Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis
Abstract
1. Introduction
1.1. Relationship between Exosomes and Ferroptosis
1.2. Exosome-Biogenesis-Modulating Genes
1.3. Ferroptosis-Modulating Genes
1.3.1. Ferroptosis-Inducing Genes
1.3.2. Ferroptosis-Inhibiting Genes
1.4. The Knowledge Gaps of miRNA-Modulating Natural Products for the Induction and Inhibition of Ferroptosis and Exosome Biogenesis
1.5. The Novelty, Rationale, and Outline of This Review
2. Ferroptosis-Modulating Natural Products
| Natural Products | Ferroptosis Modulation by Natural Products * | Ferroptosis-Inducing Genes | Ferroptosis-Inhibiting Genes | |
|---|---|---|---|---|
| Ferroptosis inducers | Artesunate [41] | Lymphoma | ATG5 [42], ATG7 [43], NCOA4 [44], TFRC [45] | SLC11A2 [46], FTH1 [47], GPX4 [48], SP1 [49] |
| Albiziabioside A [50] | Breast Ca | GPX4 [51] | ||
| Alloimperatorin [24] | Breast Ca | SLC7A11, GPX4, p-AIFM1 [24] | ||
| Amentoflavone [52] | Gastric Ca | ↓ FTH1 [31], SP1 [53] | ||
| Ardisiacrispin B [54] | Leukemia | |||
| Aridanin [55] | Liver Ca | |||
| Artemisinin [56] | Osteosarcoma | ATF3 [57] | GPX4 [58] | |
| Artenimol [59] | Leukemia | |||
| Auriculasin [25] | Colon Ca | pAIFM1 [25] | ||
| Bromelain [60] | Colon Ca | ATG5, ATG7 [61], ACSL4 [60] | ||
| Curcumin [62] | Colon Ca | ATF3 [63], ACSL4 [64], IREB2 [63], HMOX1 [65] | SLC7A11, GPX4 [62,64], HIF1A [66], NEDD4 [67,68] | |
| Dihydroartemisinin [69] | Glioma | ATF4 [70,71], NCOA4 [72], HMOX1 [69] | GPX4, SLC7A11, SLC3A2 [70], FTH1 [73] | |
| Dihydroisotanshinone I [74] | Glioma | ACSL4 [74] | GPX4 [74] | |
| Diplacone [75] | Lung Ca | |||
| DMOCPTL [76] | Breast Ca | GPX4 [76] | ||
| Epigallocatechin gallate [77] | Pancreatic Ca | ATF4 [78], ATG5, ATG7 [79], YAP1 [80] | SP1, TP53 [81] | |
| Epunctanone [82] | Leukemia | |||
| Erianin [83] | Renal Ca stem cells | ALOX12 [83] | GPX4, FTH1, SLC7A11 [83], TP53 [84] | |
| Ferroptocide [23] | Ovarian Ca | TXN [23] | ||
| Gallic acid [85] | Breast Ca | ATF4 [86] | GPX4, SLC7A11 [86] | |
| Heteronemin [87] | Liver Ca | ATG5, ATG7 [88] | GPX4 [87], TP53 [89,90] | |
| Matrine [91] | Colon Ca | ATF4 [91] | GPX4, SLC7A11 [91] | |
| Nitidine chloride [92] | Myeloma | NEDD4 [93] | ||
| Piperlongumine [94] | Pancreas Ca | ATF4 [95], HMOX1 [96] | FTH1, SLC7A11, GPX4 [97], SP1 [98] | |
| Pseudolaric acid B [99] | GBM | TFRC [31] | SLC7A11 [99] | |
| Punicic acid [100] | Colon Ca | |||
| Quercetin [101] | Breast Ca | ATF3 [102], HMOX1 [103] | SLC40A1 [104], FTL [105], SP1 [106] | |
| Ruscogenin [31] | Pancreas Ca | TF [31,107] | SLC40A1 [107] | |
| Salinomycin [108] | Head/neck Ca | ATF3 [109], ATF4 [110], ATG5, ATG7 [111], DPP4 [112], IREB2, TFRC [108,113] | FTH1, FTL [113], NFE2L2, GCLC [114], HIF1A [115], TP53 [116], FTH1 [108] | |
| Sanguinarine [117,118] | Cervical Ca | SLC7A11 [118] | ||
| Solasonine [31] | Liver Ca | GPX4, GSS [119], SLC7A11 [120] | ||
| Sulforaphane [121] | Leukemia | ATF3 [122], ATG7 [123], ALOX12 [124], HMOX1 [125], YAP1 [126] | GPX4 [124], SLC7A11 [127] | |
| Tagitinin C [128] | Colon Ca | HMOX1 [128] | ||
| Talaroconvolutin A [22] | Colon Ca | ALOXE3 [22] | SLC7A11 [22] | |
| Trigonelline [31] | Liver Ca | DPP4 [129] | NFE2L2 [129] | |
| Typhaneoside [130] | Leukemia | |||
| Ungeremine [131] | Leukemia | |||
| Withaferin A [31] | Neuroblastoma | ATF3, ATF4, HMOX1 [132], ATG5, ATG7 [133] | GPX4, NFE2L2 [31] | |
| β-Elemene [134] | Colon Ca | HIF1A [135], SP1 [136] | ||
| β-Phenethyl isothiocyanate (PEITC) [137] | Osteosarcoma | ATG4 [138], HMOX1 [139] | SLC11A2, SLC40A1, FTH1 [140], GPX4, SLC11A2 [137], HIF1A [141] | |
| Ferroptosis inhibitors | Nodosin [142] | Bladder Ca | AIFM2, GPX4 [142] | |
| Nordihydroguaiareticacid [143] | Leukemia | ALOX12, ALOX15 [143] | ||
| Cryptotanshinone [144] | Pancreas Ca | |||
| Artepillin C [145] | Neuron | |||
| Bakuchiol [146] | Neuron | |||
| Berberine [147] | Cardiomyocytes | ATG5 [148], ACSL4 [149] | Cruloplasmin [150], NFE2L2 [151], SLC7A11 [149] | |
| Glycyrrhizin [152] | Acute liver failure | GPX4 [153], SLC40A1 [154] | ||
| Psoralidin [146] | Neuron | ALOX5 [146] | ||
| Butein [155] | BMSCs | SP1 [156] | ||
| Baicalein [157] | Pancreas Ca | ATF3 [158], ACSL4 [157], ALOX12 [159], ALOX15 [160] | GPX4 [157,161], SLC7A11 [157], GCLC [162] | |
| 7-O-cinnamoyl-taxifolin [163] | Neuron | NFE2L2 [163] | ||
| 3-Hydroxybakuchiol [146] | Neuron | |||
| Morachalcone D [164] | Cardiomyocytes | SLC7A11, NFE2L2, GPX4 [164] | ||
| Sterubin [165] | Neuron | NFE2L2 [165] | ||
| Proanthocyanidin [166] | Spinal cord injury mice | ATG5, ATG7 [167], ACSL4 [168], DDP4 [169] | GPX4, SLC7A11 [168], NFE2L2 [151] | |
| Puerarin [170] | Cardiomyocyte injury | FTH1 [31], GCLC [171], NFE2L2, GPX4 [172] |
2.1. Ferroptosis-Inducing Natural Products
2.1.1. Artesunate
2.1.2. Albiziabioside A and Alloimperatorin
2.1.3. Amentoflavone, Artemisinin, Auriculasin, and Bromelain
2.1.4. Curcumin
2.1.5. Dihydroartemisinin, Dihydroisotanshinone I, and DMOCPTL
2.1.6. Epigallocatechin Gallate (EGCG)
2.1.7. Erianin and Ferroptocide
2.1.8. Gallic Acid, Heteronemin, Matrine, Nitidine Chloride, and Sanguinarine
2.1.9. Piperlongumine and Pseudolaric Acid B
2.1.10. Quercetin
2.1.11. Ruscogenin, Sulforaphane, and Solasonine
2.1.12. Salinomycin
2.1.13. Sulforaphane
2.1.14. Tagitinin C, Talaroconvolutin A, Trigonelline, and Withaferin A
2.1.15. β-Elemene and β-Phenethyl Isothiocyanate (PEITC)
2.1.16. Other Ferroptosis-Inducing Natural Products
2.2. Ferroptosis-Inhibiting Natural Products
2.2.1. Cancer Studies for Ferroptosis-Inhibiting Natural Products
Nodosin, Nordihydroguaiaretic Acid, and Cryptotanshinone
2.2.2. Cancer and Non-Cancer Studies for Ferroptosis-Inhibiting Natural Products
Artepillin C and Bakuchiol
Berberine
Glycyrrhizin, Psoralidin, and Butein
Baicalein
2.2.3. Non-Cancer Studies for Ferroptosis-Inhibiting Natural Products
2.3. Exosome Regulation by Ferroptosis-Modulating Natural Products
3. Ferroptosis-Modulating miRNAs and Their Ferroptosis-Targeting Genes
| Ferroptosis- Modulating miRNA | Cancer Cells | Targets | miRDB-Targeting Ferroptosis-Inducing/ Inhibiting Genes (Targets) | |
|---|---|---|---|---|
| Ferroptosis-inducing miRNAs | miR-1261 [211] | Liver Ca | SLC7A11 | |
| miR-143-3p [212] | Renal Ca | SLC7A11 | ||
| miR-34c-3p [213] | Oral Ca | |||
| miR-382-5p [214] | Ovarian Ca | |||
| miR-489-3p [215] | Gastric Ca | |||
| miR-25-3p [216] | Prostate Ca | SLC7A11, AIFM1, SLC11A2 | ||
| miR-409-3p [217] | Cervical Ca | SLC7A11 | ||
| miR-515-5p [217] | Cervical Ca | |||
| miR-545-3p [218] | Thyroid Ca | GCLC, SLC11A2 | ||
| miR-27a-3p [219] | Bladder Ca | SLC7A11, NEDD4, NFE2L2 | ||
| miR-375-3p [220] | Oral Ca | SLC7A11 | ||
| miR-205-5p [221] | Airway epithelial * | TXNRD1 | ||
| miR-302a-3p [222] | Lung Ca | SLC40A1 | SLC40A1, AIFM1 | |
| miR-4735-3p [223] | Renal Ca | HIF1A, NEDD4, GCLC, SLC40A1 | ||
| miR-142-3p [224] | Liver Ca | SLC3A2 | SLC7A11 | |
| miR-1231 [225] | Thyroid Ca | GPX4 | BGN | |
| miR-1287-5p [226] | Lung Ca | |||
| miR-15a-5p [227] | Prostate Ca | SLC11A2 | ||
| miR-15a-3p [228] | Colon Ca | HIF1A | ||
| miR-539-5p [229] | Colon Ca | SP1, TXNRD1, SLC11A2, SLC7A11, SLC40A1 | ||
| miR-541-3p [230] | Liver Ca | |||
| miR-324-3p [231] | Lung Ca | SLC7A11 | ||
| miR-450b-5p [232] | Liver Ca | NFE2L2, CP, SLC7A11, AIFM1 | ||
| miR-125b-5p [233] | Oral Ca | NFE2L2 | AIFM1, TXNRD1 | |
| miR-144-3p [234] | Leukemia | NFE2L2, SLC7A11, GCLC | ||
| miR-28-5p [235] | Breast Ca | NFE2L2 | ||
| miR-507 [236] | Esophageal Ca | NFE2L2 | ||
| miR-29b-1-5p [237] | Breast Ca | PROM2 | ||
| miR-365a-3p [238] | Liver Ca | |||
| miR-214-3p [239] | Liver Ca | ATF4 | TFAP2C, GPX4 | |
| miR-3200-5p [240] | Liver Ca | |||
| miR-1228-3p [241] | Breast Ca | AIFM2 | ||
| miR-429 [29] | Gastric Ca | BGN | ||
| miR-19b-3p [242] | Lung Ca | FTH1 | SLC11A2 | |
| miR-129-5p [243] | Bladder Ca | PROM2 | NFE2L2 | |
| miR-101-3p [27] | Lung Ca | TBLR1 | NFE2L2, SLC7A11, GCLC | |
