Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge
Abstract
:1. Introduction
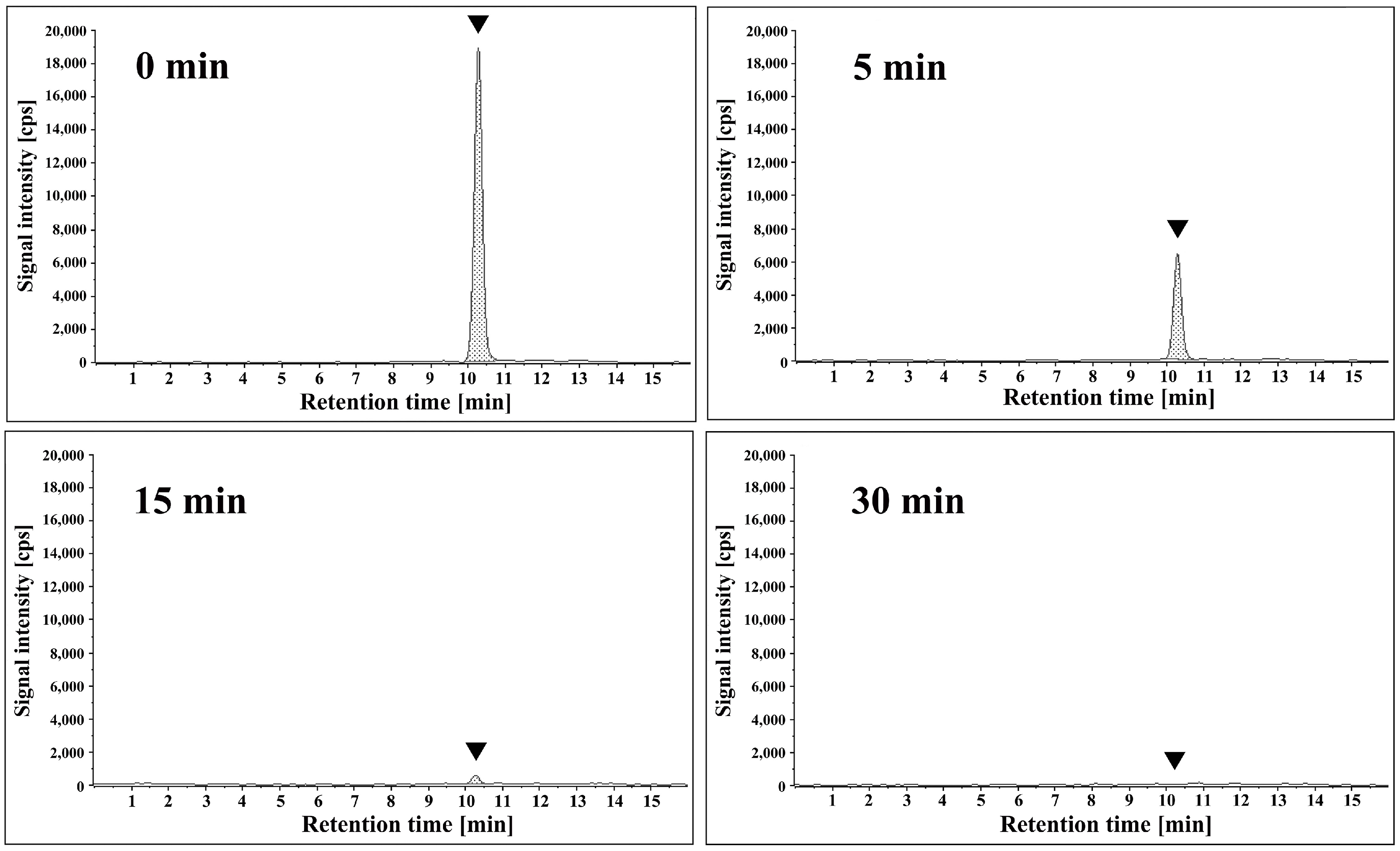
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
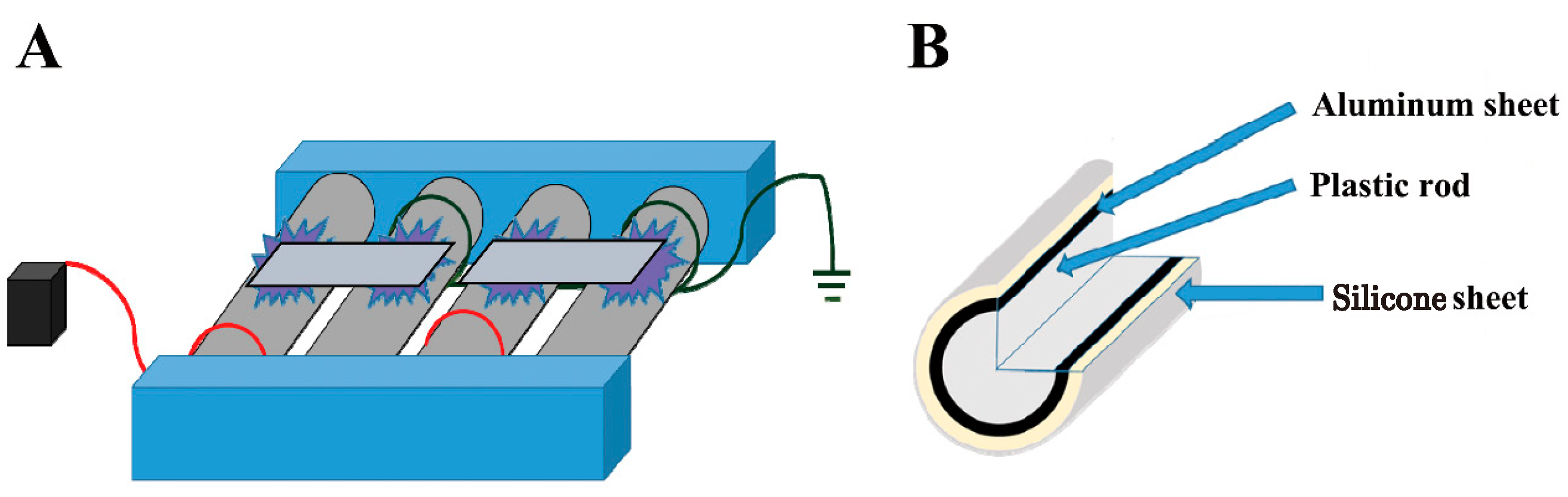
4.2. Devices
4.3. Plasma Treatment
4.4. Detection of Aflatoxin B1 by ELISA
4.5. Quantification of Stx1 and Stx2 by ELISA
4.6. Detection of S. aureus Enterotoxin by ELISA
4.7. Analysis of Cereulide by LC-MS/MS
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- World Health Organization. Food Safety—Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 15 March 2024).
- Friedman, M.; Rasooly, R. Review of the inhibition of biological activities of food-related selected toxins by natural compounds. Toxins 2013, 5, 743–775. [Google Scholar] [CrossRef]
- Jelinek, C.F.; Pohland, A.E.; Wood, G.E. Worldwide occurrence of mycotoxins in foods and feeds—An update. J. Assoc. Off. Anal. Chem. 1989, 72, 223–230. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Aflatoxins, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2012; Volume 100F, Available online: https://publications.iarc.fr/123 (accessed on 15 March 2024).
- Centers for Disease Control and Prevention. E. coli and Food Safety. 2024. Available online: https://www.cdc.gov/foodsafety/communication/ecoli-and-food-safety.html (accessed on 15 March 2024).
- Centers for Disease Control and Prevention. Staphylococcal (Staph) Food Poisoning. 2024. Available online: https://www.cdc.gov/foodsafety/diseases/staphylococcal.html (accessed on 15 March 2024).
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Reid, D.S.; Harris, L.J. Microorganisms and microbial toxins. Adv. Exp. Med. Biol. 1999, 459, 9–21. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Yu, J.; Ehrlich, K.C. Toxins of filamentous fungi. Chem. Immunol. 2002, 81, 167–206. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Hernlem, B.; He, X.; Friedman, M. Non-linear relationships between aflatoxin B1 levels and the biological response of monkey kidney vero cells. Toxins 2013, 5, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Toth, B. Novel strategies to control mycotoxins in feeds: A review. Acta Vet. Hung. 2005, 53, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Pluyer, H.R.; Ahmed, E.M.; Wei, C.I. Destruction of aflatoxins on peanuts by oven- and microwave-roasting. J. Food Prot. 1987, 50, 504–508. [Google Scholar] [CrossRef]
- Gillani, S.; Sadef, Y.; Imran, M.; Raza, H.M.F.; Ghani, A.; Anwar, S.; Ashraf, M.Y.; Hussain, S. Determination and detoxification of aflatoxin and ochratoxin in maize from different regions of Pakistan. Environ. Monit. Assess. 2022, 194, 613. [Google Scholar] [CrossRef]
