Antibacterial and Antioxidant Activity of Synthetic Polyoxygenated Flavonoids
Abstract
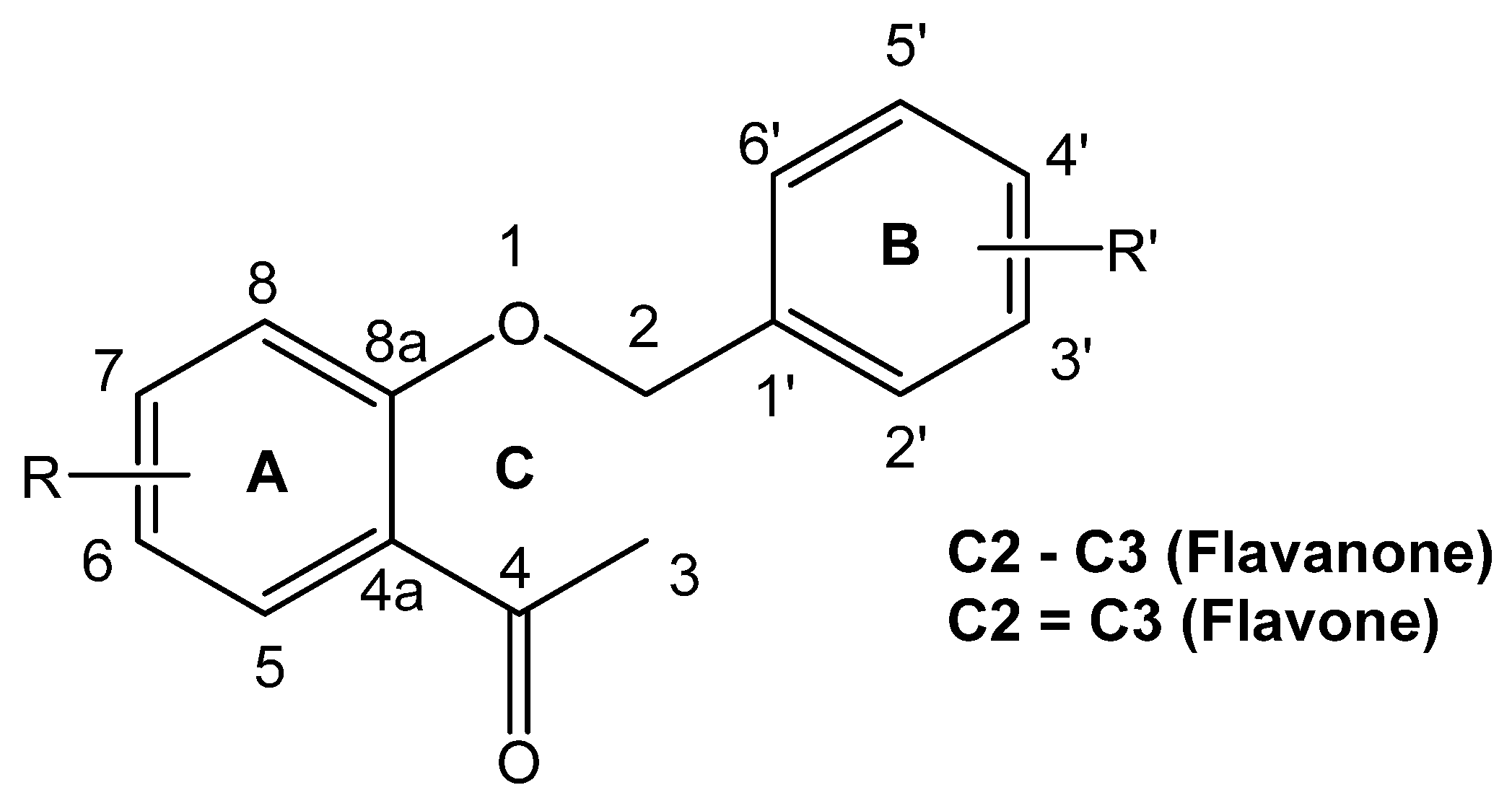
:1. Introduction
2. Results
2.1. Synthesis
2.2. In Vitro Antibacterial Effects of the Compounds on Human Pathogens
2.3. Antioxidant Activity of Synthesized Flavonoids
3. Materials and Methods
3.1. Chemistry
3.1.1. General Data
3.1.2. General Experimental Procedure for the Synthesis of Flavanones and Flavones
O-Protection with MOMCl of Acetophenone and Benzaldehyde Derivatives
General Procedure for Preparation of Chalcones CH1–CH11
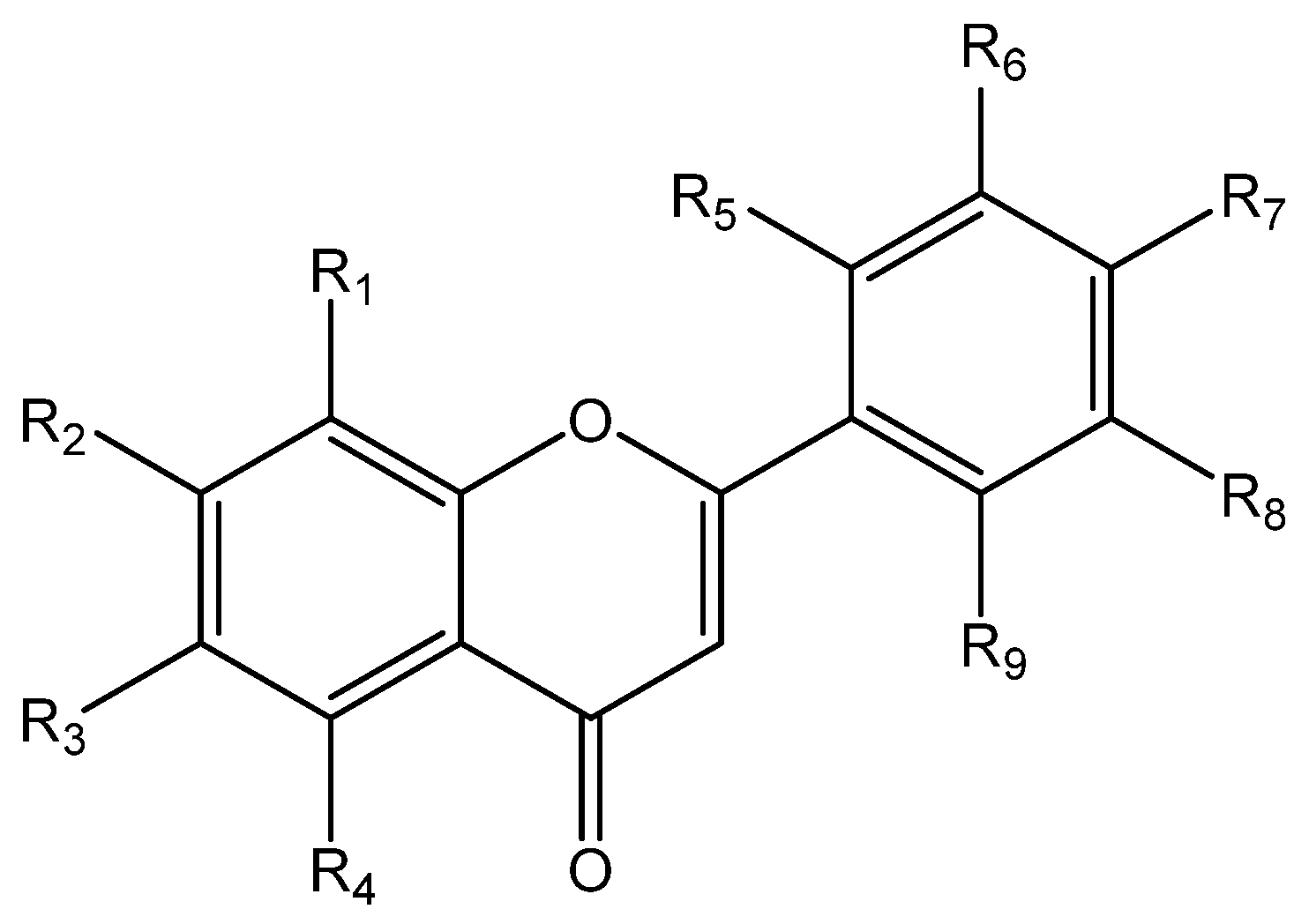
General Procedure for Preparation of Flavones FO1–FO11
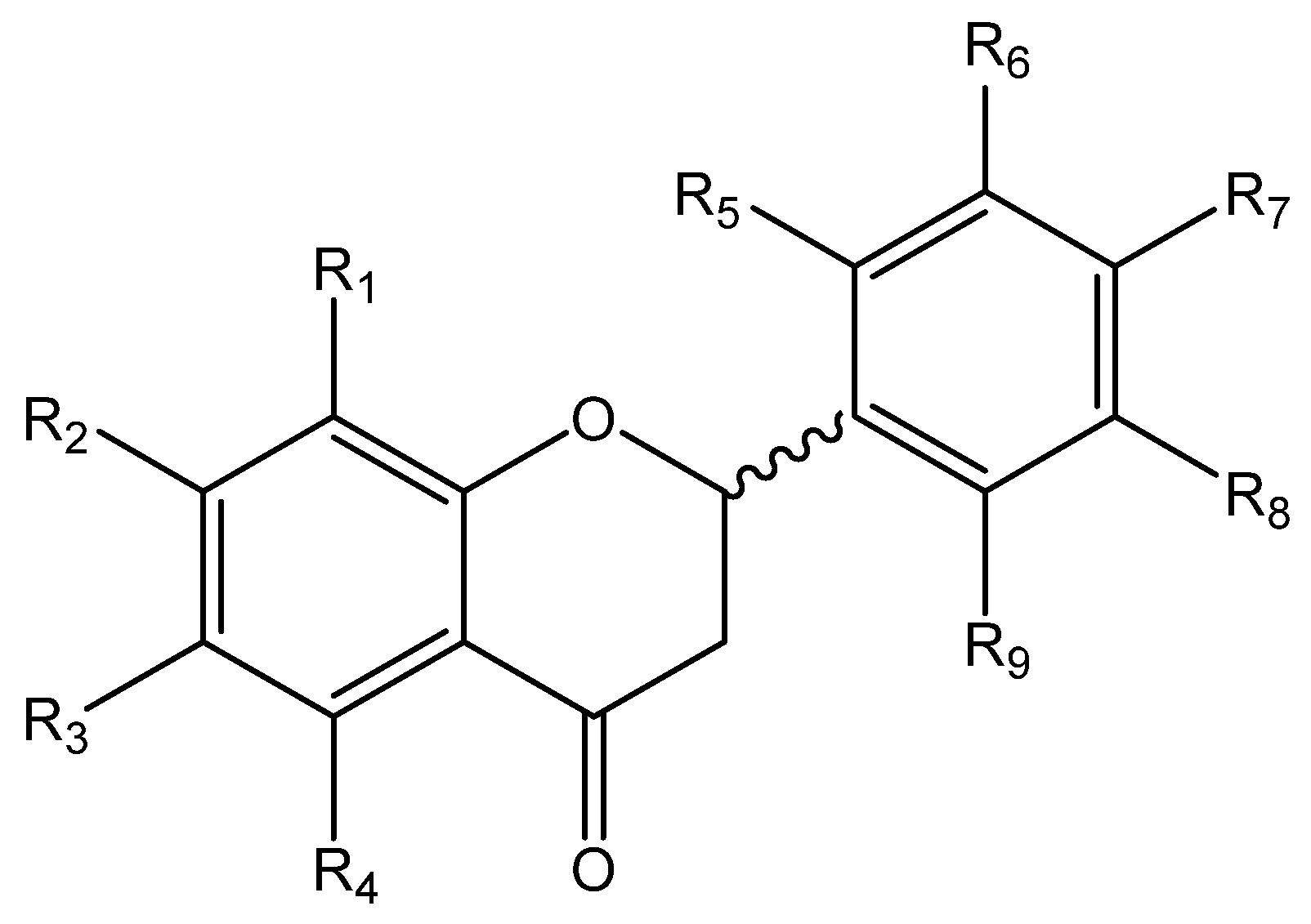
General Procedure for Preparation of Flavanones FV1–FV11
3.1.3. Physical Data of Synthesized Compound
Flavanones
Flavones
3.2. Biological Assays
3.2.1. In Vitro Antibacterial Activity Assays: Human Pathogens
Minimum Inhibitory Concentration Assay
3.3. General Procedure to Determine the DPPH Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus Phytochemical with Health-promoting Properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Ketabforoosh, S.H.M.E.; Kheirollahi, A.; Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Firoozpour, L.; Shafiee, A.; Foroumadi, A. Synthesis and Anti-Cancer Activity Evaluation of New Dimethoxylated Chalcone and Flavanone Analogs. Arch. Pharm. 