| Ferroptosis-inhibiting miRNAs | miR-23a-3p [244] | Liver Ca | ACSL4 | EPAS1 |
| miR-424-5p [245] | Ovarian Ca | ACSL4, YAP1 | ||
| miR-4291 [246] | Cervical Ca | DPP4, NCOA4, YAP1 | ||
| miR-670-3p [247] | GBM | ACSL4 | ||
| miR-18a-5p [248] | GBM | ALOXE3 | WWTR1 | |
| miR-522-3p [249] | Gastric Ca | ALOX15 | WWTR1, ACSL4 | |
| miR-19a-3p [21] | Colon Ca | IREB2 | IREB2, ACSL4, NCOA4, ATG5 |
3.1. Ferroptosis-Inducing miRNAs and Their Ferroptosis-Targeting Genes
3.1.1. SLCA11 (Ferroptosis-Inhibiting Gene)
3.1.2. SLC40A1 and SLC3A2 (Ferroptosis-Inhibiting Genes)
3.1.3. GPX4 (Ferroptosis-Inhibiting Gene)
3.1.4. NFE2L2 (Ferroptosis-Inhibiting Gene)
3.1.5. ATF4 (Ferroptosis-Inhibiting Gene)
3.1.6. AIFM2, BGN, FTH1, PROM2, and TBLR1 (Ferroptosis-Inhibiting Genes)
3.2. Ferroptosis-Inhibiting miRNAs and Their Ferroptosis-Targeting Genes
3.2.1. ACSL4 (Ferroptosis-Inducing Gene)
3.2.2. ALOXE3, IREB2, and ALOX15 (Ferroptosis-Inducing Gene)
4. Ferroptosis-Modulating miRNAs and Their Exosome-Biogenesis-Targeting Genes
| Ferroptosis- Modulating miRNA | Exosomal miRNA Studies | Exosome Biogenesis Genes (miRDB) | |
|---|---|---|---|
| Ferroptosis-inducing miRNAs | miR-101-3p [27] | Medulloblastoma [250] | RAB27A |
| miR-1231 [225] | Pancreatic ca [251] | ||
| miR-1287-5p [226] | Inflammatory injury [252] | RAB7A | |
| miR-129-5p [243] | Colon ca [253] | VPS4B, ATP9A, PDCD6IP | |
| miR-142-3p [224] | Retinoblastoma [254] | STAM, HGS | |
| miR-143-3p [212] | Lung ca [255], pancreatic ca [256] | RAB7A | |
| miR-144-3p [234] | Endothelial cells [257] | VPS4B, PDCD6IP, SMPD3 | |
| miR-15a-5p [227] | Endometrial ca [258], lung ca [259] | MYO5B, VPS4A | |
| miR-15a-3p [228] | Wound repair [260] | ||
| miR-19b-3p [242] | Lung ca [261] | SDC1, VPS4B, MYO5B | |
| miR-28-5p [235] | Lung injury [262] | SDC1 | |
| miR-29b-1-5p [237] | COPS5 | ||
| miR-302a-3p [222] | Preeclampsia [263] | SDC1, RAB11A | |
| miR-3200-5p [240] | SMPD3 | ||
| miR-324-3p [231] | RAB7B | ||
| miR-34c-3p [213] | Lung ca [264] | CD34 | |
| miR-365a-3p [238] | MYO5B | ||
| miR-409-3p [217] | Mast cells [265] | STAM | |
| miR-450b-5p [232] | Rat [266] | ATP9A, RAB11A, PDCD6IP | |
| miR-507 [236] | RAB11A, STEAP3, PDCD6IP | ||
| miR-515-5p [217] | RAB11A | ||
| miR-539-5p [229] | Stem cells [267] | STAM | |
| miR-545-3p [218] | RAB11A | ||
| Ferroptosis- inhibiting miRNAs | miR-18a-5p [248] | Osteoblast cells [268] | |
| miR-19a-3p [21] | Ischemic myocardium [269] | SDC1, VPS4B, MYO5B | |
| miR-23a-3p [244] | Cholangiocarcinoma [270] | ||
| miR-424-5p [245] | Endothelial cells [271] | VPS4A, MYO5B | |
| miR-4291 [246] | ATP9A, SMPD3, TSG101, MYO5B | ||
| miR-522-3p [249] | PDCD6IP | ||
| miR-670-3p [247] | CD34, RAB27A |
4.1. The Potential Role of the Exosome Biogenesis Modulation of Ferroptosis-Inducing miRNA in Cancer Studies
4.1.1. Anticancer Effects of Ferroptosis-Inducing miRNAs
4.1.2. miRDB Targets of Ferroptosis-Inducing miRNAs
4.2. The Potential Role of the Exosome Biogenesis Modulation of Ferroptosis-Inducing miRNA in Non-Cancer Studies
4.2.1. Non-Cancer Functions of Ferroptosis-Inducing miRNAs
4.2.2. miRDB Targets of Ferroptosis-Inducing miRNAs
4.3. The Potential Role of the Exosome Biogenesis Modulation of Ferroptosis-Inhibiting miRNA in Cancer Studies
4.4. The Potential Role of the Exosome Biogenesis Modulation of Ferroptosis-Inhibiting miRNA in Non-Cancer Studies
5. Ferroptosis-Modulating miRNAs Are Associated with Some Natural Products
| miRNAs | Ferroptosis-Modulating Natural Products | |
|---|---|---|
| Ferroptosis-inducing miRNAs | miR-101-3p | Curcumin [272] |
| miR-125b-5p | PEITC [273], quercetin [274], EGCG [275], berberine [276] | |
| miR-1287-5p | Curcumin [277] | |
| miR-129-5p | Matrine [278] | |
| miR-142-3p | Artesunate [279], quercetin [280], curcumin [281] | |
| miR-143-3p | Curcumin [282], EGCG [283,284], sulforaphane [285], quercetin [286] | |
| miR-144-3p | Curcumin [287] | |
| miR-15a-5p | Curcumin [288], baicalin [289], Withaferin A [290] | |
| miR-15a-3p | EGCG [284] | |
| miR-205-5p | Cryptotanshinone ↓ [291] proanthocyanidins ↓ [292], curcumin [293] | |
| miR-214-3p | EGCG [284], sulforaphane [285] | |
| miR-25-3p | Withaferin A [294] | |
| miR-27a-3p | β-elemene [295], quercetin [296] | |
| miR-28-5p | Curcumin [297] | |
| miR-302a-3p | Curcumin [298] | |
| miR-324-3p | Salinomycin ↓ [299] | |
| miR-365a-3p | Sulforaphane [300] | |
| miR-375-3p | Solasonine [301] | |
| miR-409-3p | Curcumin [302] | |
| miR-429 | Curcumin [303], berberine ↓ [304,305] | |
| miR-489-3p | Curcumin [306] | |
| Ferroptosis- inhibiting miRNAs | miR-18a-5p | Curcumin ↓ [307] |
| miR-19a-3p | Berberine [308], proanthocyanidins [292], matrine ↓ [309], Sulforaphane ↓ [310] | |
| miR-23a-3p | Berberine [311] | |
| miR-424-5p | Curcumin [312] | |
| miR-522-3p | EGCG ↓ [284] |
5.1. Ferroptosis-Inducing miRNAs: miR-101-3p, miR-125b-5p, miR-1287-5p, and miR-129-5p
5.2. Ferroptosis-Inducing miRNAs: miR-142-3p, miR-143-3p, miR-144-3p, and miR-15a-5p
5.3. Ferroptosis-Inducing miRNAs: miR-15a-3p, miR-205-5p, miR-214-3p, and miR-27a-3p
5.4. Ferroptosis-Inducing miRNAs: miR-28-5p, miR-302a-3p, miR-365a-3p, miR-375-3p, miR-429, and miR-489
5.5. Ferroptosis-Inducing miRNAs: miR-382-5p and miR-409-3p
5.6. Ferroptosis-Inhibiting miRNAs: miR-18a-5p, miR-19a-3p, miR-23a-3p, and miR-552-3p
5.7. Natural-Product-Centric Overview Connecting to Ferroptosis-Modulating miRNAs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crescitelli, R.; Lasser, C.; Lotvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, Q.; Yang, Y.; Li, Q.; Wang, Z. Exosome: Function and role in cancer metastasis and drug resistance. Technol. Cancer Res. Treat. 2018, 17, 1533033818763450. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, N. Role of exosomes and exosomal microRNAs in cancer. Future Sci. OA 2020, 6, FSO465. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yang, Y.; Kannisto, E.; Zeng, X.; Yu, G.; Patnaik, S.K.; Dy, G.K.; Reid, M.E.; Gan, Q.; Wu, Y. Simultaneous detection of tumor derived exosomal protein-microRNA pairs with an Exo-PROS biosensor for cancer diagnosis. ACS Nano 2023, 17, 8108–8122. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, Y.; Yu, X.; Li, X.; Fang, X. Induction and application of ferroptosis in cancer therapy. Cancer Cell Int. 2022, 22, 12. [Google Scholar] [CrossRef]
- Koeberle, S.C.; Kipp, A.P.; Stuppner, H.; Koeberle, A. Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling. Med. Res. Rev. 2023, 43, 614–682. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, B.; Huang, L.; Lin, J.; Li, Y.; Ze, Y.; Huang, C.; Yao, Y. Exosomes and ferroptosis: Roles in tumour regulation and new cancer therapies. PeerJ 2022, 10, e13238. [Google Scholar] [CrossRef]
- Zhou, Z.; You, B.; Ji, C.; Zhang, L.; Wu, F.; Qian, H. Implications of crosstalk between exosome-mediated ferroptosis and diseases for pathogenesis and treatment. Cells 2023, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Research advances in the understanding of how exosomes regulate ferroptosis in cancer. Clin. Transl. Oncol. 2023, 25, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Wu, S.; Duan, Q.; Liu, L.; Li, L.; Luo, Y.; Wang, A. Ferroptosis-dependent breast cancer cell-derived exosomes inhibit migration and invasion of breast cancer cells by suppressing M2 macrophage polarization. PeerJ 2023, 11, e15060. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Wang, H.; Ji, Z.G. Bladder cancer tissue-derived exosomes suppress ferroptosis of T24 bladder cancer cells by transporting miR-217. Environ. Mol. Mutagen. 2023, 64, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Kapanova, G.; Kalmakhanov, S.; Kussainov, A.Z.; Datkhayeva, Z. Regulation of ferroptosis by non-coding RNAs: Mechanistic insights. J. Pharmacol. Exp. Ther. 2023, 384, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, Y.; Lin, R.; Liu, T.; Wang, S.; Feng, Z.; Wang, X.; Cao, H.; Chen, X.; Miao, J.; et al. Ferroptosis: A promising candidate for exosome-mediated regulation in different diseases. Cell Commun. Signal 2024, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Si, L.; Bian, J.; Pan, C.; Guo, W.; Qin, P.; Zhu, W.; Xia, Y.; Zhang, Q.; Wei, K. Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radic. Biol. Med. 2022, 182, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Bult, C.J.; Blake, J.A.; Smith, C.L.; Kadin, J.A.; Richardson, J.E.; Mouse Genome Database, G. Mouse Genome Database (MGD) 2019. Nucleic Acids Res 2019, 47, D801–D806. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, Y.; Kim, S.E.; An, J.Y. Ferroptosis-related genes in neurodevelopment and central nervous system. Biology 2021, 10, 35. [Google Scholar] [CrossRef]