- Cucullu, A.F.; Lee, L.S.; Pons, W.A., Jr.; Stanley, J.B. Ammoniation of aflatoxin B1: Isolation and characterization of a product with molecular weight 206. J. Agric. Food Chem. 1976, 24, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Vesonder, R.F.; Beckwith, A.C.; Ciegler, A.; Dimler, R.J. Ammonium hydroxide treatment of aflatoxin B1. Some chemical characteristics and biological effects. J. Agric. Food Chem. 1975, 23, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Fremy, J.M.; Gleizes, E.; de Méo, M.; Laget, M. Degradation of patulin using ammoniation. IARC Sci. Publ. 1991, 113, 41–45. [Google Scholar]
- Maeba, H.; Takamoto, Y.; Kamimura, M.; Miura, T. Destruction and detoxification of aflatoxins with ozone. J. Food Sci. 1988, 53, 667–668. [Google Scholar] [CrossRef]
- Sun, C.; Yang, F.; Xiao, J.; Zhou, W.; Li, J.; Gu, X. Simulating ozone degradation of deoxynivalenol and its bio-safety assessment by mouse model. Front. Microbiol. 2023, 14, 1286503. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, U.; Sen, A.C.; Fernando, S.Y.; Ahmed, E.M.; Wei, C.I. Inactivation of aflatoxin B1 in corn meal, copra meal and peanuts by chlorine gas treatment. Food Chem. Toxicol. 1991, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lu, T.; Shi, J.; Li, X.; Wu, K.; Xiong, Y. Identification of deoxynivalenol and degradation products during maize germ oil refining process. Foods 2022, 11, 1720. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Diao, E.; Li, X.; Zhang, Z.; Dong, H. Detoxification and safety evaluation of aflatoxin B1 in peanut oil using alkali refining. J. Sci. Food Agric. 2016, 96, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.S. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014, 45, 127–133. [Google Scholar] [CrossRef]
- Bata, A.; Lásztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Misawa, T.; Imanishi, Y. Degradation and detoxification of aflatoxin B1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 2017, 73, 619–626. [Google Scholar] [CrossRef]
- Sakudo, A.; Imanishi, Y. Degradation and inactivation of Shiga toxins by nitrogen gas plasma. AMB Express 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, J.; Yang, X.; Yu, B.; Zhuang, J.; Xu, H.; Xiang, Q.; Ma, R.; Jiao, Z. Recent advances in the degradation efficacy and mechanisms of mycotoxins in food by atmospheric cold plasma. Ecotoxicol. Environ. Saf. 2024, 270, 115944. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Keener, K.M. Degradation of Aflatoxin M1 in skim and whole milk using high voltage atmospheric cold plasma (HVACP) and quality assessment. Food Res. Int. 2022, 162, 112009. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Q.; Yang, Y.; Zhang, J.; Han, R.; Wang, J. Research progress and future trends of low temperature plasma application in food industry: A review. Molecules 2023, 28, 4714. [Google Scholar] [CrossRef]
- Shintani, H.; Sakudo, A. Gas Plasma Sterilization in Microbiology: Theory, Applications, Pitfalls and New Perspectives; Caister Academic Press: Poole, UK, 2016. [Google Scholar]
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731–738. [Google Scholar] [CrossRef]