2014, 347, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ndoile, M.M.; van Heerden, F.R. Total Synthesis of Ochnaflavone. Beilstein J. Org. Chem. 2013, 9, 1346–1351. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, Y.E.; Sohn, J.-H.; Ryu, D.H. A Facile Method for the Rapid and Selective Deprotection of Methoxymethyl (MOM) Ethers. Tetrahedron 2010, 66, 1673–1677. [Google Scholar] [CrossRef]
- Valdés, E.; González, C.; Díaz, K.; Vásquez-Martínez, Y.; Mascayano, C.; Torrent, C.; Cabezas, F.; Mejias, S.; Montoya, M.; Cortez-San Martín, M.; et al. Biological Properties and Absolute Configuration of Flavanones from Calceolariathyrsiflora Graham. Front. Pharmacol. 2020, 11, 1125. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ravindhiran, R.; Sivarajan, K.; Sekar, J.N.; Murugesan, R.; Dhandapani, K. Listeria Monocytogenes an Emerging Pathogen: A Comprehensive Overview on Listeriosis, Virulence Determinants, Detection, and Anti-Listerial Interventions. Microb. Ecol. 2023, 86, 2231–2251. [Google Scholar] [CrossRef]
- Kim, G.; Xu, Y.; Zhang, J.; Sui, Z.; Corke, H. Antibacterial Activity and Multi-Targeting Mechanism of Dehydrocorydaline from Corydalis turtschaninovii Bess. against Listeria monocytogenes. Front. Microbiol. 2022, 12, 799094. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic Adjuvants: An Alternative Approach to Overcome Multi-Drug Resistant Gram-Negative Bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Martín, M.C.