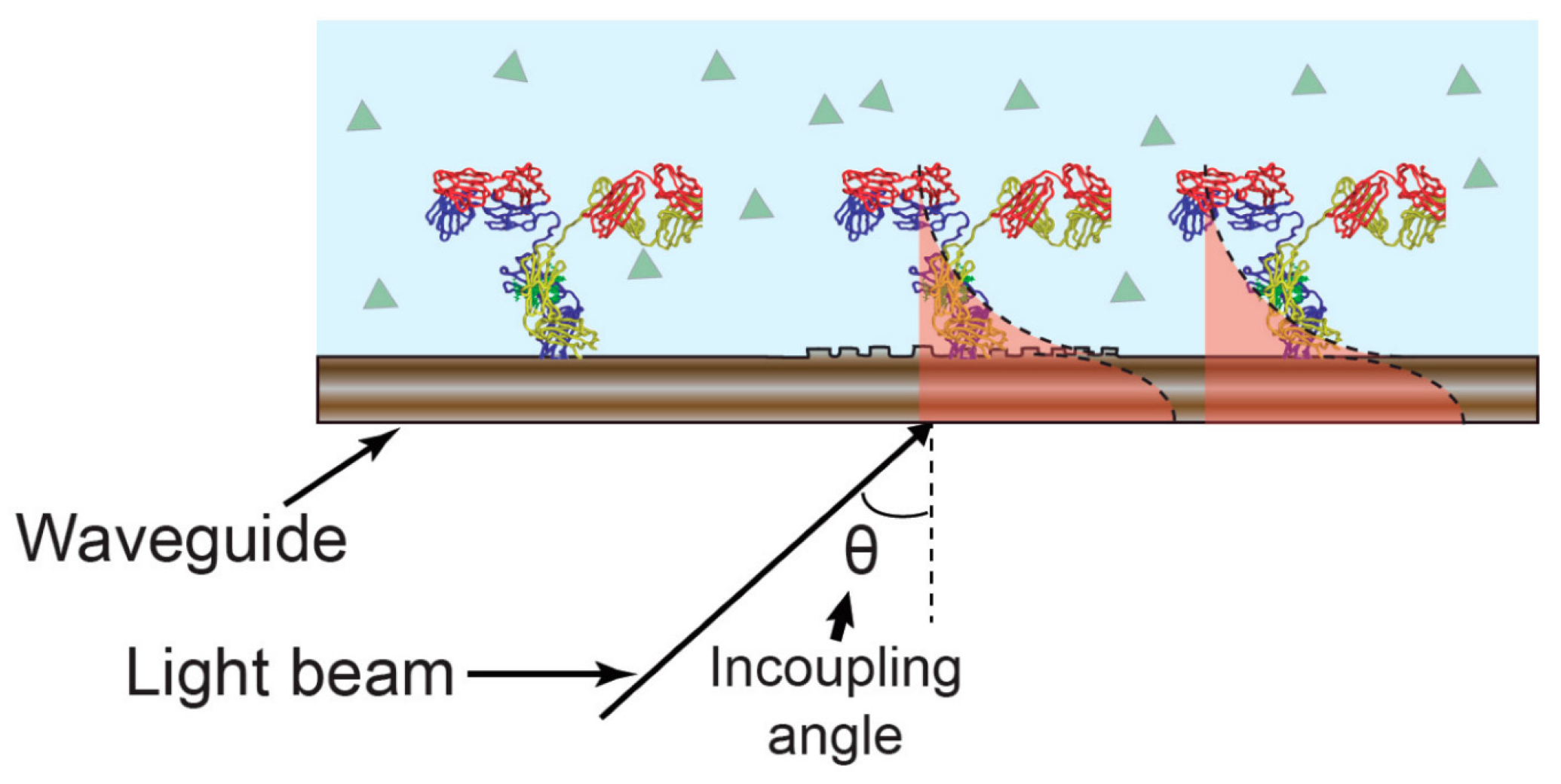
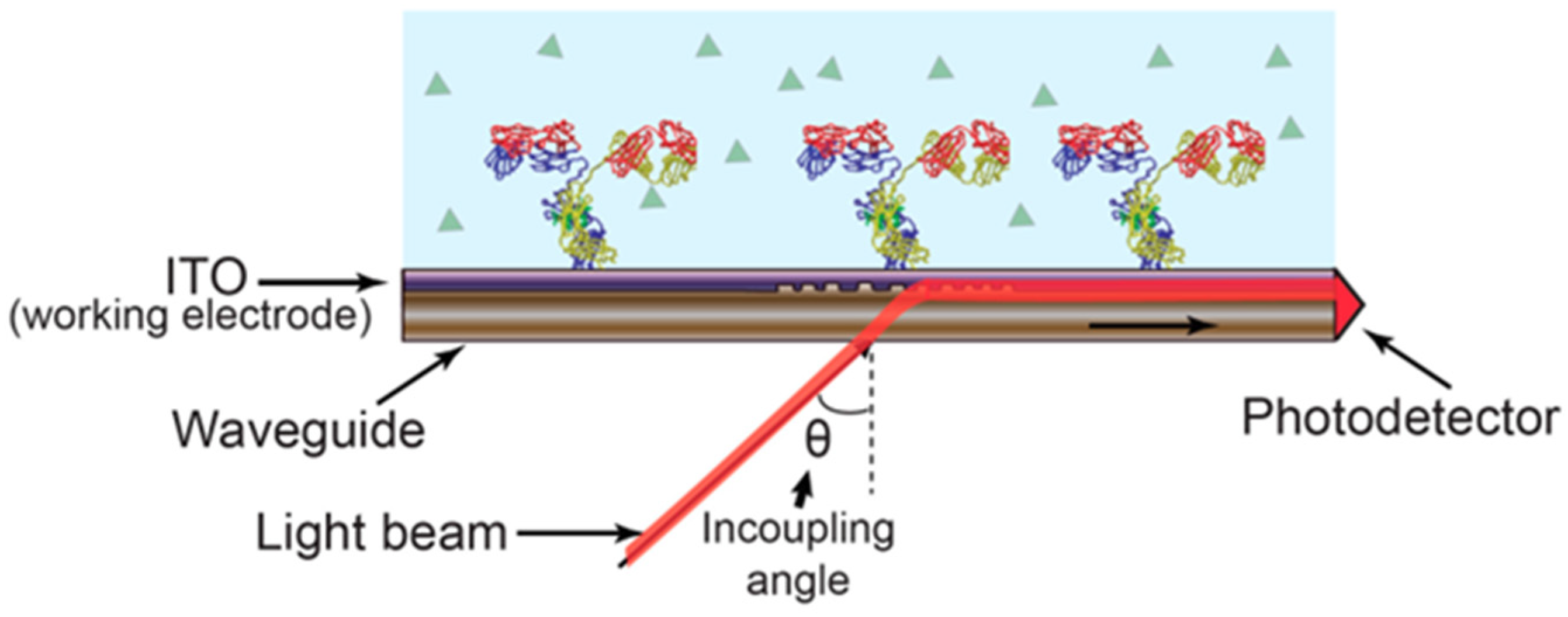
2.1. Fusion of Optical and Electrochemical Techniques
2.2. Combination of Optical- and Nanomaterial-Based Methods
2.3. Combination of Multiple Nanomaterials
2.4. Hybrid Biosensing with Microfluidics
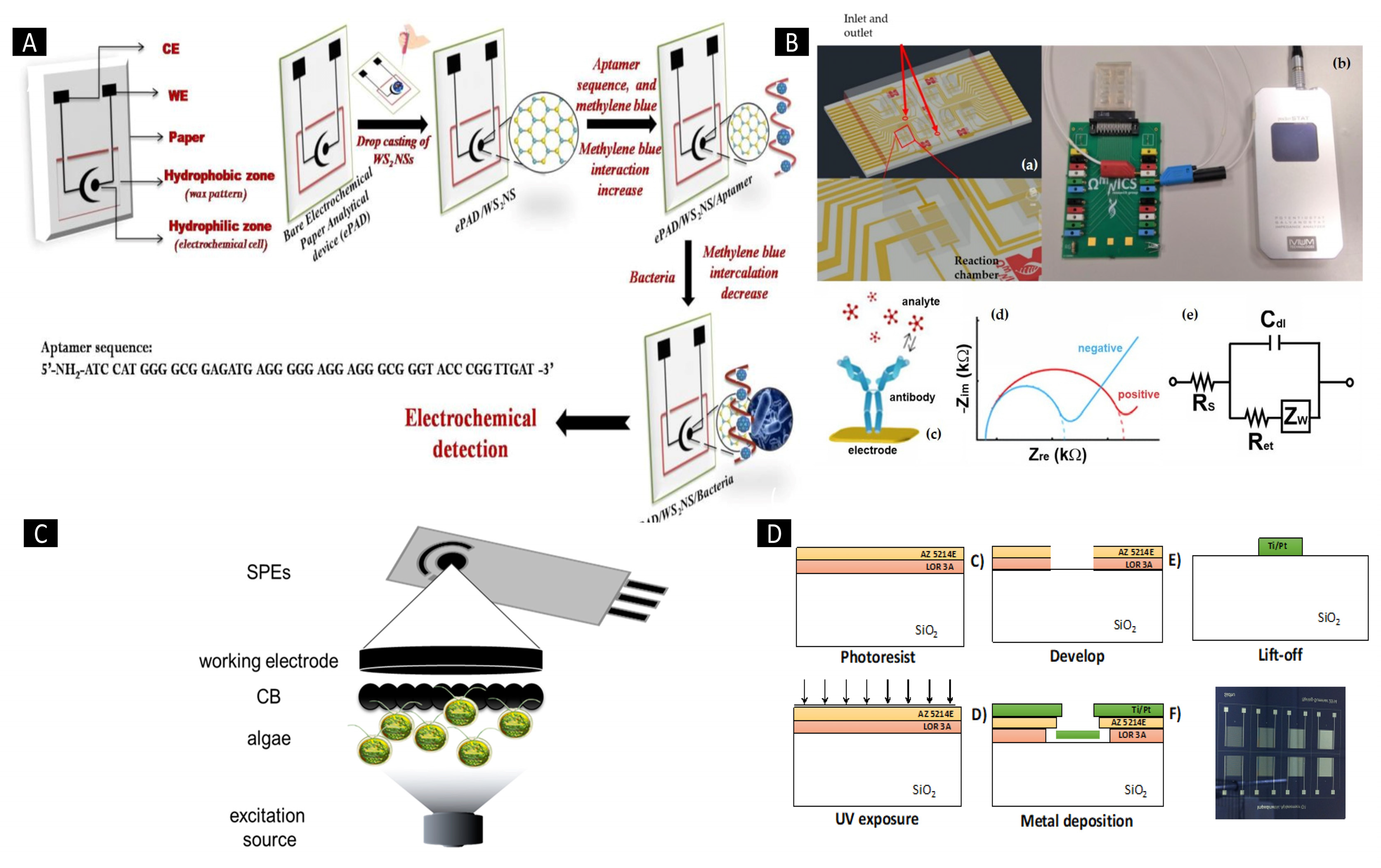
2.5. Integration of Nanoparticle-Based Screen Printing Technology
3. Advantages and Challenges of Multimodal Biosensing
3.1. Enhanced Sensitivity and Specificity
3.2. Improved Analytical Performance
3.3. Minimization of False Positives and Negatives
3.4. Challenges and Limitations
3.4.1. Integration and Miniaturization
3.4.2. Cost and Scalability
4. Applications in Foodborne Pathogen Detection
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Lang, T.; Cao, B.; Shen, C.; Shi, G. Multimode-coreless-multimode fiber biosensor based on surface plasmon resonance. J. Phys. D Appl. Phys. 2019, 52, 195204. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, Y.; Chen, J.; Guo, Y.; Wu, W.; He, Y.; Xu, L.; Fu, F. A microfluidic chip-based fluorescent biosensor for the sensitive and specific detection of label-free single-base mismatch via magnetic beads-based “sandwich” hybridization strategy. Electrophoresis 2013, 34, 2177–2184. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef]
- Sevenler, D.; Daaboul, G.G.; Kanik, F.E.; Ünlü, N.L.; Ünlü, M.S. Digital microarrays: Single-molecule readout with interferometric detection of plasmonic nanorod labels. ACS Nano 2018, 12, 5880–5887. [Google Scholar] [CrossRef] [PubMed]
- Sevenler, D.; Avci, O.; Ünlü, M.S. Quantitative interferometric reflectance imaging for the detection and measurement of biological nanoparticles. Biomed. Opt. Express 2017, 8, 2976–2989. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, H.; Wang, Z.; Jeong, S.; Jo, M.-C.; Park, M.-H.; Duan, X. Nanoparticle–membrane interactions: Real-time detection of nanoparticles interaction with lipid membranes using an integrated acoustical and electrical multimode biosensor (part. part. syst. charact. 2/2019). Part. Part. Syst. Charact. 2019, 36, 1970004. [Google Scholar] [CrossRef]
- Lukose, J.; Barik, A.K.; Mithun, N.; Pavithran, S.; George, S.D.; Murukeshan, V.M.; Chidangil, S. Raman spectroscopy for viral diagnostics. Biophys. Rev. 2023, 15, 199–221. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Zhao, J.; Huang, C. Advances in the Bacteriophage-Based Precise Identification and Magnetic Relaxation Switch Sensor for Rapid Detection of Foodborne Pathogens. In Foodborne Pathogens-Recent Advances in Control and Detection; IntechOpen: London, UK, 2022. [Google Scholar]
- Zhang, J.; Wang, Y.; Lu, X. Molecular imprinting technology for sensing foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2021, 413, 4581–4598. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Cabaj, J.; Malecha, K. Biosensors Technologies for Point-of-Care Testing–A Review. Period. Polytech. Electr. Eng. Comput. Sci. 2020, 64, 325–333. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Chen, J.; Yang, L.; Qiu, Y.; Du, Q.; Wang, C.; Teng, M.; Wang, T.; Dong, Y. A novel strategy for therapeutic drug monitoring: Application of biosensors to quantify antimicrobials in biological matrices. J. Antimicrob. Chemother. 2023, 78, 2612–2629. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, W.; Zhang, X.; Wang, B.; Jiang, H. ZnO-based amperometric enzyme biosensors. Sensors 2010, 10, 1216–1231. [Google Scholar] [CrossRef]