- Fan, H.; Ai, R.; Mu, S.; Niu, X.; Guo, Z.; Liu, L. MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2. Bioengineered 2022, 13, 12021–12029. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, S.; Li, C.; Ai, Z.; Shen, W.; Ren, W.; Yang, X. Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo. Cell Death Dis. 2020, 11, 988. [Google Scholar] [CrossRef]
- Llabani, E.; Hicklin, R.W.; Lee, H.Y.; Motika, S.E.; Crawford, L.A.; Weerapana, E.; Hergenrother, P.J. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem. 2019, 11, 521–532. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, R.F.; Li, J.; Yu, K.D.; Bi, K.X. Alloimperatorin activates apoptosis, ferroptosis, and oxeiptosis to inhibit the growth and invasion of breast cancer cells in vitro. Biochem. Cell Biol. 2022, 100, 213–222. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, L.H.; Zhuang, H.B.; Shi, Z.S.; Chen, Z.C.; Pan, J.P.; Hong, Z.S. Auriculasin enhances ROS generation to regulate colorectal cancer cell apoptosis, ferroptosis, oxeiptosis, invasion and colony formation. Biochem. Biophys. Res. Commun. 2022, 587, 99–106. [Google Scholar] [CrossRef]
- Bao, W.D.; Pang, P.; Zhou, X.T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, G.; Yi, H.; Li, Q.; Wu, Z.; Wang, J.; Yang, J.; Li, B.; Peng, Y.; Liang, Y.; et al. Nanomedicine promotes ferroptosis to inhibit tumour proliferation in vivo. Redox Biol. 2021, 42, 101908. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381. [Google Scholar] [CrossRef]
- Wu, C.; Hou, X.; Li, S.; Luo, S. Long noncoding RNA ZEB1-AS1 attenuates ferroptosis of gastric cancer cells through modulating miR-429/BGN axis. J. Biochem. Mol. Toxicol. 2023, 37, e23381. [Google Scholar] [CrossRef]
- Chuang, Y.T.; Tang, J.Y.; Shiau, J.P.; Yen, C.Y.; Chang, F.R.; Yang, K.H.; Hou, M.F.; Farooqi, A.A.; Chang, H.W. Modulating effects of cancer-derived exosomal miRNAs and exosomal processing by natural products. Cancers 2023, 15, 318. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, S.; Mu, H.; Zheng, S.; Tan, Z.; Huang, X.; Xu, C.; Zou, J.; Zhu, Y.; Feng, D.; et al. Emerging mechanisms and disease implications of ferroptosis: Potential applications of natural products. Front. Cell Dev. Biol. 2021, 9, 774957. [Google Scholar] [CrossRef]
- Zheng, K.; Dong, Y.; Yang, R.; Liang, Y.; Wu, H.; He, Z. Regulation of ferroptosis by bioactive phytochemicals: Implications for medical nutritional therapy. Pharmacol. Res. 2021, 168, 105580. [Google Scholar] [CrossRef]
- Greco, G.; Catanzaro, E.; Fimognari, C. Natural products as inducers of non-canonical cell death: A weapon against cancer. Cancers 2021, 13, 304. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, M.; Liu, Y.; Xiong, Y.; Gao, Z.; Ma, J.; Zhuang, G.; Hong, X. Application of natural products for inducing ferroptosis in tumor cells. Biotechnol. Appl. Biochem. 2022, 69, 190–197. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Q.; Liu, Y. The role of microRNAs in ferroptosis. Front. Mol. Biosci. 2022, 9, 1003045. [Google Scholar] [CrossRef]
- Zhi, Y.; Gao, L.; Wang, B.; Ren, W.; Liang, K.X.; Zhi, K. Ferroptosis holds novel promise in treatment of cancer mediated by non-coding RNAs. Front. Cell Dev. Biol. 2021, 9, 686906. [Google Scholar] [CrossRef]
- Dai, S.M.; Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. Relationship between miRNA and ferroptosis in tumors. Front. Pharmacol. 2022, 13, 977062. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef]
- Pandrangi, S.L.; Chittineedi, P.; Chalumuri, S.S.; Meena, A.S.; Neira Mosquera, J.A.; Sanchez Llaguno, S.N.; Pamuru, R.R.; Mohiddin, G.J.; Mohammad, A. Role of intracellular iron in switching apoptosis to ferroptosis to target therapy-resistant cancer stem cells. Molecules 2022, 27, 3011. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, G.Z.; Yin, J.L.; Bian, Z.X. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s Lymphoma. Biochem. Biophys. Res. Commun. 2019, 519, 533–539. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Xiang, J.D.; Jin, C.S.; Li, M.Q.; He, Y.Y. Artesunate-induced ATG5-related autophagy enhances the cytotoxicity of NK92 cells on endometrial cancer cells via interactions between CD155 and CD226/TIGIT. Int. Immunopharmacol. 2021, 97, 107705. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Wang, L.; Wong, Y.K.; Lee, Y.M.; Zhou, C.; Wu, G.; Zhao, T.; Yang, L.; Lu, L.; et al. Artesunate-induced mitophagy alters cellular redox status. Redox Biol. 2018, 19, 263–273. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef]
- Jeong, D.E.; Song, H.J.; Lim, S.; Lee, S.J.; Lim, J.E.; Nam, D.H.; Joo, K.M.; Jeong, B.C.; Jeon, S.S.; Choi, H.Y.; et al. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 2015, 6, 33046–33064. [Google Scholar] [CrossRef]
- Liu, X.; Gong, J.; Du, Q.; Li, R.; Zhang, S.; Zhu, H.; Liu, Z.; Ouyang, Z.; Ouyang, L. Study on the change of iron transporter expression in k562 cells apoptosis induced by artesunate. Blood 2008, 112, 5019. [Google Scholar]
- Kong, Z.; Liu, R.; Cheng, Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 2019, 109, 2043–2053. [Google Scholar] [CrossRef]
- Liu, J.; Pan, Z.; Tong, B.; Wang, C.; Yang, J.; Zou, J.; Jiang, J.; Zhang, L.; Jiang, B. Artesunate protects against ocular fibrosis by suppressing fibroblast activation and inducing mitochondria-dependent ferroptosis. FASEB J. 2023, 37, e22954. [Google Scholar] [CrossRef]
- Drouot, E.; Piret, J.; Boivin, G. Artesunate demonstrates in vitro synergism with several antiviral agents against human cytomegalovirus. Antivir. Ther. 2016, 21, 535–539. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Hou, Z.; Luan, W.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Novel antitumor compound optimized from natural saponin Albiziabioside A induced caspase-dependent apoptosis and ferroptosis as a p53 activator through the mitochondrial pathway. Eur. J. Med. Chem. 2018, 157, 759–772. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Luan, W.; Hou, Z.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis-M2-TAMs polarization for combined cancer therapy. J. Med. Chem. 2019, 62, 8760–8772. [Google Scholar] [CrossRef]
- Tang, F.; Xu, Y.; Gao, E.; Zhang, W.; Zhang, F.; Xiang, Y.; Xu, L.; Dong, F. Amentoflavone attenuates cell proliferation and induces ferroptosis in human gastric cancer by miR-496/ATF2 axis. Chem. Biol. Drug Des. 2023, 102, 782–792. [Google Scholar]
- Zhaohui, W.; Yingli, N.; Hongli, L.; Haijing, W.; Xiaohua, Z.; Chao, F.; Liugeng, W.; Hui, Z.; Feng, T.; Linfeng, Y. Amentoflavone induces apoptosis and suppresses glycolysis in glioma cells by targeting miR-124-3p. Neurosci. Lett. 2018, 686, 1–9. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.M.; Celik, I.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018, 43, 78–85. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Chi, G.F.; Bonsou, I.N.; Abdelfatah, S.; Tamfu, A.N.; Yeboah, E.M.O.; Kuete, V.; Efferth, T. N-acetylglycoside of oleanolic acid (aridanin) displays promising cytotoxicity towards human and animal cancer cells, inducing apoptotic, ferroptotic and necroptotic cell death. Phytomedicine 2020, 76, 153261. [Google Scholar] [CrossRef]