-S.; Vásquez-Martínez, Y. Prenylated Flavonoids with Potential Antimicrobial Activity: Synthesis, Biological Activity, and In Silico Study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Lambert, P.A. Antibiotics and Antimicrobial Action; Edward Arnold: London, UK, 1981. [Google Scholar]
- Komape, N.; Aderogba, M.; Bagla, V.; Masoko, P.; Eloff, J. Anti-Bacterial and Anti-Oxidant Activities of Leaf Extracts of Combretum Vende (Combretecacea) and the Isolation of an Anti-Bacterial Compound. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, X.; Wei, L.; Wang, L.; Lv, Q. Acacetin Alleviates Listeria monocytogenes Virulence Both In Vitro and In Vivo via the Inhibition of Listeriolysin O. Foodborne Pathog. Dis. 2022, 19, 115–125. [Google Scholar] [CrossRef]
- Cha, J.-D.; Choi, S.-M.; Park, J.H. Combination of Acacetin with Antibiotics against Methicillin Resistant Staphylococcus aureus Isolated from Clinical Specimens. Adv. Biosci. Biotechnol. 2014, 5, 398–408. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a Flavone with Diverse Therapeutic Potential in Cancer, Inflammation, Infections and Other Metabolic Disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A Simple Flavone Exhibiting Diverse Pharmacological Activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus aureus and Listeria monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.-E.; El Omari, N.