- Gheorghiu, M.; Polonschii, C.; Popescu, O.; Gheorghiu, E. Advanced optogenetic-based biosensing and related biomaterials. Materials 2021, 14, 4151. [Google Scholar] [CrossRef] [PubMed]
- Syahir, A.; Mihara, H.; Kajikawa, K. A new optical label-free biosensing platform based on a metal-insulator-metal structure. Langmuir 2010, 26, 6053–6057. [Google Scholar] [CrossRef]
- Lin, Y.; Chapman, R.; Stevens, M.M. Label-free multimodal protease detection based on protein/perylene dye coassembly and enzyme-triggered disassembly. Anal. Chem. 2014, 86, 6410–6417. [Google Scholar] [CrossRef]
- Juan-Colás, J.; Johnson, S.; Krauss, T.F. Dual-mode electro-optical techniques for biosensing applications: A review. Sensors 2017, 17, 2047. [Google Scholar] [CrossRef]
- Vörös, J.; Ramsden, J.; Csúcs, G.; Szendrő, I.; De Paul, S.; Textor, M.; Spencer, N. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Piehler, J.; Brandenburg, A.; Brecht, A.; Wagner, E.; Gauglitz, G. Characterization of grating couplers for affinity-based pesticide sensing. Appl. Opt. 1997, 36, 6554–6562. [Google Scholar] [CrossRef] [PubMed]
- Bearinger, J.P.; Vörös, J.; Hubbell, J.A.; Textor, M. Electrochemical optical waveguide lightmode spectroscopy (EC-OWLS): A pilot study using evanescent-field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)-coated waveguide chips. Biotechnol. Bioeng. 2003, 82, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Schmutz, P.; Petronis, S.; Textor, M.; Keller, B.; Vörös, J. Locally Addressable Electrochemical Patterning Technique (LAEPT) applied to poly(L-lysine)-graft-poly(ethylene glycol) adlayers on titanium and silicon oxide surfaces. Biotechnol. Bioeng. 2005, 91, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Szendrő, I.; Erdélyi, K.; Fábián, M.; Puskás, Z.; Adányi, N.; Somogyi, K. Combination of the optical waveguide lightmode spectroscopy method with electrochemical measurements. Thin Solid Film. 2008, 516, 8165–8169. [Google Scholar] [CrossRef]
- Erdélyi, K.; Frutos, A.G.; Ramsden, J.J.; Szendro, I.; Voirin, G. Grating-Based Optical Biosensors. In Handbook of Biosensors and Biochips; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; ISBN 9780470061565. [Google Scholar]
- Ngankam, A.P.; Van Tassel, P.R. In situ layer-by-layer film formation kinetics under an applied voltage measured by optical waveguide lightmode spectroscopy. Langmuir 2005, 21, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Boulmedais, F.; Tang, C.S.; Keller, B.; Vörös, J. Controlled Electrodissolution of Polyelectrolyte Multilayers: A Platform Technology Towards the Surface-Initiated Delivery of Drugs. Adv. Funct. Mater. 2006, 16, 63–70. [Google Scholar] [CrossRef]
