Molecular Basis of CO2 Sensing in Hyphantria cunea
Abstract
1. Introduction
2. Results
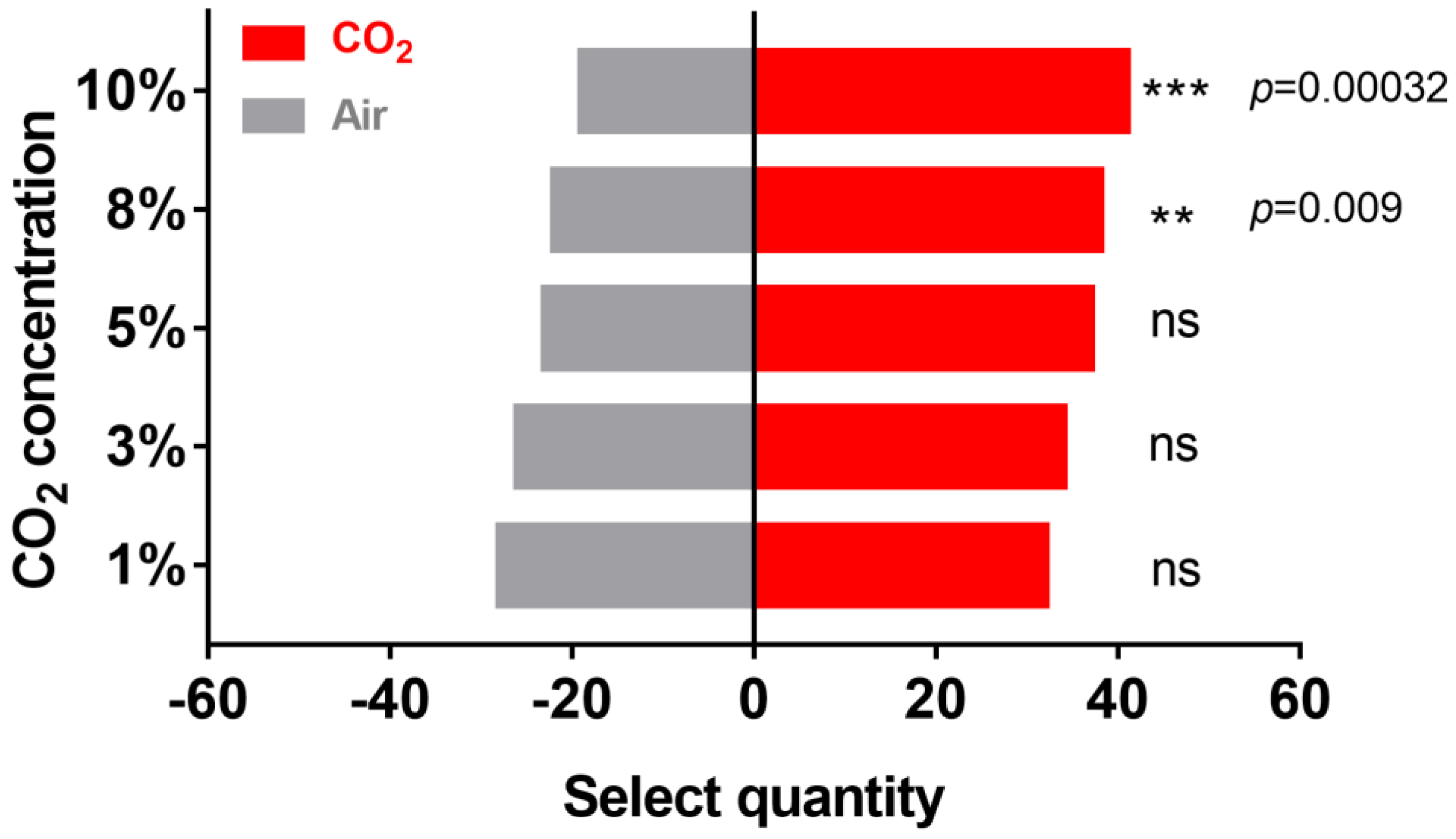
2.1. Effect of CO2 on H. cunea Behavior
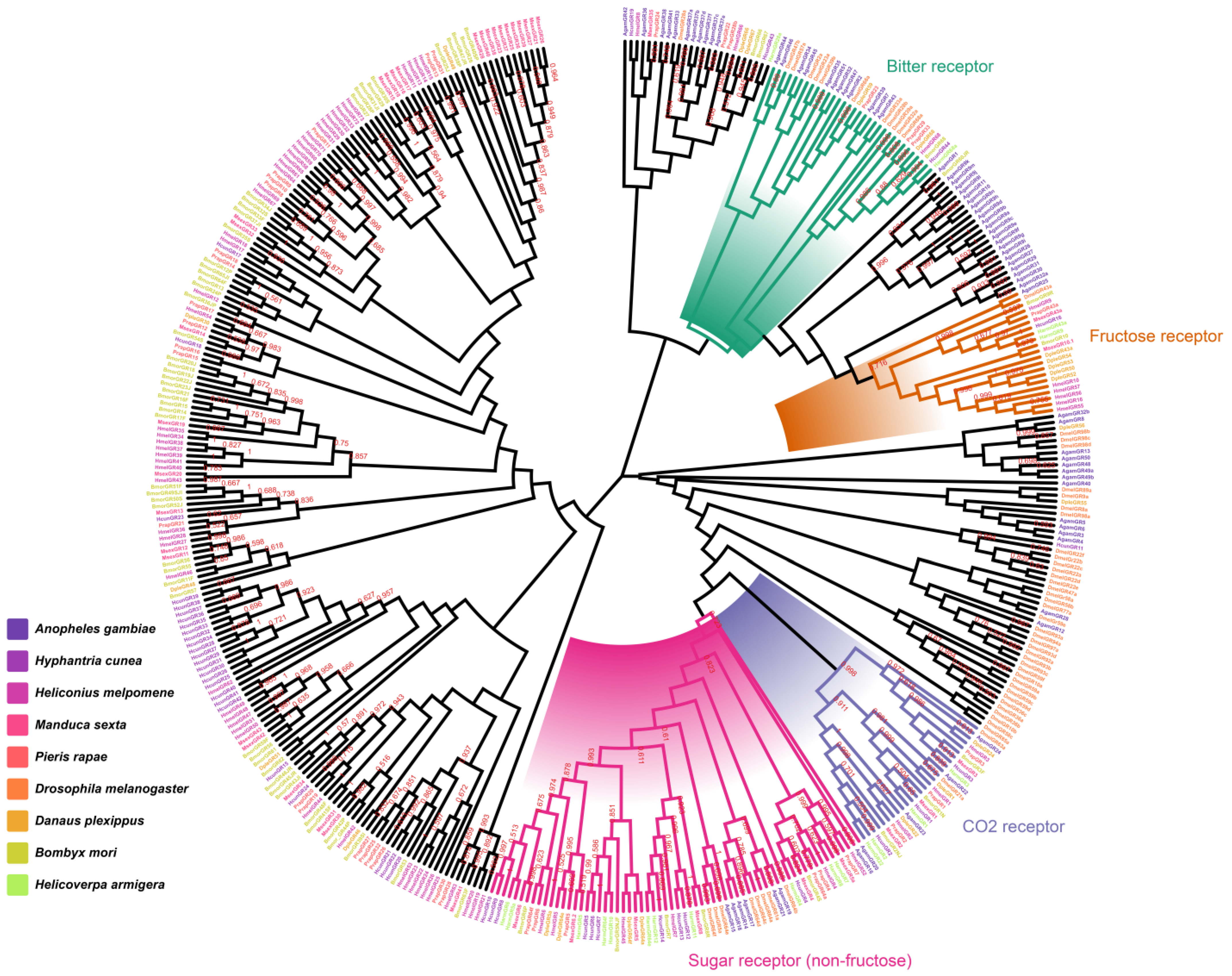
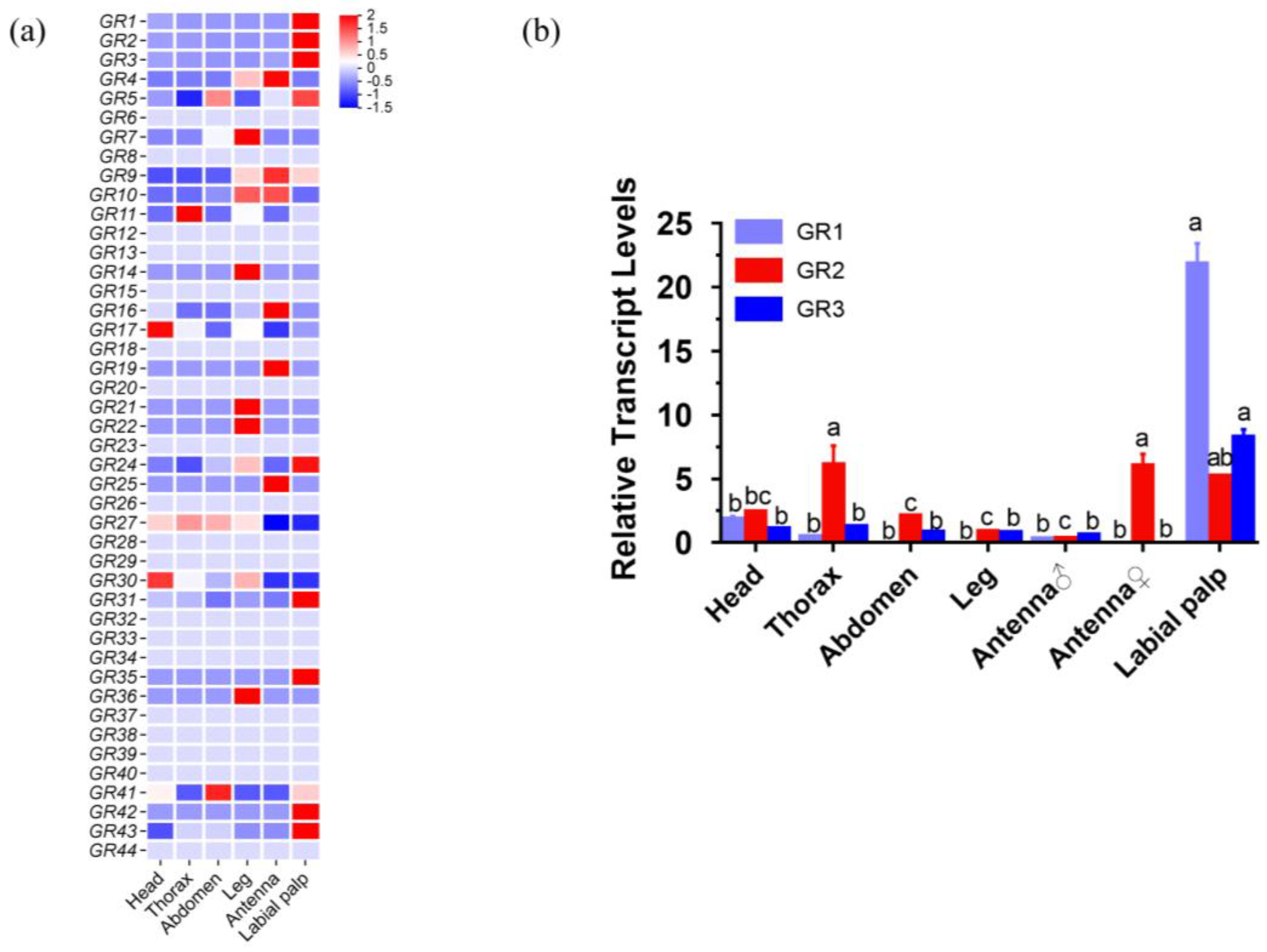
2.2. Identification and Homology Analysis of HcunGRs
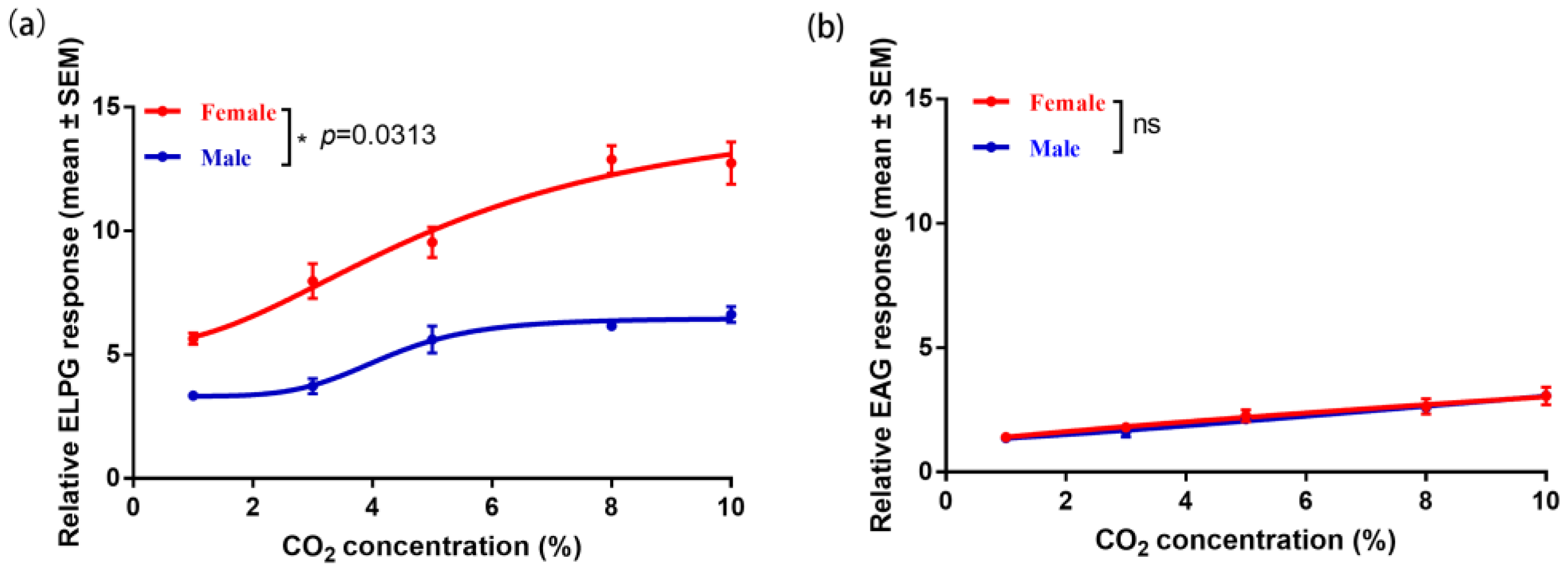
2.3. Electrophysiological Response of the Antennae and Labial Palp to CO2
2.4. Two-Electrode Voltage Clamp (TEVC) Response of HcunGr1, HcunGr2, HcunGr3, and Their Combinations
2.5. Anterograde Dye Filling of Labial Palps in H. cunea
3. Discussion
4. Materials and Methods
4.1. Insect
4.2. Binary Choice Assay
4.3. Homology Analysis of Gustatory Receptors (GRs)
4.4. Electrophysiological Recording
4.5. RNA Extraction, Expression Pattern Analysis, Quantitative PCR, and Cloning
4.6. cRNA Synthesis and Oocyte Microinjection
4.7. CO2 Quantification and TEVC
4.8. Anterograde Dye Filling and Immunohistochemical Staining of the Labial Palps
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerenstein, P.G.; Hildebrand, J.G. Roles and Effects of Environmental Carbon Dioxide in Insect Life. Annu. Rev. Entomol. 2008, 53, 161–178. [Google Scholar] [CrossRef]
- Seeley, T.D. Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. J. Insect Physiol. 1974, 20, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.F. The Sensory Physiology of Host-Seeking Behavior in Mosquitoes. Annu. Rev. Entomol. 1991, 36, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bull. Entomol. Res. 1980, 70, 525–532. [Google Scholar] [CrossRef]