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Hou, Y.; Wang, R.; Ji, M.; Cai, Y.; Ao, J.; Shen, H.; Li, M.; Wang, J.; Luo, A. Potential Application of Luteolin as an Active Antibacterial Composition in the Development of Hand Sanitizer Products. Molecules 2022, 27, 7342. [Google Scholar] [CrossRef]
- Osorio, M.E.; Qiuroz, K.A.; Carvajal, M.A.; Vergara, A.P.; Sánchez, E.Y.; González, C.E.; Catalán, K.S. Synthesis, Anti-Phytopathogenic and DPPH Radical Scavenging Activities of C-Prenylated Ace Tophenones and Benzaldehydes. J. Chil. Chem. Soc. 2016, 61, 3095–3101. [Google Scholar] [CrossRef]
- Becerra, M.C.; Albesa, I. Oxidative Stress Induced by Ciprofloxacin in Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2002, 297, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Baharfar, R.; Azimi, R.; Mohseni, M. Antioxidant and Antibacterial Activity of Flavonoid-, Polyphenol- and Anthocyanin-Rich Extracts from Thymus kotschyanus Boiss & Hohen Aerial Parts. J. Food Sci. Technol. 2015, 52, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, M.; Takeda, M.; Takahashi, A.; Tazaki, T.; Tamura, H.; Shioiri, T. An Effective Synthesis of 5,4′-Disubstituted Flavones via a Cesium Enolate Assisted Intramolecular Ipso-Substitution Reaction. Chem. Pharm. Bull. 2010, 58, 1107–1110. [Google Scholar] [CrossRef]
- Sánchez-González, R.; Leyton, P.; Aguilar, L.F.; Reyna-Jeldes, M.; Coddou, C.; Díaz, K.; Mellado, M. Resveratrol-Schiff Base Hybrid Compounds with Selective Antibacterial Activity: Synthesis, Biological Activity, and Computational Study. Microorganisms 2022, 10, 1483. [Google Scholar] [CrossRef]