- Sakudo, A.; Misawa, T.; Yagyu, Y. Equipment design for cold plasma disinfection of food products. In Advances in Cold Plasma Applications for Food Safety and Preservation; Daniela Bermudez-Aguirre, Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 289–307. [Google Scholar]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavus spores and biofilms: A comparative study. Appl. Environ. Microbiol. 2020, 86, e02619-19. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Toyokawa, Y.; Shimizu, N.; Imanishi, Y.; Sakudo, A. Inactivation of Salmonella by nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 2015, 52, 54–59. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Nakamura, T.; Yagyu, Y.; Imanishi, Y. Nitrogen gas plasma treatment of bacterial spores induces oxidative stress that damages the genomic DNA. Mol. Med. Rep. 2017, 15, 396–402. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Yu, B.; Du, M.; Feng, J.; Zhuang, J.; Ma, R.; Jiao, Z. Comparative analysis of helium and air surface micro-discharge plasma treatment on the microbial reduction and quality attributes of beef slices. Meat Sci. 2023, 204, 109259. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y. Application of a roller conveyor type plasma disinfection device with fungus-contaminated citrus fruits. AMB Express 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, Y.; Yagyu, Y.; Misawa, T.; Sakudo, A. A new roller conveyer system of non-thermal gas plasma as a potential control measure of plant pathogenic bacteria in primary food production. Food Control 2017, 72, 62–72. [Google Scholar] [CrossRef]
- Toyokawa, Y.; Yagyu, Y.; Yamashiro, R.; Ninomiya, K.; Sakudo, A. Roller conveyer system for the reduction of pesticides using non-thermal gas plasma—A potential food safety control measure? Food Control 2018, 87, 211–217. [Google Scholar] [CrossRef]
- Schneider, M.; Bláha, L. Advanced oxidation processes for the removal of cyanobacterial toxins from drinking water. Environ. Sci. Eur. 2020, 32, 94. [Google Scholar] [CrossRef]
- Kovacic, A.; Modic, M.; Hojnik, N.; Stampar, M.; Gulin, M.R.; Nannou, C.; Koronaiou, L.A.; Heath, D.; Walsh, J.L.; Zegura, B.; et al. Degradation and toxicity of bisphenol A and S during cold atmospheric pressure plasma treatment. J. Hazard. Mater. 2023, 454, 131478. [Google Scholar] [CrossRef] [PubMed]
- Oliinychenko, Y.K.; Ekonomou, S.I.; Tiwari, B.K.; Stratakos, A.C. Assessing the effects of cold atmospheric plasma on the natural microbiota and quality of pork during storage. Foods 2024, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Kutasi, K.; Recek, N.; Zaplotnik, R.; Mozetic, M.; Krajnc, M.; Gselman, P.; Primc, G. Approaches to inactivating aflatoxins-a review and challenges. Int. J. Mol. Sci. 2021, 22, 13322. [Google Scholar] [CrossRef]
- Pitt, J.; Taniwaki, M.H.; Cole, M. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Mycotoxins. Toxins Found in Food Infected by Certain Molds or Fungi. 2024. Available online: https://www.fda.gov/food/natural-toxins-food/mycotoxins (accessed on 15 March 2024).