- Huang, X.; Mack, T.P. Artificial Carbon Dioxide Source to Attract Lesser Cornstalk Borer (Lepidoptera: Pyralidae) Larvae. J. Econ. Entomol. 2001, 94, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Abrell, L.; Guerenstein, P.G.; Mechaber, W.L.; Stange, G.; Christensen, T.A.; Nakanishi, K.; Hildebrand, J.G. Effect of elevated atmospheric CO2 on oviposition behavior in Manduca sexta moths. Glob. Chang. Biol. 2005, 11, 1272–1282. [Google Scholar] [CrossRef]
- Stange, G.; Monro, J.; Stowe, S.; Osmond, C.B. The CO2 sense of the moth Cactoblastis cactorum and its probable role in the biological control of the CAM plant Opuntia stricta. Oecologia 1995, 102, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Loewy, K.J.; Flansburg, A.L.; Grenis, K.; Kjeldgaard, M.K.; Mccarty, J.; Montesano, L.; Vernick, J.; Murphy, S.M. Life History Traits and Rearing Techniques for Fall Webworms (Hyphantria cunea Drury) in Colorado. J. Lepid. Soc. 2013, 67, 196–205. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Ring, D.R. Biology and Management of the Fall Webworm, Hyphantria cunea (Lepidoptera: Erebidae). J. Integr. Pest Manag. 2017, 8. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, S.; Li, X.; Cao, Y.; Liu, X.; Wang, Q.; Liu, Q.; Liu, H.; Hu, X.; Zhou, X.J.; et al. Fall webworm genomes yield insights into rapid adaptation of invasive species. Nat. Ecol. Evol. 2019, 3, 105–115. [Google Scholar] [CrossRef]
- Jones, W.D.; Cayirlioglu, P.; Kadow, I.G.; Vosshall, L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 2006, 445, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Dahanukar, A.; Weiss, L.A.; Carlson, J.R. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. USA 2007, 104, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M.; Kent, L.B. Evolution of the Gene Lineage Encoding the Carbon Dioxide Receptor in Insects. J. Insect Sci. 2009, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhong, C.; Ding, C.; Chi, Q.; Walz, A.; Mombaerts, P.; Matsunami, H.; Luo, M. Detection of Near-Atmospheric Concentrations of CO2 by an Olfactory Subsystem in the Mouse. Science 2007, 317, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Fischler, W.; Kong, P.; Marella, S.; Scott, K. The detection of carbonation by the Drosophila gustatory system. Nature 2007, 448, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Engsontia, P.; Sangket, U.; Chotigeat, W.; Satasook, C. Molecular Evolution of the Odorant and Gustatory Receptor Genes in Lepidopteran Insects: Implications for Their Adaptation and Speciation. J. Mol. Evol. 2014, 79, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Anderson, A. Carbon dioxide receptor genes in cotton bollworm Helicoverpa armigera. Sci. Nat. 2015, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Yang, K.; Xu, M.; Huang, L.-Q.; Wang, C.-Z. Functional validation of the carbon dioxide receptor in labial palps of Helicoverpa armigera moths. Insect Biochem. Mol. Biol. 2016, 73, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F.; Singh, R.N.; Schorderet, M.; Siddiqi, O. Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster. Cell Tissue Res. 1983, 232, 237–248. [Google Scholar] [CrossRef]
- Suh, G.S.B.; Wong, A.M.; Hergarden, A.C.; Wang, J.W.; Simon, A.F.; Benzer, S.; Axel, R.; Anderson, D.J. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 2004, 431, 854–859. [Google Scholar] [CrossRef]
- Distler, P.G.; Boeckh, J. Central Projections of the Maxillary and Antennal Nerves in the Mosquito Aedes Aegypti. J. Exp. Biol. 1997, 200, 1873–1879. [Google Scholar] [CrossRef]