- De La Fuente, R.; Sonawane, N.D.; Arumainayagam, D.; Verkman, A.S. Small Molecules with Antimicrobial Activity against E. coli and P. aeruginosa Identified by High-Throughput Screening. Br. J. Pharmacol. 2006, 149, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Iten, F.; Saller, R.; Abel, G.; Reichling, J. Additive Antmicrobial Effects of the Active Components of the Essential Oil of Thymus vulgaris—Chemotype Carvacrol. Planta Med. 2009, 75, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Martínez, Y.; Osorio, M.E.; San Martín, D.; Carvajal, M.A.; Vergara, A.P.; Sanchez, E.; Raimondi, M.; Zacchino, S.A.; Mascayano, C.; Torrent, C.; et al. Antimicrobial, Anti-Inflammatory and Antioxidant Activities of Polyoxygenated Chalcones. J. Braz. Chem. Soc. 2019, 30, 286–304. [Google Scholar] [CrossRef]
- BioLabTests. Microbial Top Facts: E. coli. Available online: https://biolabtests.com/top-facts-ecoli/ (accessed on 2 May 2024).
- Centers for Disease Control and Prevention. Staphylococcus Aureus in Healthcare Settings. Available online: https://www.cdc.gov/hai/organisms/staph.html#print (accessed on 2 May 2024).
- Centros para el Control y la Prevención de Enfermedades Listeria (Listeriosis). Available online: https://www.cdc.gov/spanish/listeria/index.html (accessed on 2 May 2024).