- Gabi, M.; Sannomiya, T.; Larmagnac, A.; Puttaswamy, M.; Vörös, J. Influence of applied currents on the viability of cells close to microelectrodes. Integr. Biol. 2009, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, M.; Reuter, F.; Vörös, J. Review of transducer principles for label-free biomolecular interaction analysis. Biosensors 2011, 1, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Brusatori, M.A.; Tie, Y.; Van Tassel, P.R. Protein Adsorption Kinetics under an Applied Electric Field: An Optical Waveguide Lightmode Spectroscopy Study. Langmuir 2003, 19, 5089–5097. [Google Scholar] [CrossRef]
- Adányi, N.; Németh, E.; Halász, A.; Szendrő, I.; Váradi, M. Application of electrochemical optical waveguide lightmode spectroscopy for studying the effect of different stress factors on lactic acid bacteria. Anal. Chim. Acta 2006, 573–574, 41–47. [Google Scholar] [CrossRef]
- Sugihara, K.; Delai, M.; Szendro, I.; Guillaume-Gentil, O.; Vörös, J.; Zambelli, T. Simultaneous OWLS and EIS monitoring of supported lipid bilayers with the pore forming peptide melittin. Sens. Actuators B Chem. 2012, 161, 600–606. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2-based advanced biosensors: A review. ACS Appl. Nano Mater. 2017, 1, 2–25. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic alumina: A versatile platform for optical biosensors. Materials 2014, 7, 4297–4320. [Google Scholar] [CrossRef]
- Zhong, W. Nanomaterials in fluorescence-based biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Du, T.; Qi, Y. Recent advances in integrated dual-mode optical sensors for food safety detection. Trends Food Sci. Technol. 2023, 135, 14–31. [Google Scholar] [CrossRef]
- Tian, J.; An, M.; Zhao, X.; Wang, Y.; Hasan, M. Advances in fluorescent sensing carbon dots: An account of food analysis. ACS Omega 2023, 8, 9031–9039. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.; Wang, Z.; Wu, S. An aptasensor based on fluorescence resonance energy transfer for multiplexed pathogenic bacteria determination. Anal. Methods 2016, 8, 1390–1395. [Google Scholar] [CrossRef]
- Kalkal, A.; Tiwari, A.; Sharma, D.; Baghel, M.K.; Kumar, P.; Pradhan, R.; Packirisamy, G. Air-brush spray coated Ti3C2-MXene-graphene nanohybrid thin film based electrochemical biosensor for cancer biomarker detection. Int. J. Biol. Macromol. 2023, 253, 127260. [Google Scholar] [CrossRef] [PubMed]
- Kalkal, A.; Pradhan, R.; Packirisamy, G. Gold nanoparticles modified reduced graphene oxide nanosheets based dual-quencher for highly sensitive detection of carcinoembryonic antigen. Int. J. Biol. Macromol. 2023, 5, 125157. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, C.; Wu, S.; Li, H.; Guo, H.; Yang, B.; Qiu, S.; Li, J.; Liu, L.; Zeng, H.; et al. Development of a lateral flow colloidal gold immunoassay strip for the simultaneous detection of Shigella boydii and Escherichia coli O157:H7 in bread, milk and jelly samples. Food Control 2016, 59, 345–351. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yao, S.; Shang, M.; Zhao, C.; Li, J.; Wang, J. A multicolor sensing system for simultaneous detection of four foodborne pathogenic bacteria based on Fe3O4/MnO2 nanocomposites and the etching of gold nanorods. Food Chem. Toxicol. 2021, 149, 112035. [Google Scholar] [CrossRef] [PubMed]