- Anton, S.; van Loon, J.J.; Meijerink, J.; Smid, H.M.; Takken, W.; Rospars, J.-P. Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes. Arthropod Struct. Dev. 2003, 32, 319–327. [Google Scholar] [CrossRef]
- Anton, S.; Rospars, J. Quantitative analysis of olfactory receptor neuron projections in the antennal lobe of the malaria mosquito, Anopheles gambiae. J. Comp. Neurol. 2004, 475, 315–326. [Google Scholar] [CrossRef]
- Zhao, X.-C.; Tang, Q.-B.; Berg, B.G.; Liu, Y.; Wang, Y.-R.; Yan, F.-M.; Wang, G.-R. Fine structure and primary sensory projections of sensilla located in the labial-palp pit organ of Helicoverpa armigera (Insecta). Cell Tissue Res. 2013, 353, 399–408. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Christensen, T.A.; Hildebrand, J.G. Sensory processing of ambient CO2 information in the brain of the moth Manduca sexta. J. Comp. Physiol. A 2004, 190, 707–725. [Google Scholar] [CrossRef]
- Yu, H.; Khashaveh, A.; Li, Y.; Li, X.; Zhang, Y. Field Trapping of Predaceous Insects With Synthetic Herbivore-Induced Plant Volatiles in Cotton Fields. Environ. Entomol. 2017, 47, 114–120. [Google Scholar] [CrossRef]
- Slone, J.; Daniels, J.; Amrein, H. Sugar Receptors in Drosophila. Curr. Biol. 2007, 17, 1809–1816. [Google Scholar] [CrossRef]
- Xu, P.; Wen, X.; Leal, W.S. CO2 per se activates carbon dioxide receptors. Insect Biochem. Mol. Biol. 2019, 117, 103284. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Zachariah, E.J.; Hussain, A.A. Nepenthes pitchers are CO2-enriched cavities, emit CO2 to attract preys. Sci. Rep. 2017, 7, 11281. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar] [CrossRef]