- Anand, P.; Singh, B. Synthesis and Evaluation of Novel Carbamate-Substituted Flavanone Derivatives as Potent Acetylcholinesterase Inhibitors and Anti-Amnestic Agents. Med. Chem. Res. 2013, 22, 1648–1659. [Google Scholar] [CrossRef]
- Barontini, M.; Bernini, R.; Crisante, F.; Fabrizi, G. Selective and Efficient Oxidative Modifications of Flavonoids with 2-Iodoxybenzoic Acid (IBX). Tetrahedron 2010, 66, 6047–6053. [Google Scholar] [CrossRef]
- Zhao, D.-H.; Sui, X.; Qu, Y.-L.; Yang, L.-Y.; Wang, X.; Guan, L.-P. Synthesis and Studies on Antidepressant Effect of 5,7-Dihydroxyflavanone Derivatives. Asian J. Chem. 2011, 23, 1129–1132. [Google Scholar]
- Khan, M.K.; Rakotomanomana, N.; Loonis, M.; Dangles, O. Chemical Synthesis of Citrus Flavanone Glucuronides. J. Agric. Food Chem. 2010, 58, 8437–8443. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, J.; Kawagoe, M.; Sato, D. New Synthesis of Polyhydroxychalcones, Polyhydroxyhydrochalcones, Polyhydroxyflavanones. XIII. Synthesis of 3′,4′,5′-Trimethoxy-5,7-Dihydroxyflavanone and 3′,4′,5′,5,7-Pentahydroxyflavanone. Yakugaku Zasshi 1931, 51, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Cameroni, R. Pigments of Spartium junceum. III. Isolation and Constitution of a New Luteolin Glucoside. Gazz. Chim. Ital. 1958, 88, 204–213. [Google Scholar]
- Wang, Q.; Zhang, J.; Liu, M.; Yang, J.; Zhang, X.; Zhou, L.; Cao, L.; Liao, X. Modified Syntheses of the Dietary Flavonoid Luteolin. J. Chem. Res. 2015, 39, 550–552. [Google Scholar] [CrossRef]
- Awaad, A.S.; Maitland, D.J.; Soliman, G.A. Hepatoprotective Activity of Schouwia thebica Webb. Bioorg. Med. Chem. Lett. 2006, 16, 4624–4628. [Google Scholar] [CrossRef] [PubMed]
- Seijas, J.A.; Vázquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-Free Synthesis of Functionalized Flavones under Microwave Irradiation. J. Org. Chem. 2005, 70, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Nagarathnam, D. A Method for the Facile Synthesis of Ring-A Hydroxylated Flavones. Tetrahedron Lett. 1990, 31, 6497–6500. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Lei, X.; Wang, L.; Guo, L.; Zhao, G.; Lin, G. Discovery and Synthesis of Novel Luteolin Derivatives as DAT Agonists. Bioorg. Med. Chem. 2010, 18, 7842–7848. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, S.; Hanaya, K.; Shoji, M.; Sugai, T. Synthesis of Acacetin and Resveratrol 3,5-Di-O-β-Glucopyranoside Using Lipase-Catalyzed Regioselective Deacetylation of Polyphenol Glycoside Peracetates as the Key Step. J. Mol. Catal. B Enzym. 2016, 128, 19–26. [Google Scholar] [CrossRef]