- Isani, G.; Bertocchi, M.; Andreani, G.; Farruggia, G.; Cappadone, C.; Salaroli, R.; Forni, M.; Bernardini, C. Cytotoxic effects of Artemisia annua L. and pure artemisinin on the D-17 canine osteosarcoma cell line. Oxid. Med. Cell. Longev. 2019, 2019, 1615758. [Google Scholar] [CrossRef]
- Ma, G.T.; Lee, S.K.; Park, K.K.; Park, J.; Son, S.H.; Jung, M.; Chung, W.Y. Artemisinin-daumone hybrid inhibits cancer cell-mediated osteolysis by targeting cancer cells and osteoclasts. Cell Physiol. Biochem. 2018, 49, 1460–1475. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Q.; Huo, C.; Li, Y.; He, L.; Ran, B.; Chen, J.; Li, Y.; Liu, W. Ferroptosis: A novel mechanism of artemisinin and its derivatives in cancer therapy. Curr. Med. Chem. 2021, 28, 329–345. [Google Scholar] [CrossRef]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.; Kim, M.; Jin, E.J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim. Cells Syst. 2018, 22, 334–340. [Google Scholar] [CrossRef]
- Hu, P.A.; Hsu, M.C.; Chen, S.H.; Chen, C.H.; Kou, Y.R.; Huang, J.W.; Lee, T.S. Bromelain activates the AMP-activated protein kinase-autophagy pathway to alleviate hepatic lipid accumulation. J. Food Drug Anal. 2014, 30, 357. [Google Scholar] [CrossRef]
- Chen, M.; Tan, A.H.; Li, J. Curcumin represses colorectal cancer cell proliferation by triggering ferroptosis via PI3K/Akt/mTOR signaling. Nutr. Cancer 2023, 75, 726–733. [Google Scholar] [CrossRef]
- Malik, M.; Mendoza, M.; Payson, M.; Catherino, W.H. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil. Steril. 2009, 91, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef]
- Kreutz, D.; Sinthuvanich, C.; Bileck, A.; Janker, L.; Muqaku, B.; Slany, A.; Gerner, C. Curcumin exerts its antitumor effects in a context dependent fashion. J. Proteom. 2018, 182, 65–72. [Google Scholar] [CrossRef]
- Shan, B.; Schaaf, C.; Schmidt, A.; Lucia, K.; Buchfelder, M.; Losa, M.; Kuhlen, D.; Kreutzer, J.; Perone, M.J.; Arzt, E.; et al. Curcumin suppresses HIF1A synthesis and VEGFA release in pituitary adenomas. J. Endocrinol. 2012, 214, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, J.; Yuan, J.; Tang, X.; Wang, Y.; Chen, H.; Liu, Y.; Zhou, L. Curcumin exerts its tumor suppressive function via inhibition of NEDD4 oncoprotein in glioma cancer cells. Int. J. Oncol. 2017, 51, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhou, X.; Yin, X.; Wang, L.; Zhao, Z.; Hou, Y.; Zheng, N.; Xia, J.; Wang, Z. The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochem. Pharmacol. 2017, 140, 28–40. [Google Scholar] [CrossRef]
- Gong, H.; Gao, M.; Lin, Y.; Liu, J.; Hu, Z.; Liu, J. TUG1/MAZ/FTH1 axis attenuates the antiglioma effect of dihydroartemisinin by inhibiting ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 7843863. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Q.; Zhang, L.; Zhuan, Q.; Meng, L.; Fu, X.; Hou, Y. Dihydroartemisinin exposure impairs porcine ovarian granulosa cells by activating PERK-eIF2alpha-ATF4 through endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2020, 403, 115159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, Z.; Zhang, Z.; Cao, Y.; Wei, Z.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Dihydroartemisinin alleviates hepatic fibrosis through inducing ferroptosis in hepatic stellate cells. Biofactors 2021, 47, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Grignano, E.; Cantero-Aguilar, L.; Tuerdi, Z.; Chabane, T.; Vazquez, R.; Johnson, N.; Zerbit, J.; Decroocq, J.; Birsen, R.; Fontenay, M.; et al. Dihydroartemisinin-induced ferroptosis in acute myeloid leukemia: Links to iron metabolism and metallothionein. Cell Death Discov. 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Hou, X.; Mei, L. Dihydrotanshinone I inhibits human glioma cell proliferation via the activation of ferroptosis. Oncol. Lett. 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Ryu, H.W.; Song, Y.N.; Kang, M.J.; Huh, Y.H.; Park, J.Y.; Oh, S.M.; Lee, S.Y.; Park, Y.J.; Kim, D.Y.; et al. Diplacone isolated from Paulownia tomentosa mature fruit induces ferroptosis-mediated cell death through mitochondrial Ca2+ influx and mitochondrial permeability transition. Int. J. Mol. Sci. 2023, 24, 7057. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Q.; Sun, X.; Zeh, H.J., 3rd; Lotze, M.T.; Kang, R.; Tang, D. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Md Nesran, Z.N.; Shafie, N.H.; Ishak, A.H.; Mohd Esa, N.; Ismail, A.; Md Tohid, S.F. Induction of endoplasmic reticulum stress pathway by green tea epigallocatechin-3-gallate (EGCG) in colorectal cancer cells: Activation of PERK/p-eIF2alpha/ATF4 and IRE1alpha. Biomed. Res. Int. 2019, 2019, 3480569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhao, Y.; Zhao, J.; Zhang, B.; Xu, K. EGCG regulates cell apoptosis of human umbilical vein endothelial cells grown on 316L stainless steel for stent implantation. Drug Des. Dev. Ther. 2021, 15, 493–499. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, Y.; Li, Y.; Jin, X.; Wang, D.; Lei, M.; Chen, F.; Liu, Y.; Xu, J.; Yao, G.; et al. Cytoplasmic YAP1-mediated ESCRT-III assembly promotes autophagic cell death and is ubiquitinated by NEDD4L in breast cancer. Cancer Commun. 2023, 43, 582–612. [Google Scholar] [CrossRef]
- Sivakumar, A.; Emerson, I.A.; Jayaraman, G. Docking studies on transcription factor sp1: The transcriptional down-regulation of TACO gene by EGCG and the Importance of TACO in M. tuberculosis survival. Int. J. Drug Des. Discov. 2010, 1, 265–272. [Google Scholar]
- Mbaveng, A.T.; Fotso, G.W.; Ngnintedo, D.; Kuete, V.; Ngadjui, B.T.; Keumedjio, F.; Andrae-Marobela, K.; Efferth, T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine 2018, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Geng, Z.; Nie, X.; Liu, T. Erianin induces ferroptosis of renal cancer stem cells via promoting ALOX12/P53 mRNA N6-methyladenosine modification. J. Cancer 2023, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Y.; Peng, C.; Xie, X.; Hu, G. Erianin inhibits human cervical cancer cell through regulation of tumor protein p53 via the extracellular signal-regulated kinase signaling pathway. Oncol. Lett. 2018, 16, 5006–5012. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Tang, P.; Liu, B.; Ran, C.; Yuan, C.; Zhang, Y.; Lu, Y.; Duan, X.; Yang, Y.; Wu, H. Ferroptosis-related genes for overall survival prediction in patients with colorectal cancer can be inhibited by gallic acid. Int. J. Biol. Sci. 2021, 17, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Bow, Y.D.; Fu, P.J.; Li, C.Y.; Wu, C.Y.; Chang, Y.H.; Teng, Y.N.; Li, R.N.; Lu, M.C.; Liu, Y.C.; et al. A marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ROS and MAPK pathways. Oxid. Med. Cell. Longev. 2021, 2021, 7689045. [Google Scholar] [CrossRef] [PubMed]
- Kaftan, G.; Erdogan, M.A.; El-Shazly, M.; Lu, M.C.; Shih, S.P.; Lin, H.Y.; Saso, L.; Armagan, G. Heteronemin promotes iron-dependent cell death in pancreatic cancer. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.; Huang, T.Y.; Chu, H.R.; De Luca, R.; Candelotti, E.; Huang, C.H.; Yang, Y.S.H.; Incerpi, S.; Pedersen, J.Z.; Lin, C.Y.; et al. Heteronemin and tetrac derivatives suppress non-small cell lung cancer growth via ERK1/2 inhibition. Food Chem. Toxicol. 2022, 161, 112850. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, T.Y.; Chang, W.J.; Pan, Y.S.; Chu, H.R.; Li, Z.L.; Unson, S.; Chin, Y.T.; Lin, C.Y.; Huang, H.M.; et al. Combined treatment of heteronemin and tetrac induces antiproliferation in oral cancer cells. Mar. Drugs 2020, 18, 348. [Google Scholar] [CrossRef]
- Wang, J.; Tang, P.; Cai, Q.; Xie, S.; Duan, X.; Pan, Y. Matrine can inhibit the growth of colorectal cancer cells by inducing ferroptosis. Nat. Prod. Commun. 2020, 15, 1934578X20982779. [Google Scholar] [CrossRef]