- Krenn, H.W. Feeding Mechanisms of Adult Lepidoptera: Structure, Function, and Evolution of the Mouthparts. Annu. Rev. Entomol. 2010, 55, 307–327. [Google Scholar] [CrossRef]
- Morris, R.F. Fecundity and colony size in natural populations of hyphantria cunea. Can. Entomol. 1972, 104, 399–409. [Google Scholar] [CrossRef]
- Clark, D. Images of Leaf Stomata: Little Things that Matter. Microsc. Today 2019, 27, 12–17. [Google Scholar] [CrossRef]
- Eshun, O.; Gerry, A.; Hayes, W.K. Mosquito Capture Rate Using CO2-Baited Traps in Relation to Distance From Water and Height: Implications for Avian Disease Transmission. J. Med Entomol. 2016, 53, 1378–1384. [Google Scholar] [CrossRef]
- Chen, Y.-C.D.; Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci. 2019, 77, 1087–1101. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef]
- Sánchez-Alcañiz, J.A.; Silbering, A.F.; Croset, V.; Zappia, G.; Sivasubramaniam, A.K.; Abuin, L.; Sahai, S.Y.; Münch, D.; Steck, K.; Auer, T.O.; et al. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat. Commun. 2018, 9, 4252, Correction in Nat. Commun. 2020, 11, 1131. [Google Scholar] [CrossRef]
- Ai, M.; Min, S.; Grosjean, Y.; Leblanc, C.; Bell, R.; Benton, R.; Suh, G.S.B. Acid sensing by the Drosophila olfactory system. Nature 2010, 468, 691–695. [Google Scholar] [CrossRef]
- van Breugel, F.; Huda, A.; Dickinson, M.H. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature 2018, 564, 420–424. [Google Scholar] [CrossRef]
- Terhag, J.; Cavara, N.A.; Hollmann, M. Cave Canalem: How endogenous ion channels may interfere with heterologous expression in Xenopus oocytes. Methods 2010, 51, 66–74. [Google Scholar] [CrossRef]