- Zahertar, S.; Wang, Y.; Tao, R.; Xie, J.; Fu, Y.Q.; Torun, H. A fully integrated biosensing platform combining acoustofluidics and electromagnetic metamaterials. J. Phys. D Appl. Phys. 2019, 52, 485004. [Google Scholar] [CrossRef]
- Mishra, A.; Pilloton, R.; Jain, S.; Roy, S.; Khanuja, M.; Mathur, A.; Narang, J. Based Electrodes Conjugated with Tungsten Disulfide Nanostructure and Aptamer for Impedimetric Detection of Listeria monocytogenes. Biosensors 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Rizzato, S.; De Bellis, L.; Elicio, V.; Formica, L.; Luvisi, A.; Maruccio, G. Detection of Ampelovirus and Nepovirus by Lab-on-a-Chip: A Promising Alternative to ELISA Test for Large Scale Health Screening of Grapevine. Biosensors 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, A.; Arduini, F.; Attaallah, R.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A proof-of-concept electrochemical cytosensor based on Chlamydomonas reinhardtii functionalized carbon black screen-printed electrodes: Detection of Escherichia coli in wastewater as a case study. Biosensors 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.K.; Cavallaro, N.D.; Pola, C.C.; Danyluk, M.D.; McLamore, E.S.; Gomes, C.L. Planar interdigitated aptasensor for flow-through detection of Listeria spp. in hydroponic lettuce growth media. Sensors 2020, 20, 5773. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.; Cepeda, D.I.; Torres, A.F.L.; Cortes, M.M.A.; Monroy, Z.J.R.; Castaneda, J.E.G. Nucleic acid-based biosensors: Analytical devices for prevention, diagnosis and treatment of diseases. Rev. Vitae 2021, 28, 1–27. [Google Scholar]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advances in Microfluidics-Based Electrochemical Sensors for Foodborne Pathogen Detection. Biosensors 2023, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Knoll, W.; Dostalek, J. Bacterial pathogen surface plasmon resonance biosensor advanced by long range surface plasmons and magnetic nanoparticle assays. Anal. Chem. 2012, 84, 8345–8350. [Google Scholar] [CrossRef]
- Gao, J.; Li, L.; Ho, P.; Mak, G.C.; Gu, H.; Xu, B. Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv. Mater. 2006, 18, 3145–3148. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Wang, Y.; Hua, T.; Guo, R.; Miao, D.; Jiang, S. Flexible, stable and sensitive surface-enhanced Raman scattering of graphite/titanium-cotton substrate for conformal rapid food safety detection. Cellulose 2020, 27, 941–954. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal. Chem. 2023, 75, 2075–2079. [Google Scholar] [CrossRef]
- Huang, F.; Xue, L.; Zhang, H.; Guo, R.; Li, Y.; Liao, M.; Wang, M.; Lin, J. An enzyme-free biosensor for sensitive detection of Salmonella using curcumin as signal reporter and click chemistry for signal amplification. Theranostics 2018, 8, 6263. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Kaaliveetil, S.; Yang, J.; Alssaidy, S.; Li, Z.; Cheng, Y.-H.; Menon, N.H.; Chande, C.; Basuray, S. Microfluidic Gas Sensors: Detection Principle and Applications. Micromachines 2022, 13, 1716. [Google Scholar] [CrossRef]