- Yin, Z.; Lv, Y.; Deng, L.; Li, G.; Ou, R.; Chen, L.; Zhu, Y.; Zhong, Q.; Liu, Z.; Huang, J.; et al. Targeting ABCB6 with nitidine chloride inhibits PI3K/AKT signaling pathway to promote ferroptosis in multiple myeloma. Free Radic. Biol. Med. 2023, 203, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, R.; Lian, C.; Cao, T.; Shi, Y.; Ma, J.; Wang, P.; Xia, J. Nitidine chloride suppresses NEDD4 expression in lung cancer cells. Aging 2021, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Lv, X.; Weng, Q.; Chen, M.; Cui, R.; Liang, G.; Ji, J. Piperlongumine, a novel TrxR1 inhibitor, induces apoptosis in hepatocellular carcinoma cells by ROS-mediated ER stress. Front. Pharmacol. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, J.; Singh, R.R.; Riggle, C.; Haugrud, B.; Abdalla, M.Y.; Reindl, K.M. JNK inhibition blocks piperlongumine-induced cell death and transcriptional activation of heme oxygenase-1 in pancreatic cancer cells. Apoptosis 2019, 24, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Li, Y.Q.; Wang, D.Y.; Shen, Y.Q. Natural product piperlongumine inhibits proliferation of oral squamous carcinoma cells by inducing ferroptosis and inhibiting intracellular antioxidant capacity. Transl. Cancer Res. 2023, 12, 2911–2922. [Google Scholar] [CrossRef] [PubMed]
- Karki, K.; Hedrick, E.; Kasiappan, R.; Jin, U.H.; Safe, S. Piperlongumine induces reactive oxygen species (ROS)-dependent downregulation of specificity protein transcription factors. Cancer Prev. Res. 2017, 10, 467–477. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Wang, X.; Lu, S.; Wang, C.; He, C.; Wang, L.; Piao, M.; Chi, G.; Luo, Y.; et al. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 2018, 428, 21–33. [Google Scholar] [CrossRef]
- Vermonden, P.; Vancoppenolle, M.; Dierge, E.; Mignolet, E.; Cuvelier, G.; Knoops, B.; Page, M.; Debier, C.; Feron, O.; Larondelle, Y. Punicic acid triggers ferroptotic cell death in carcinoma cells. Nutrients 2021, 13, 2751. [Google Scholar] [CrossRef]
- An, S.; Hu, M. Quercetin promotes TFEB nuclear translocation and activates lysosomal degradation of ferritin to induce ferroptosis in breast cancer cells. Comput. Intell. Neurosci. 2022, 2022, 5299218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fan, S.; Lu, Y.; Wei, Y.; Tang, J.; Yang, Y.; Li, F.; Chen, Q.; Zheng, J.; Liu, X. Quercetin confers protection of murine sepsis by inducing macrophage M2 polarization via the TRPM2 dependent calcium influx and AMPK/ATF3 activation. J. Funct. Foods 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Lin, H.; Peng, S.; Chen, L.; Cheng, X.; Yao, P.; Tang, Y. Iron-frataxin involved in the protective effect of quercetin against alcohol-induced liver mitochondrial dysfunction. J. Nutr. Biochem. 2023, 114, 109258. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.I.; Cho, J.H.; Lee, K.A.; Choi, N.J.; Seo, K.S.; Kim, S.B.; Lee, S.H.; Shim, J.H. Role of transcription factor Sp1 in the quercetin-mediated inhibitory effect on human malignant pleural mesothelioma. Int. J. Mol. Med. 2012, 30, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiang, X.; Li, J.; Deng, J.; Fang, Z.; Zhang, L.; Xiong, J. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol. Rep. 2020, 43, 516–524. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Promotion of ferroptosis in head and neck cancer with divalent metal transporter 1 inhibition or salinomycin. Hum. Cell 2023, 36, 1090–1098. [Google Scholar] [CrossRef]
- Ketola, K.; Hilvo, M.; Hyotylainen, T.; Vuoristo, A.; Ruskeepaa, A.L.; Oresic, M.; Kallioniemi, O.; Iljin, K. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br. J. Cancer 2012, 106, 99–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Liu, L.; Jiang, H.; Hu, H.; Du, X.; Ge, X.; Cao, J.; Wang, Y. Salinomycin triggers endoplasmic reticulum stress through ATP2A3 upregulation in PC-3 cells. BMC Cancer 2019, 19, 381. [Google Scholar] [CrossRef]
- Verdoodt, B.; Vogt, M.; Schmitz, I.; Liffers, S.T.; Tannapfel, A.; Mirmohammadsadegh, A. Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species. PLoS ONE 2012, 7, e44132. [Google Scholar] [CrossRef] [PubMed]
- Skeberdyte, A.; Sarapiniene, I.; Aleksander-Krasko, J.; Stankevicius, V.; Suziedelis, K.; Jarmalaite, S. Dichloroacetate and salinomycin exert a synergistic cytotoxic effect in colorectal cancer cell lines. Sci. Rep. 2018, 8, 17744. [Google Scholar] [CrossRef]
- Mai, T.T.; Hamai, A.; Hienzsch, A.; Caneque, T.; Muller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.; Li, S.; Meng, P. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp. Ther. Med. 2021, 22, 946. [Google Scholar] [CrossRef]
- Januszyk, S.; Mieszczanski, P.; Lurka, H.; Sagan, D.; Boron, D.; Grabarek, B.O. Expression profile of mRNAs and miRNAs related to the oxidative-stress phenomenon in the ishikawa cell line treated either cisplatin or salinomycin. Biomedicines 2022, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Niwa, A.M.; Semprebon, S.C.; D’Epiro, G.F.R.; Marques, L.A.; Zanetti, T.A.; Mantovani, M.S. Salinomycin induces cell cycle arrest and apoptosis and modulates hepatic cytochrome P450 mRNA expression in HepG2/C3a cells. Toxicol. Mech. Methods 2022, 32, 341–351. [Google Scholar] [CrossRef]
- Peng, R.; Xu, M.; Xie, B.; Min, Q.; Hui, S.; Du, Z.; Liu, Y.; Yu, W.; Wang, S.; Chen, X.; et al. Insights on antitumor activity and mechanism of natural benzophenanthridine alkaloids. Molecules 2023, 28, 6588. [Google Scholar] [CrossRef]
- Alakkal, A.; Thayyullathil, F.; Pallichankandy, S.; Subburayan, K.; Cheratta, A.R.; Galadari, S. Sanguinarine induces H2O2-dependent apoptosis and ferroptosis in human cervical cancer. Biomedicines 2022, 10, 1795. [Google Scholar] [CrossRef]
- Jin, M.; Shi, C.; Li, T.; Wu, Y.; Hu, C.; Huang, G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed. Pharmacother. 2020, 129, 110282. [Google Scholar] [CrossRef]
- Liang, X.; Hu, C.; Han, M.; Liu, C.; Sun, X.; Yu, K.; Gu, H.; Zhang, J. Solasonine inhibits pancreatic cancer progression with involvement of ferroptosis induction. Front. Oncol. 2022, 12, 834729. [Google Scholar] [CrossRef]
- Greco, G.; Schnekenburger, M.; Catanzaro, E.; Turrini, E.; Ferrini, F.; Sestili, P.; Diederich, M.; Fimognari, C. Discovery of sulforaphane as an inducer of ferroptosis in U-937 leukemia cells: Expanding its anticancer potential. Cancers 2021, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.G.; Kim, S.Y.; Lee, K.J.; Kim, J.S. Up-regulation of NAG-1 and p21 genes by sulforaphane. J. Life Sci. 2012, 22, 360–365. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Y.; Zhao, X.; Qiu, Z. Anticancer activity of sulforaphane against human hepatoblastoma cells involves apoptosis, autophagy and inhibition of β-catenin signaling pathway. Arch. Med. Sci. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Khan, M.A.; Alalami, U.; Somvanshi, P.; Bhardwaj, T.; Pramodh, S.; Raina, R.; Shekfeh, Z.; Haque, S.; Hussain, A. Phytochemicals induce apoptosis by modulation of nitric oxide signaling pathway in cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11827–11844. [Google Scholar] [PubMed]
- Hu, L.; Li, H.; Lee, E.D.; Grandis, J.R.; Bauman, J.E.; Johnson, D.E. Gene targets of sulforaphane in head and neck squamous cell carcinoma. Mol. Med. Rep. 2019, 20, 5335–5344. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.L.; Ciavattone, N.; Grun, D.; Adhikary, G.; Eckert, R.L. Sulforaphane reduces YAP/∆Np63alpha signaling to reduce cancer stem cell survival and tumor formation. Oncotarget 2017, 8, 73407–73418. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Okamoto-Katsuyama, M.; Maruoka, S.; Mizumura, K.; Shimizu, T.; Shikano, S.; Hikichi, M.; Takahashi, M.; Tsuya, K.; Okamoto, S.; et al. Effective ferroptotic small-cell lung cancer cell death from SLC7A11 inhibition by sulforaphane. Oncol. Lett. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y.; et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021, 17, 2703–2717. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Fu, M.; Dong, R.; Yang, Y.; Luo, J.; Hu, S.; Li, W.; Xu, X.; Tu, L. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 2020, 177, 113951. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Huang, Z.X.; Chen, G.Q.; Sheng, F.; Zheng, Y.S. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem. Biophys. Res. Commun. 2019, 516, 1265–1271. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Bitchagno, G.T.M.; Kuete, V.; Tane, P.; Efferth, T. Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy. Phytomedicine 2019, 60, 152832. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, S.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant inhibition of cytoprotective autophagy augments the efficacy of withaferin A in hepatocellular carcinoma. Cancers 2019, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, B.; Ji, Y.; Zhu, L.; Zhu, Y.; Zhao, H. beta-elemene inhibits the generation of peritoneum effusion in pancreatic cancer via suppression of the HIF1A-VEGFA pathway based on network pharmacology. Oncol. Rep. 2019, 42, 2561–2571. [Google Scholar] [PubMed]