- Badhwar, I.C.; Kang, K.S.; Venkataraman, K. Synthetical Experiments in the Chromone Group. Part VII. Synthesis of 7 : 8 : 4′-Trihydroxy-, 7 : 8 : 3′ : 4′-Tetrahydroxy-, and 7 : 8 : 3′ : 4′ : 5′-, 5 : 7 : 3′ : 4′ : 5′-, and 3 : 7 : 3′ : 4′ : 5′-Pentahydroxyflavones. J. Chem. Soc. 1932, 1107–1112. [Google Scholar] [CrossRef]
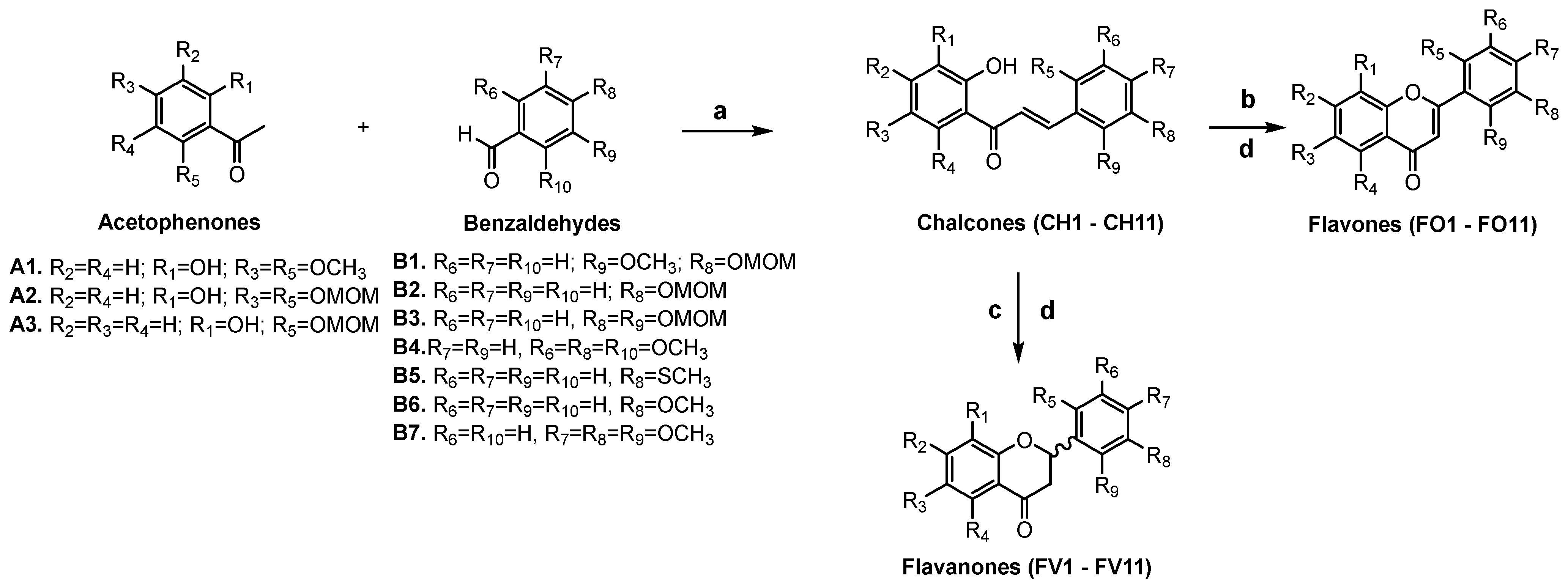

| Entry | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | Yield (CH1–CH11; %) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | OCH3 | H | OCH3 | H | H | OMOM | OCH3 | H | 75 |
| 2 | H | OMOM | H | OMOM | H | H | OMOM | OMOM | H | 86 |
| 3 | H | OMOM | H | OMOM | H | H | OMOM | OCH3 | H | 98 |
| 4 | H | OMOM | H | OMOM | OCH3 | H | OCH3 | H | OCH3 | 98 |
| 5 | H | OMOM | H | OMOM | H | H | OMOM | H | H | 90 |
| 6 | H | OMOM | H | OMOM | H | H | SCH3 | H | H | 99 |
| 7 | H | H | H | OMOM | H | H | SCH3 | H | H | 99 |
| 8 | H | OMOM | H | H | H | OCH3 | OCH3 | OCH3 | H | 49 |
| 9 | H | OCH3 | H | OCH3 | H | H | OMOM | OMOM | H | 44 |
| 10 | H | OMOM | H | OMOM | H | H | OCH3 | H | H | 98 |
| 11 | H | OMOM | H | OMOM | H | OCH3 | OCH3 | OCH3 | H | 77 |
| Entry | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | Yield (FV1–FV11; %) | Yield (FO1–FO11; %) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | OCH3 | H | OCH3 | H | H | OH | OCH3 | H | 62 | 25 |
| 2 | H | OH | H | OH | H | H | OH | OH | H | 69 | 15 |
| 3 | H | OH | H | OH | H | H | OH | OCH3 | H | 46 | 17 |
| 4 | H | OH | H | OH | OCH3 | H | OCH3 | H | OCH3 | 14 | 28 |
| 5 | H | OH | H | OH | H | H | OH | H | H | 56 | 24 |
| 6 | H | OH | H | OH | H | H | SCH3 | H | H | 51 | 14 |
| 7 | H | H | H | OH | H | H | SCH3 | H | H | 58 | 59 |
| 8 | H | OH | H | H | H | OCH3 | OCH3 | OCH3 | H | 38 | 10 |
| 9 | H | OCH3 | H | OCH3 | H | H | OH | OH | H | 27 | 23 |
| 10 | H | OH | H | OH | H | H | OCH3 | H | H | 23 | 29 |
| 11 | H | OH | H | OH | H | OCH3 | OCH3 | OCH3 | H | 51 | 17 |
| Compound | MRSA 97-7 (% Inhibition) | MRSA 97-7 MIC (µg/mL) | E. coli (% Inhibition) | E. coli MIC (µg/mL) | L. monocytogenes (% Inhibition) | L. monocytogenes MIC (µg/mL) |
|---|---|---|---|---|---|---|
| FV 1 | 0 | >50 | 57 | >50 | 100 | 25 |
| FV 2 | 59 | >50 | 100 | 25 | 50 | >50 |