- Raj, V.; Vijayan, A.N.; Joseph, K. Cysteine capped gold nanoparticles for naked eye detection of E. coli bacteria in UTI patients. Sens. Bio-Sens. Res. 2015, 5, 33–36. [Google Scholar] [CrossRef]
- Sepunaru, L.; Tschulik, K.; Batchelor-McAuley, C.; Gavish, R.; Compton, R.G. Electrochemical detection of single E. coli bacteria labeled with silver nanoparticles. Biomater. Sci. 2015, 3, 816–820. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Shi, Z.; Fang, C.; Wang, Z. Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal. Chem. 2014, 86, 3100–3107. [Google Scholar] [CrossRef]
- Hormsombut, T.; Mekjinda, N.; Kalasin, S.; Surareungchai, W.; Rijiravanich, P. Mesoporous silica nanoparticles-enhanced microarray technology for highly sensitive simultaneous detection of multiplex foodborne pathogens. ACS Appl. Bio Mater. 2014, 7, 2367–2377. [Google Scholar] [CrossRef]
- Chen, K.; Ma, B.; Li, J.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.; Zhang, M. A rapid and sensitive europium nanoparticle-based lateral flow immunoassay combined with recombinase polymerase amplification for simultaneous detection of three food-borne pathogens. Int. J. Environ. Res. Public Health 2021, 18, 4574. [Google Scholar] [CrossRef]
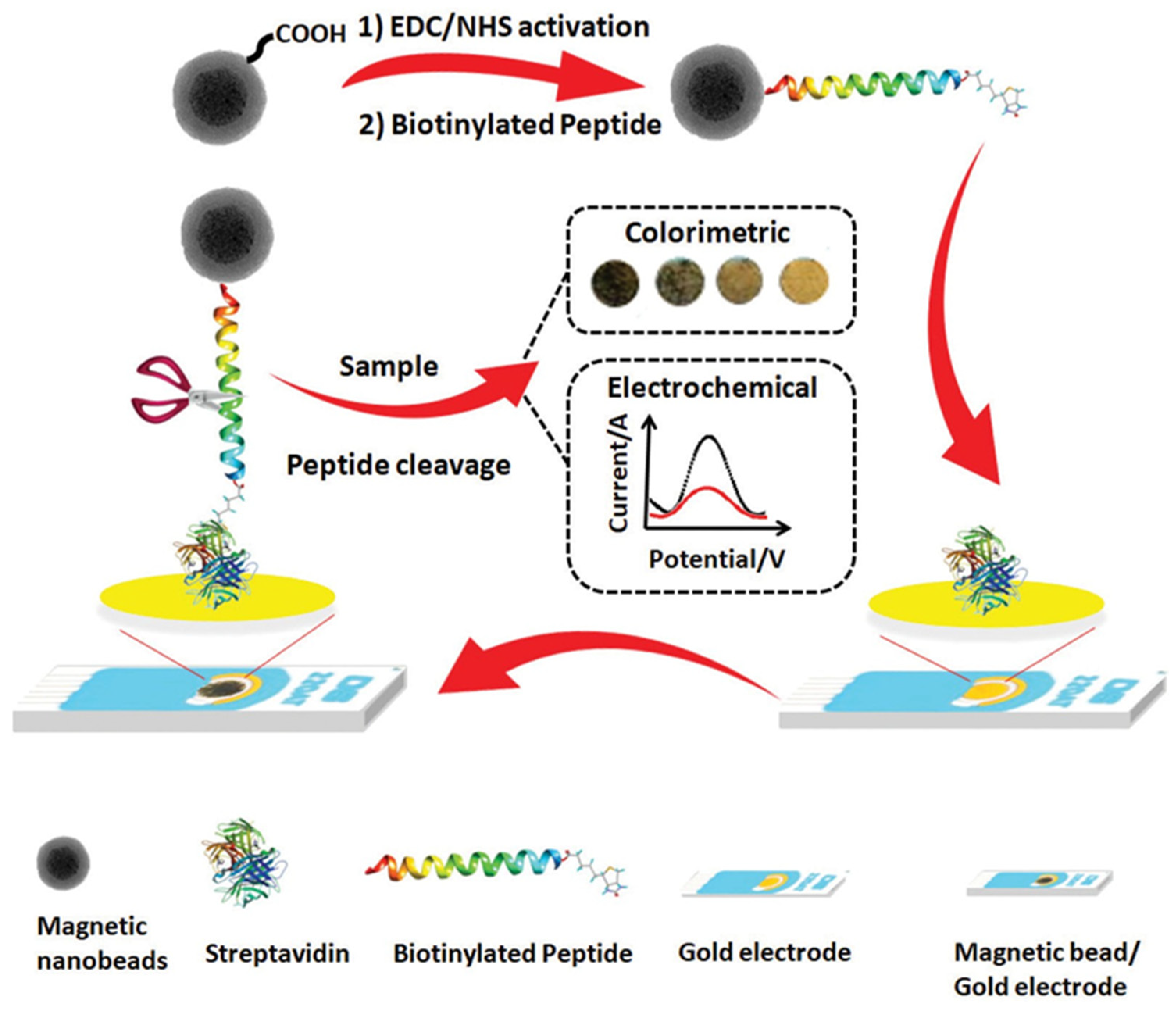
- Eissa, S.; Zourob, M. A dual electrochemical/colorimetric magnetic nanoparticle/peptide-based platform for the detection of Staphylococcus aureus. Analyst 2020, 145, 4606–4614. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta 2007, 74, 308–317. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of monodisperse particles by using microfluidics: Control over size, shape, and composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Siqueira, J.R., Jr.; Molinnus, D.; Beging, S.; Schöning, M.J. Incorporating a hybrid urease-carbon nanotubes sensitive nanofilm on capacitive field-effect sensors for urea detection. Anal. Chem. 2014, 86, 5370–5375. [Google Scholar] [CrossRef]
- Kim, Y.; Gonzales, J.; Zheng, Y. Sensitivity-Enhancing Strategies in Optical Biosensing. Small 2021, 17, 2004988. [Google Scholar] [CrossRef]
- Xu, Y.; Ang, Y.S.; Wu, L.; Ang, L.K. High sensitivity surface plasmon resonance sensor based on two-dimensional MXene and transition metal dichalcogenide: A theoretical study. Nanomaterials 2019, 9, 165. [Google Scholar] [CrossRef]
- Ullah, N.; Chen, W.; Noureen, B.; Tian, Y.; Du, L.; Wu, C.; Ma, J. An electrochemical Ti3C2Tx aptasensor for sensitive and label-free detection of marine biological toxins. Sensors 2021, 21, 4938. [Google Scholar] [CrossRef]
- Ullah, N.; Noureen, B.; Tian, Y.; Du, L.; Chen, W.; Wu, C. Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor. Nanomaterials 2022, 12, 1505. [Google Scholar] [CrossRef]
- Noureen, B.; Ullah, N.; Tian, Y.; Du, L.; Chen, W.; Wu, C.; Wang, P. An electrochemical PAH-modified aptasensor for the label-free and highly-sensitive detection of saxitoxin. Talanta 2022, 240, 123185. [Google Scholar] [CrossRef]