- Zhao, S.; Wu, J.; Zheng, F.; Tang, Q.; Yang, L.; Li, L.; Wu, W.; Hann, S.S. β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKalpha signalling pathways in human lung cancer cells: The role of Sp1. J. Cell. Mol. Med. 2015, 19, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.H.; Zhen, C.X.; Liu, J.Y.; Shang, P. PEITC triggers multiple forms of cell death by GSH-iron-ROS regulation in K7M2 murine osteosarcoma cells. Acta Pharmacol. Sin. 2020, 41, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, A.; Hahm, E.R.; Xiao, D.; Powolny, A.A.; Fisher, A.L.; Jiang, Y.; Singh, S.V. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009, 69, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Lamsa, V.; Levonen, A.L.; Leinonen, H.; Yla-Herttuala, S.; Yamamoto, M.; Hakkola, J. Cytochrome P450 2A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem. Res. Toxicol. 2010, 23, 977–985. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Shang, P. beta-Phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5021983. [Google Scholar] [CrossRef]
- Wang, X.H.; Cavell, B.E.; Syed Alwi, S.S.; Packham, G. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem. Pharmacol. 2009, 78, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Fan, H.; Yang, J.; Tang, J.; Zhou, J.; Zhao, Y.; Huang, L.; Xia, Y. Network pharmacology research and dual-omic analyses reveal the molecular mechanism of natural product nodosin inhibiting muscle-invasive bladder cancer in vitro and in vivo. J. Nat. Prod. 2022, 85, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Dachert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, Y.; Jia, Y.; Zhao, Y.; Kang, R.; Tang, D.; Dai, E. Ferroptosis is a lysosomal cell death process. Biochem. Biophys. Res. Commun. 2018, 503, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Ichihara, K.; Hirata, Y. Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food Chem. Toxicol. 2019, 132, 110669. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, A.; Yang, F.; Zhang, X.; Li, F.; Chen, B.; Faraag, A.H.I.; Wang, M.K.; Shen, X.F.; Wang, L. Ferroptosis inhibitory constituents from the fruits of Cullen corylifolium. Nat. Prod. Res. 2021, 35, 5364–5368. [Google Scholar] [CrossRef]
- Yang, K.T.; Chao, T.H.; Wang, I.C.; Luo, Y.P.; Ting, P.C.; Lin, J.H.; Chang, J.C. Berberine protects cardiac cells against ferroptosis. Tzu Chi Med. J. 2022, 34, 310–317. [Google Scholar] [PubMed]
- Tan, Y.; Li, C.; Zhou, J.; Deng, F.; Liu, Y. Berberine attenuates liver fibrosis by autophagy inhibition triggering apoptosis via the miR-30a-5p/ATG5 axis. Exp. Cell Res. 2023, 427, 113600. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, S.; Song, Z.; Sun, H.; Wu, F.; Lin, X.; Jin, K.; Jin, X.; Wang, W.; et al. Berberine modulates gut microbiota to attenuate cerebral ferroptosis induced by ischemia-reperfusion in mice. Eur. J. Pharmacol. 2023, 953, 175782. [Google Scholar] [CrossRef]
- Punitha, I.S.R.; Shirwaikar, A.; Shirwaikar, A. Antidiabetic activity of benzyl tetra isoquinoline alkaloid berberine in streptozotocin-nicotinamide induced type 2 diabetic rats. Diabetol. Croat. 2005, 34, 117–128. [Google Scholar]
- Zhang, X.; Liang, D.; Lian, X.; Jiang, Y.; He, H.; Liang, W.; Zhao, Y.; Chi, Z.H. Berberine activates Nrf2 nuclear translocation and inhibits apoptosis induced by high glucose in renal tubular epithelial cells through a phosphatidylinositol 3-kinase/Akt-dependent mechanism. Apoptosis 2016, 21, 721–736. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, X.; Liu, S.; Yu, J.; Wu, S.; Hei, M. Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting ferroptosis and neuroinflammation in neonatal rats via the HMGB1/GPX4 pathway. Oxid. Med. Cell. Longev. 2022, 2022, 8438528. [Google Scholar] [CrossRef]
- Gowda, P.; Patrick, S.; Joshi, S.D.; Kumawat, R.K.; Sen, E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 2021, 142, 155496. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Cai, R.; Ren, Z.; Zhang, A.; Deng, F.; Chen, D. Simultaneous study of anti-ferroptosis and antioxidant mechanisms of butein and (S)-butin. Molecules 2020, 25, 674. [Google Scholar] [CrossRef]
- Jayasooriya, R.; Molagoda, I.M.N.; Park, C.; Jeong, J.W.; Choi, Y.H.; Moon, D.O.; Kim, M.O.; Kim, G.Y. Molecular chemotherapeutic potential of butein: A concise review. Food Chem. Toxicol. 2018, 112, 1–10. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Yu, S.; Li, J.; Wang, Y.; Yang, W.; Wu, H. Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 2022, 366, 110137. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.J.; Yang, D.; Hwang, Y.; Jun, H.S.; Cheon, H.G. Baicalein protects rat insulinoma INS-1 cells from palmitate-induced lipotoxicity by inducing HO-1. PLoS ONE 2017, 12, e0176432. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of oxidative stress and ALOX12 and NF-kappaB pathways contribute to the protective effect of baicalein on carbon tetrachloride-induced acute liver injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Guo, Y.Z.; Lu, D.H.; Pan, M.H.; Liu, H.Z.; Jiao, G.L.; Bi, W.; Kurihara, H.; Li, Y.F.; Duan, W.J.; et al. Tianma Gouteng granules decreases the susceptibility of Parkinson’s disease by inhibiting ALOX15-mediated lipid peroxidation. J. Ethnopharmacol. 2020, 256, 112824. [Google Scholar] [CrossRef]
- Yi, Z.H.; Li, S.Q.; Ke, J.Y.; Wang, Y.; Zhao, M.Z.; Li, J.; Li, M.Q.; Zhu, Z.L. Baicalein relieves ferroptosis-mediated phagocytosis inhibition of macrophages in ovarian endometriosis. Curr. Issues Mol. Biol. 2022, 44, 6189–6204. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hao, Z.; Zhang, S.; Wei, M.; Lu, B.; Wang, Z.; Ji, L. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem. Pharmacol. 2018, 150, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Gunesch, S.; Hoffmann, M.; Kiermeier, C.; Fischer, W.; Pinto, A.F.M.; Maurice, T.; Maher, P.; Decker, M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox Biol. 2020, 29, 101378. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Shi, D.; Zhou, T.; Tu, J.; He, M.; Jiang, Y.; Yang, B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020, 315, 126236. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Maher, P. Structural requirements for the neuroprotective and anti-inflammatory activities of the flavanone sterubin. Antioxidants 2022, 11, 2197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yin, C.; Zhang, Z.; Tang, H.; Shen, W.; Zha, X.; Gao, M.; Sun, J.; Xu, X.; Chen, Q. Proanthocyanidin promotes functional recovery of spinal cord injury via inhibiting ferroptosis. J. Chem. Neuroanat. 2020, 107, 101807. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A.; Sidor, A.; Kulczyński, B. Berries as a potential anti-influenza factor—A review. J. Funct. Foods 2017, 37, 116–137. [Google Scholar] [CrossRef]