- The European Parliament and the Council of the European Union. Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91. OJEU (The Official Journal of the European Union), L 189. 2007. Available online: https://eur-lex.europa.eu/eli/reg/2007/834/oj (accessed on 15 March 2024).
- Liu, R.; Jin, Q.; Huang, J.; Liu, Y.; Wang, X.; Zhou, X.; Mao, W.; Wang, S. In vitro toxicity of aflatoxin B1 and its photodegradation products in HepG2 cells. J. Appl. Toxicol. 2012, 32, 276–281. [Google Scholar] [CrossRef]
- Jalili, M.; Jinap, S.; Noranizan, M.A. Aflatoxins and ochratoxin a reduction in black and white pepper by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1786–1788. [Google Scholar] [CrossRef]
- Mobeen, A.K.; Aftab, A.; Asif, A.; Zuzzer, A.S. Aflatoxins B1 and B2 contamination of peanut and peanut products and subsequent microwave detoxification. J. Pharm. Nutr. Sci. 2011, 1, 1–3. [Google Scholar] [CrossRef]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.H.; Lee, M.H.; Han, D.W.; Chung, K.H.; Hyun, S.O.; Park, J.C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Tech. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Worldwide Regulations for Mycotoxins 1995, A Compendium, FAO Food and Nutrition Paper 64; Food and Agriculture Organization: Italy, Rome, 1995. [Google Scholar]
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- European Commission. RASFF (Rapid Alert System for Food and Feed) Annual Report 2020. 2020. Available online: https://food.ec.europa.eu/document/download/4b178b4f-c40c-4405-9c01-db558aa1392a_en?filename=rasff_pub_annual-report_2020.pdf (accessed on 15 March 2024).
- European Commission. RASFF Consumers Portal. 2014. Available online: https://webgate.ec.europa.eu/rasff-window/screen/consumers (accessed on 15 March 2024).
- GLOBALGAP. 2024. Available online: http://www.globalgap.org/ (accessed on 15 March 2024).
- EC (European Commission). Plasma Gas Technique as Electronic Preservation Practice of Organic Food and Feed, EGTOP/2014, Directorate-General for Agriculture and Rural Development, Directorate, B. Multilateral Relations, Quality Policy, B.4. Organics, Expert Group for Technical Advice on Organic Production EGTOP, Final Report on Food (III). 2014. Available online: https://ec.europa.eu/agriculture/organic/sites/orgfarming/files/docs/body/egtop-final-report-food-iii_en.pdf (accessed on 15 May 2020).
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Schantz, E.J.; Roessler, W.G.; Wagman, J.; Spero, L.; Dunnery, D.A.; Bergdoll, M.S. Purification of staphylococcal enterotoxin B. Biochemistry 1965, 4, 1011–1016. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakudo, A.; Yagyu, Y. Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge. Int. J. Mol. Sci. 2024, 25, 5986. https://doi.org/10.3390/ijms25115986
Sakudo A, Yagyu Y. Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge. International Journal of Molecular Sciences. 2024; 25(11):5986. https://doi.org/10.3390/ijms25115986
Chicago/Turabian StyleSakudo, Akikazu, and Yoshihito Yagyu. 2024. "Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge" International Journal of Molecular Sciences 25, no. 11: 5986. https://doi.org/10.3390/ijms25115986
APA StyleSakudo, A., & Yagyu, Y. (2024). Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge. International Journal of Molecular Sciences, 25(11), 5986. https://doi.org/10.3390/ijms25115986