- Ullah, N.; Noureen, B.; Zahra, Q.U.A.; Aziz, T.; Shehzadi, S.; Alfaifif, M.Y.; Elbehairif, S.E.I.; Thebo, K.H.; Ullah, A.; Iqbal, H. A Novel Fluorescent Aptasensor Based on Mesoporous Silica Nanoparticles for Selective and Sensitive Detection of Saxitoxin in Shellfish. Curr. Anal. Chem. 2023, 19, 677–684. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio. Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Zargartalebi, H.; Yousefi, H.; Flynn, C.D.; Gomis, S.; Das, J.; Young, T.L.; Chien, E.; Mubareka, S.; McGeer, A.; Wang, H.; et al. Capillary-assisted molecular pendulum bioanalysis. J. Am. Chem. Soc. 2022, 144, 18338–18349. [Google Scholar] [CrossRef]
- Wang, J.; Cui, X.; Liang, L.; Li, J.; Pang, B.; Li, J. Advances in DNA-based electrochemical biosensors for the detection of foodborne pathogenic bacteria. Talanta 2024, 275, 126072. [Google Scholar] [CrossRef]
- Sadabadi, H.; Packirisamy, M. Nano-integrated suspended polymeric microfluidics (SPMF) platform for ultra-sensitive bio-molecular recognition of bovine growth hormones. Sci. Rep. 2017, 7, 10969. [Google Scholar] [CrossRef]
- Pappa, A.M.; Curto, V.F.; Braendlein, M.; Strakosas, X.; Donahue, M.J.; Fiocchi, M.; Malliaras, G.G.; Owens, R.M. Organic transistor arrays integrated with finger-powered microfluidics for multianalyte saliva testing. Adv. Healthc. Mater. 2016, 5, 2295–2302. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, D.; Zhu, H.; Chen, X. Lanthanide-doped luminescent nanoprobes: Controlled synthesis, optical spectroscopy, and bioapplications. Chem. Soc. Rev. 2013, 42, 6924–6958. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Frasco, M.F.; Serrano, V.; Fortunato, E.; Sales, M.G.F. Molecular imprinting on nanozymes for sensing applications. Biosensors 2021, 11, 152. [Google Scholar] [CrossRef]
- Soleymani, L.; Li, F. Mechanistic challenges and advantages of biosensor miniaturization into the nanoscale. ACS Sens. 2017, 2, 458–467. [Google Scholar] [CrossRef]
- Tzouvadaki, I.; Prodromakis, T. Large-scale nano-biosensing technologies. Front. Nanotechnol. 2023, 5, 1127363. [Google Scholar] [CrossRef]
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R. Biosensors for sustainable food engineering: Challenges and perspectives. Biosensors 2018, 8, 23. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, H.; Deng, S.; Xiao, X.; Xiong, Y.; Peng, J.; Lai, W. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag–Au urchin-like hollow structures for the sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2023, 225, 115090. [Google Scholar] [CrossRef]
- Cho, I.H.; Mauer, L.; Irudayaraj, J. In-situ fluorescent immunomagnetic multiplex detection of foodborne pathogens in very low numbers. Biosens. Bioelectron. 2014, 57, 143–148. [Google Scholar] [CrossRef]
- Qi, W.; Zheng, L.; Hou, Y.; Duan, H.; Wang, L.; Wang, S.; Liu, Y.; Li, Y.; Liao, M.; Lin, J. A finger-actuated microfluidic biosensor for colorimetric detection of foodborne pathogens. Food Chem. 2014, 381, 131801. [Google Scholar] [CrossRef]