- Lv, Y.W.; Du, Y.; Ma, S.S.; Shi, Y.C.; Xu, H.C.; Deng, L.; Chen, X.Y. Proanthocyanidins attenuates ferroptosis against influenza-induced acute lung injury in mice by reducing IFN-gamma. Life Sci. 2023, 314, 121279. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, M.; Nakaishi, S.; Usuda, A.; Miyahara, Y.; Katsumoto, K.; Katsura, K.; Terakado, I.; Jindo, M.; Nakajima, S.; Ogawa, S.; et al. Analysis of anti-obesity and anti-diabetic effects of acacia bark-derived proanthocyanidins in type 2 diabetes model KKAy mice. J. Nat. Med. 2021, 75, 893–906. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, C.; Li, H.; Chen, X.; Ding, Y.; Xu, S. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 2018, 497, 233–240. [Google Scholar] [CrossRef]
- Li, C.; Pan, Z.; Xu, T.; Zhang, C.; Wu, Q.; Niu, Y. Puerarin induces the upregulation of glutathione levels and nuclear translocation of Nrf2 through PI3K/Akt/GSK-3beta signaling events in PC12 cells exposed to lead. Neurotoxicol. Teratol. 2014, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Hu, Y.; Zhou, C.; Wu, J.; Wu, Y.; Wang, H.; Lenahan, C.; Huang, L.; Nie, S.; et al. Puerarin attenuates oxidative stress and ferroptosis via AMPK/PGC1alpha/Nrf2 pathway after subarachnoid hemorrhage in rats. Antioxidants 2022, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wang, D.; Shen, T.; Liu, X.; Dai, B.; Zhou, D.; Shen, H.; Gong, J.; Li, G.; Hu, Y.; et al. PDIA4 confers resistance to ferroptosis via induction of ATF4/SLC7A11 in renal cell carcinoma. Cell Death Dis. 2023, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, S.; Xu, W.; Zhang, Y.; Yang, R.; Ma, K.; Zhang, J.; Xu, J. Piperlongumine inhibits thioredoxin reductase 1 by targeting selenocysteine residues and sensitizes cancer cells to erastin. Antioxidants 2022, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lyu, H.; Ouyang, Q.; Shi, H.; Zhang, R.; Xiao, S.; Guo, D.; Zhang, Q.; Chen, X.Z.; Zhou, C.; et al. Insights into the roles of epigenetic modifications in ferroptosis. Biology 2024, 13, 122. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Nair, M.G.; Kirn, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Chang, S.Y.; Wu, Q.; Gou, Y.J.; Jia, L.P.; Cui, Y.M.; Yu, P.; Shi, Z.H.; Wu, W.S.; Gao, G.F.; et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front. Aging Neurosci. 2016, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Yang, X.; Cheng, L.; He, Z.; Xin, Y.; Huang, S.; Meng, F.; Zhang, P.; Luo, L. Activation of ALOX12 by a multi-organelle-orienting photosensitizer drives ACSL4-independent cell ferroptosis. Cell Death Dis. 2022, 13, 1040. [Google Scholar] [CrossRef]
- Yang, W.H.; Huang, Z.; Wu, J.; Ding, C.C.; Murphy, S.K.; Chi, J.T. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol. Cancer Res. 2020, 18, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, C.; Shi, D.; Xiao, X.; Chen, X.; Zeng, Y.; Li, X.; Xie, R. Ferroptosis related genes participate in the pathogenesis of spinal cord injury via HIF-1 signaling pathway. Brain Res. Bull. 2023, 192, 192–202. [Google Scholar] [CrossRef]
- Balihodzic, A.; Prinz, F.; Dengler, M.A.; Calin, G.A.; Jost, P.J.; Pichler, M. Non-coding RNAs and ferroptosis: Potential implications for cancer therapy. Cell Death Differ. 2022, 29, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Santana-Codina, N.; Mancias, J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Yi, C.; He, Y.; Chen, X.; Zhao, W.; Yu, D. Ferroptosis-related gene signature predicts the prognosis in oral squamous cell carcinoma patients. BMC Cancer 2021, 21, 835. [Google Scholar] [CrossRef]
- Vabulas, R.M. Ferroptosis-related flavoproteins: Their function and stability. Int. J. Mol. Sci. 2021, 22, 430. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef]
- Turcu, A.L.; Versini, A.; Khene, N.; Gaillet, C.; Caneque, T.; Muller, S.; Rodriguez, R. DMT1 inhibitors kill cancer stem cells by blocking lysosomal iron translocation. Chemistry 2020, 26, 7369–7373. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.L.; Schreiber, S.; Schafer, H. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayir, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Messerli, S.M.; Ahn, M.R.; Kunimasa, K.; Yanagihara, M.; Tatefuji, T.; Hashimoto, K.; Mautner, V.; Uto, Y.; Hori, H.; Kumazawa, S.; et al. Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother. Res. 2009, 23, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.H.; Lee, Y.; Yang, H.; Heo, Y.S.; Bode, A.M.; Lee, K.W.; Dong, Z. Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase. Oncotarget 2016, 7, 14616–14627. [Google Scholar] [CrossRef]
- Guo, X.H.; Jiang, S.S.; Zhang, L.L.; Hu, J.; Edelbek, D.; Feng, Y.Q.; Yang, Z.X.; Hu, P.C.; Zhong, H.; Yang, G.H.; et al. Berberine exerts its antineoplastic effects by reversing the Warburg effect via downregulation of the Akt/mTOR/GLUT1 signaling pathway. Oncol. Rep. 2021, 46, 253. [Google Scholar] [CrossRef] [PubMed]
- El-Senduny, F.F.; Zidane, M.M.; Youssef, M.M.; Badria, F.A. An approach to treatment of liver cancer by novel glycyrrhizin derivative. Anticancer. Agents Med. Chem. 2019, 19, 1863–1873. [Google Scholar] [CrossRef]
- Amani, D.; Shakiba, E.; Motaghi, E.; Alipanah, H.; Jalalpourroodsari, M.; Rashidi, M. Psoralidin exerts anti-tumor, anti-angiogenic, and immunostimulatory activities in 4T1 tumor-bearing balb/c mice. Horm. Mol. Biol. Clin. Investig. 2021, 43, 71–79. [Google Scholar] [CrossRef]
- Xie, Y.; Song, X.; Sun, X.; Huang, J.; Zhong, M.; Lotze, M.T.; Zeh, H.J.R.; Kang, R.; Tang, D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Huang, M.Z.; Chen, H.Y.; Peng, G.X.; Sun, H.; Peng, H.C.; Li, H.Y.; Liu, X.H.; Li, Q. Exosomes from artesunate-treated bone marrow-derived mesenchymal stem cells transferring SNHG7 to promote osteogenesis via TAF15-RUNX2 pathway. Regen. Med. 2022, 17, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, J.C.; Zeng, C.; Wu, D.; Mu, Z.M.; Chen, B.K.; Xie, Y.C.; Ye, Y.W.; Liu, J.X. Curcumin increases exosomal TCF21 thus suppressing exosome-induced lung cancer. Oncotarget 2016, 7, 87081–87090. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Lee, J.K.; Jeon, Y.K.; Kim, C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef]
- Jabbari, N.; Feghhi, M.; Esnaashari, O.; Soraya, H.; Rezaie, J. Inhibitory effects of gallic acid on the activity of exosomal secretory pathway in breast cancer cell lines: A possible anticancer impact. Bioimpacts 2022, 12, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lu, H.; Qian, Z. Matrine reduces the secretion of exosomal circSLC7A6 from cancer-associated fibroblast to inhibit tumorigenesis of colorectal cancer by regulating CXCR5. Biochem. Biophys. Res. Commun. 2020, 527, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Becer, E.; Özsoy, S.; Kabadayı, H.; Vatansever, H.S. Effect of quercetin on tumor-derived exosomal miRNA circulation in primary (Colo 320) and metastatic (Colo 741) colon cancer cell lines. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Zheng, K.; Ma, J.; Wang, Y.; He, Z.; Deng, K. Sulforaphane inhibits autophagy and induces exosome-mediated paracrine senescence via regulating mTOR/TFE3. Mol. Nutr. Food Res. 2020, 64, e1901231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as emerging drug delivery and diagnostic modality for breast cancer: Recent advances in isolation and application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.D.; Yao, Y.F.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. beta-Elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via Exosomes. Cell Physiol. Biochem. 2015, 36, 2274–2286. [Google Scholar] [CrossRef]
- Sun, Q.; Shan, R.; Qi, T.; Yang, P. Berberine reverses the tumorigenic function of colon cancer cell-derived exosomes. Tohoku J. Exp. Med. 2023, 260, 75–85. [Google Scholar] [CrossRef]