| FV 3 | 35 | >50 | 36 | >50 | 69 | >50 |
| FV 4 | 27 | >50 | 14 | >50 | 94 | >50 |
| FV 5 | 26 | >50 | 73 | >50 | 52 | >50 |
| FV 6 | 22 | >50 | 100 | 25 | 80 | >50 |
| FV 7 | 0 | >50 | 71 | >50 | 84 | >50 |
| FV 8 | 28 | >50 | 34 | >50 | 57 | >50 |
| FV 9 | 16 | >50 | 50 | >50 | 42 | >50 |
| FV 10 | 48 | >50 | 43 | >50 | 74 | >50 |
| FV 11 | 49 | >50 | 32 | >50 | 91 | >50 |
| FO 1 | 63 | >50 | 18 | >50 | 46 | >50 |
| FO 2 | 100 | 50 | 31 | >50 | 43 | >50 |
| FO 3 | 100 | 12 | 6 | >50 | 59 | >50 |
| FO 4 | 12 | >50 | 9 | >50 | 46 | >50 |
| FO 5 | 94 | 50 | 8 | >50 | 64 | >50 |
| FO 6 | 87 | >50 | 4 | >50 | 37 | >50 |
| FO 7 | 0 | >50 | 0 | >50 | 57 | >50 |
| FO 8 | 37 | >50 | 4 | >50 | 73 | >50 |
| FO 9 | 54 | >50 | 20 | >50 | 31 | >50 |
| FO 10 | 42 | >50 | 100 | 25 | 100 | 15 |
| FO 11 | 10 | >50 | 62 | >50 | 56 | >50 |
| Vancomycin a | 100 | 25 | ||||
| Chloramphenicol b | - | 100 | 25 | |||
| Ciprofloxacin c | 100 | 5 |
| Compound | IC50 µM ± SD | Compound | IC50 µM ± SD |
|---|---|---|---|
| FV 1 | Inactive | FO 1 | Inactive |
| FV 2 | 22.20 ± 0.18 | FO 2 | 19.21 ± 1.66 |
| FV 3 | Inactive | FO 3 | Inactive |
| FV 4 | Inactive | FO 4 | Inactive |
| FV 5 | Inactive | FO 5 | Inactive |
| FV 6 | Inactive | FO 6 | Inactive |
| FV 7 | Inactive | FO 7 | Inactive |
| FV 8 | Inactive | FO 8 | Inactive |
| FV 9 | 23.69 ± 0.73 | FO 9 | 14.95 ± 0.51 |
| FV 10 | Inactive | FO 10 | Inactive |
| FV 11 | Inactive | FO 11 | Inactive |
| Trolox 1 | 17.81 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Olivares, M.E.; Vásquez-Martínez, Y.; Díaz, K.; Canelo, J.; Taborga, L.; Espinoza-Catalán, L. Antibacterial and Antioxidant Activity of Synthetic Polyoxygenated Flavonoids. Int. J. Mol. Sci. 2024, 25, 5999. https://doi.org/10.3390/ijms25115999
Osorio-Olivares ME, Vásquez-Martínez Y, Díaz K, Canelo J, Taborga L, Espinoza-Catalán L. Antibacterial and Antioxidant Activity of Synthetic Polyoxygenated Flavonoids. International Journal of Molecular Sciences. 2024; 25(11):5999. https://doi.org/10.3390/ijms25115999
Chicago/Turabian StyleOsorio-Olivares, Mauricio Enrique, Yesseny Vásquez-Martínez, Katy Díaz, Javiera Canelo, Lautaro Taborga, and Luis Espinoza-Catalán. 2024. "Antibacterial and Antioxidant Activity of Synthetic Polyoxygenated Flavonoids" International Journal of Molecular Sciences 25, no. 11: 5999. https://doi.org/10.3390/ijms25115999