- Wen, T.; Wang, R.; Sotero, A.; Li, Y. A portable impedance immunosensing system for rapid detection of Salmonella typhimurium. Sensors 2017, 17, 1973. [Google Scholar] [CrossRef]
- Ravariu, C.; Manea, E.; Babarada, F. Masks and metallic electrodes compounds for silicon biosensor integration. J. Alloys Compd. 2017, 697, 72–79. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. Nanotechnology in food science—Food packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Pinel, O.; Jian, P.; de Araújo, R.M.; Feng, J.; Chalopin, B.; Fabre, C.; Treps, N. Generation and characterization of multimode quantum frequency combs. Phys. Rev. Lett. 2012, 108, 083601. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Nguyen, T.G.; Chu, S.T.; Little, B.E.; Morandotti, R.; Mitchell, A.; Moss, D.J. RF photonics: An optical microcombs’ perspective. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1–20. [Google Scholar] [CrossRef]
- Aamer, A.M.; Al-Awlaqi, M.A.; Affia, I.; Arumsari, S.; Mandahawi, N. The internet of things in the food supply chain: Adoption challenges. Benchmarking Int. J. 2021, 28, 2521. [Google Scholar] [CrossRef]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef]
- Ali, A.; Altemimi, A.B.; Alhelfi, N.; Ibrahim, S.A. Application of Biosensors for Detection of Pathogenic Food Bacteria: A Review. Biosensors 2020, 10, 58. [Google Scholar] [CrossRef]
- Xiao, F.; Li, W.; Xu, H. Advances in magnetic nanoparticles for the separation of foodborne pathogens: Recognition, separation strategy, and application. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4478–4504. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Xu, F.; Yang, X. Powerful CRISPR-based biosensing techniques and their integration with microfluidic platforms. Front. Bioeng. Biotechnol. 2022, 10, 851712. [Google Scholar] [CrossRef]
- Lu, L.; Ge, Y.; Wang, X.; Lu, Z.; Wang, T.; Zhang, H.; Du, S. Rapid and sensitive multimode detection of Salmonella typhimurium based on the photothermal effect and peroxidase-like activity of MoS2@ Au nanocomposite. Sens. Actuators B Chem. 2021, 326, 128807. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, X.; Qi, L.; Yang, H.; Wang, Y.; Zhang, M.; Huang, K.; Yuan, X. Smartphone readable colorimetry and ICP-MS dual-mode sensing platform for ultrasensitive and label-free detection of Escherichia coli based on filter-assisted separation. Talanta 2023, 251, 123760. [Google Scholar] [CrossRef]
- Raham, H.; Al-Bassam, S.S. Optical fiber sensor based on surface plasmon resonance for detection of Escherichia coli (E. coli). J. Opt. 2022, 52, 631–636. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Gan, L.; Gong, A.; Zhang, H.; Liu, D.; Sun, Q. Selective and sensitive Escherichia coli detection based on a T4 bacteriophage-immobilized multimode microfiber. J. Biophotonics 2018, 11, e201800012. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Y.-Z.; Tang, M.; Wu, L.-L.; Xie, H.-Y.; Zhang, Z.-L.; Pang, D.-W. Colorimetric-fluorescent-magnetic nanosphere-based multimodal assay platform for salmonella detection. Anal. Chem. 2018, 91, 1178–1184. [Google Scholar] [CrossRef]