- Amat, C.; Marion-Poll, F.; Navarro-Roldán, M.A.; Gemeno, C. Gustatory function of sensilla chaetica on the labial palps and antennae of three tortricid moths (Lepidoptera: Tortricidae). Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Gaudry, Q.; Hong, E.J.; Kain, J.; de Bivort, B.L.; Wilson, R.I. Asymmetric neurotransmitter release enables rapid odour lateralization in Drosophila. Nature 2012, 493, 424–428. [Google Scholar] [CrossRef]
- Mohamed, A.A.M.; Hansson, B.S.; Sachse, S. Third-Order Neurons in the Lateral Horn Enhance Bilateral Contrast of Odor Inputs Through Contralateral Inhibition in Drosophila. Front. Physiol. 2019, 10, 851. [Google Scholar] [CrossRef]
- Mysore, K.; Subramanian, K.; Sarasij, R.; Suresh, A.; Shyamala, B.V.; VijayRaghavan, K.; Rodrigues, V. Caste and sex specific olfactory glomerular organization and brain architecture in two sympatric ant species Camponotus sericeus and Camponotus compressus (Fabricius, 1798). Arthropod Struct. Dev. 2009, 38, 485–497. [Google Scholar] [CrossRef]
- Varela, N.; Avilla, J.; Gemeno, C.; Anton, S. Ordinary glomeruli in the antennal lobe of male and female tortricid moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae) process sex pheromone and host-plant volatiles. J. Exp. Biol. 2011, 214, 637–645. [Google Scholar] [CrossRef]
- Kent, K.S.; Harrow, I.D.; Quartararo, P.; Hildebrand, J.G. An accessory olfactory pathway in Lepidoptera: The labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res. 1986, 245, 237–245. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, H.; Wen, M.; Li, J.; Zhou, H.; Wang, J.; Zhou, Y.; Liu, Y.; Du, L.; Kang, H.; et al. Genome of the webworm Hyphantria cunea unveils genetic adaptations supporting its rapid invasion and spread. BMC Genom. 2020, 21, 1–22. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, F.; Zhang, X.; Zhang, S.; Guo, S.; Zhu, G.; Liu, Q.; Li, M. Transcriptome and Expression Patterns of Chemosensory Genes in Antennae of the Parasitoid Wasp Chouioia cunea. PLoS ONE 2016, 11, e0148159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Q.; Feng, K.; Wang, J.; Zhou, H.; Wen, M.; Duan, H.; Wang, Y.; Ren, B. New binding sites of nicotinic acetylcholine receptors from Myzus persicae. Entomol. Gen. 2023, 43, 461–470. [Google Scholar] [CrossRef]
- Ignell, R.; Anton, S.; Hansson, B.S. The maxillary palp sensory pathway of Orthoptera. Arthropod Struct. Dev. 2000, 29, 295–305. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, F.; Liu, N. Three-dimensional structure of the antennal lobe in the Southern house mosquito Culex quinquefasciatus. Insect Sci. 2021, 28, 93–102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Duan, S.; Wang, W.; Liu, D.; Wang, Y. Molecular Basis of CO2 Sensing in Hyphantria cunea. Int. J. Mol. Sci. 2024, 25, 5987. https://doi.org/10.3390/ijms25115987
Zhang J, Duan S, Wang W, Liu D, Wang Y. Molecular Basis of CO2 Sensing in Hyphantria cunea. International Journal of Molecular Sciences. 2024; 25(11):5987. https://doi.org/10.3390/ijms25115987
Chicago/Turabian StyleZhang, Jian, Shiwen Duan, Wenlong Wang, Duo Liu, and Yinliang Wang. 2024. "Molecular Basis of CO2 Sensing in Hyphantria cunea" International Journal of Molecular Sciences 25, no. 11: 5987. https://doi.org/10.3390/ijms25115987
APA StyleZhang, J., Duan, S., Wang, W., Liu, D., & Wang, Y. (2024). Molecular Basis of CO2 Sensing in Hyphantria cunea. International Journal of Molecular Sciences, 25(11), 5987. https://doi.org/10.3390/ijms25115987