- Gao, L.; Xu, X.; Liu, W.; Xie, J.; Zhang, H.; Du, S. A sensitive multimode dot-filtration strip for the detection of Salmonella typhimurium using MoS2@Fe3O4. Microchim. Acta 2022, 189, 475. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, L.; Li, B.; Zhang, X. Dual-Modal Biosensor for Staphylococcus aureus Detection Based on a Porphyrin-Based Porous Organic Polymer FePor-TPA with Excellent Peroxidase-like, Catalase-like, and Photoelectrochemical Properties. Anal. Chem. 2023, 95, 13855–13863. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, B.; Fan, Y.; Zhou, M.; Wen, H.; Huang, B.; Lu, K.; Ren, J. Fluorescent aptasensor for highly sensitive detection of staphylococcus aureus based on dual-amplification strategy by integrating DNA walking and hybridization chain reaction. Talanta 2024, 270, 125624. [Google Scholar] [CrossRef]
- Welling, M.; de Korne, C.M.; Spa, S.J.; van Willigen, D.M.; Hensbergen, A.W.; Bunschoten, A.; Duszenko, N.; Smits, W.K.; Roestenberg, M.; van Leeuwen, F.W.B. Multimodal tracking of controlled staphylococcus aureus infections in mice. ACS Infect. Dis. 2019, 5, 1160–1168. [Google Scholar] [CrossRef]


| Pathogen | Sample | Detection Method | Analysis Time | LOD | References |
|---|---|---|---|---|---|
| E. coli O157-H7 | Food samples | Surface plasmon resonance | 30 min | 50 CFU/mL | [51] |
| E. coli, S. aureus | Human blood | Fluorescence-based biosensors | 120 min | 4 CFU/mL | [52] |
| S.typhimurium | Food samples | Surface-enhanced Raman spectroscopy | Not Stated | 35 CFU/mL | [53] |
| Shiga toxin E. coli | Water sample | Electrochemical impedance spectroscopy | 1 h | 10–102 CFU/mL | [54] |
| Salmonella typhimurium | Chicken sample | Magnetic biosensors | Not Stated | 50 CFU/mL | [55] |
| Salmonella | Milk samples | MicrofluidicBiosensors | 2 h | 10 CFU/mL | [56] |
| E. coli O157-H7 | Apple juice samples | Microfluidic-based immunoassay | 1 h | 10 CFU/mL | [57] |
| E. coli | Urine Sample | Colorimetric biosensors | Not Stated | 35 CFU/mL | [58] |
| E. coli | Water sample | Anodic particle Colorimetry technique | Not Stated | 1 CFU/mL | [59] |
| Salmonella typhimurium | Food samples | Luminescence bioassay method | Not Stated | 10–15 CFU/mL | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, N.; Bruce-Tagoe, T.A.; Asamoah, G.A.; Danquah, M.K. Multimodal Biosensing of Foodborne Pathogens. Int. J. Mol. Sci. 2024, 25, 5959. https://doi.org/10.3390/ijms25115959
Ullah N, Bruce-Tagoe TA, Asamoah GA, Danquah MK. Multimodal Biosensing of Foodborne Pathogens. International Journal of Molecular Sciences. 2024; 25(11):5959. https://doi.org/10.3390/ijms25115959
Chicago/Turabian StyleUllah, Najeeb, Tracy Ann Bruce-Tagoe, George Adu Asamoah, and Michael K. Danquah. 2024. "Multimodal Biosensing of Foodborne Pathogens" International Journal of Molecular Sciences 25, no. 11: 5959. https://doi.org/10.3390/ijms25115959
APA StyleUllah, N., Bruce-Tagoe, T. A., Asamoah, G. A., & Danquah, M. K. (2024). Multimodal Biosensing of Foodborne Pathogens. International Journal of Molecular Sciences, 25(11), 5959. https://doi.org/10.3390/ijms25115959