- Lyu, N.; Zeng, Y.; Kong, Y.; Chen, Q.; Deng, H.; Ou, S.; Bai, Y.; Tang, H.; Wang, X.; Zhao, M. Ferroptosis is involved in the progression of hepatocellular carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann. Transl. Med. 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Zhu, H.C.; Du, Y.; Zhao, H.C.; Wang, L. Silencing lncRNA SLC16A1-AS1 induced ferroptosis in renal cell carcinoma through miR-143-3p/SLC7A11 signaling. Technol. Cancer Res. Treat. 2022, 21, 15330338221077803. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Ren, W.; Li, S.; Zheng, J.; Huang, Y.; Zhi, K.; Gao, L. MiR-34c-3p upregulates erastin-induced ferroptosis to inhibit proliferation in oral squamous cell carcinomas by targeting SLC7A11. Pathol. Res. Pract. 2022, 231, 153778. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, Y.C.; Zhang, X.Y. Lidocaine promoted ferroptosis by targeting miR-382-5p/SLC7A11 axis in ovarian and breast cancer. Front. Pharmacol. 2021, 12, 681223. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.H.; Zhu, C.H.; Nie, Y.; Yu, J.; Wang, L. Levobupivacaine induces ferroptosis by miR-489-3p/SLC7A11 signaling in gastric cancer. Front. Pharmacol. 2021, 12, 681338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guo, S.; Xu, M.; Ma, B.; Liu, R.; Xu, Y.; Zhang, Y. TFAP2C-mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front. Oncol. 2022, 12, 862015. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, C.; Ye, D.M.; Yu, K.; Li, Y.; Tang, H.; Xu, G.; Yi, S.; Zhang, Z. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging 2021, 13, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Ma, J.N.; Zhan, X.R. Circular RNA Circ_0067934 attenuates ferroptosis of thyroid cancer cells by miR-545-3p/SLC7A11 signaling. Front. Endocrinol. 2021, 12, 670031. [Google Scholar] [CrossRef] [PubMed]
- Drayton, R.M.; Dudziec, E.; Peter, S.; Bertz, S.; Hartmann, A.; Bryant, H.E.; Catto, J.W. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin. Cancer Res. 2014, 20, 1990–2000. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Song, B.; Qiu, X.; Zhao, J. MiR-375/SLC7A11 axis regulates oral squamous cell carcinoma proliferation and invasion. Cancer Med. 2017, 6, 1686–1697. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, Y.; Guan, L.; Qin, L.; Ding, J.; Zhang, Q.; Zhou, L. Seven ferroptosis-specific expressed genes are considered as potential biomarkers for the diagnosis and treatment of cigarette smoke-induced chronic obstructive pulmonary disease. Ann. Transl. Med. 2022, 10, 331. [Google Scholar] [CrossRef]
- Wei, D.; Ke, Y.Q.; Duan, P.; Zhou, L.; Wang, C.Y.; Cao, P. MicroRNA-302a-3p induces ferroptosis of non-small cell lung cancer cells via targeting ferroportin. Free Radic. Res. 2021, 55, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Song, Z.; Chen, Z.; Lin, T.; Lin, H.; Xu, Z.; Ai, F.; Zheng, S. MicroRNA-4735-3p facilitates ferroptosis in clear cell renal cell carcinoma by targeting SLC40A1. Anal. Cell. Pathol. 2022, 2022, 4213401. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yin, Y.; Jiang, J.; Yan, C.; Wang, Y.; Wang, D.; Li, L. Exosomal miR-142-3p secreted by hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of M1-type macrophages through SLC3A2 and the mechanism of HCC progression. J. Gastrointest. Oncol. 2022, 13, 754–767. [Google Scholar] [CrossRef]
- Chen, W.; Fu, J.; Chen, Y.; Li, Y.; Ning, L.; Huang, D.; Yan, S.; Zhang, Q. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging 2021, 13, 16500–16512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, L.; Wang, C.; Zhang, L.; Xu, W. MicroRNA-1287-5p promotes ferroptosis of osteosarcoma cells through inhibiting GPX4. Free Radic. Res. 2021, 55, 1119–1129. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Deng, Z.; Tan, Z.; Pei, X. MicroRNA-15a promotes prostate cancer cell ferroptosis by inhibiting GPX4 expression. Oncol. Lett. 2022, 23, 67. [Google Scholar] [CrossRef]
- Liu, L.; Yao, H.; Zhou, X.; Chen, J.; Chen, G.; Shi, X.; Wu, G.; Zhou, G.; He, S. MiR-15a-3p regulates ferroptosis via targeting glutathione peroxidase GPX4 in colorectal cancer. Mol. Carcinog. 2022, 61, 301–310. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Han, Z.; Wu, Z.; Hua, J.; Zhong, R.; Zhao, R.; Ran, H.; Qu, K.; Huang, H.; et al. miR-539 activates the SAPK/JNK signaling pathway to promote ferropotosis in colorectal cancer by directly targeting TIPE. Cell Death Discov. 2021, 7, 272. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, L.; Yang, G.; Meng, F.; Wan, Y.; Wang, L.; Zhang, L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol. Int. 2020, 44, 2344–2356. [Google Scholar] [CrossRef]
- Deng, S.H.; Wu, D.M.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; He, M.; Zhao, Y.Y.; Han, R.; et al. miR-324-3p reverses cisplatin resistance by inducing GPX4-mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem. Biophys. Res. Commun. 2021, 549, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lian, P.; Lv, Q.; Liu, F. Silencing lncRNA HCG18 regulates GPX4-inhibited ferroptosis by adsorbing miR-450b-5p to avert sorafenib resistance in hepatocellular carcinoma. Hum. Exp. Toxicol. 2023, 42, 9603271221142818. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wei, Y.Y.; Yang, C.C.; Liu, C.J.; Yeh, L.Y.; Chou, C.H.; Chang, K.W.; Lin, S.C. miR-125b suppresses oral oncogenicity by targeting the anti-oxidative gene PRXL2A. Redox Biol. 2019, 22, 101140. [Google Scholar] [CrossRef]
- Sun, X.; Liu, D.; Xue, Y.; Hu, X. Enforced miR-144-3p expression as a non-invasive biomarker for the acute myeloid leukemia patients mainly by targeting NRF2. Clin. Lab. 2017, 63, 679–687. [Google Scholar] [CrossRef]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res. Treat. 2011, 129, 983–991. [Google Scholar] [CrossRef]
- Yamamoto, S.; Inoue, J.; Kawano, T.; Kozaki, K.; Omura, K.; Inazawa, J. The impact of miRNA-based molecular diagnostics and treatment of NRF2-stabilized tumors. Mol. Cancer Res. 2014, 12, 58–68. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, A.; Di Fiore, R.; Pratelli, G.; Drago-Ferrante, R.; Saliba, C.; Baldacchino, S.; Grech, G.; Scerri, C.; Vento, R.; Tesoriere, G. A loop involving NRF2, miR-29b-1-5p and AKT, regulates cell fate of MDA-MB-231 triple-negative breast cancer cells. J. Cell. Physiol. 2020, 235, 629–637. [Google Scholar] [CrossRef]
- Gao, M.; Li, C.; Xu, M.; Liu, Y.; Cong, M.; Liu, S. LncRNA MT1DP aggravates cadmium-induced oxidative stress by repressing the function of Nrf2 and is dependent on interaction with miR-365. Adv. Sci. 2018, 5, 1800087. [Google Scholar] [CrossRef]
- Bai, T.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J. Cell. Physiol. 2020, 235, 5637–5648. [Google Scholar] [CrossRef]
- Guan, L.; Wang, F.; Wang, M.; Han, S.; Cui, Z.; Xi, S.; Xu, H.; Li, S. Downregulation of HULC induces ferroptosis in hepatocellular carcinoma via targeting of the miR-3200-5p/ATF4 axis. Oxid. Med. Cell. Longev. 2022, 2022, 9613095. [Google Scholar] [CrossRef]
- Bazhabayi, M.; Qiu, X.; Li, X.; Yang, A.; Wen, W.; Zhang, X.; Xiao, X.; He, R.; Liu, P. CircGFRA1 facilitates the malignant progression of HER-2-positive breast cancer via acting as a sponge of miR-1228 and enhancing AIFM2 expression. J. Cell. Mol. Med. 2021, 25, 10248–10256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Xie, H.; Liang, Z.; Chen, B.; Xu, C.; Wang, J.; Huang, X.; et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater. 2022, 13, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, J.; Xu, W.; Ma, C.; Wan, F.; Huang, Y.; Yao, M.; Zhang, H.; Qu, Y.; Ye, D.; et al. LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis. 2021, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.T.; Tan, H.Y.; Zhang, C.; Guo, W.; Xu, Y.; Sharma, R.; Chen, Z.S.; Zheng, Y.C.; Wang, N.; et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Liang, L.; Zhou, D.; Wang, S.W. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma 2021, 68, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Lu, S.; Wang, L.; Wang, Y.; Lv, M.; Li, T.; Xu, Y.; Lu, J.; Ge, R.S. Circular RNA circLMO1 suppresses cervical cancer growth and metastasis by triggering miR-4291/ACSL4-mediated ferroptosis. Front. Oncol. 2022, 12, 858598. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhang, J.; Xian, S.Y.; Chen, F. MicroRNA-670-3p suppresses ferroptosis of human glioblastoma cells through targeting ACSL4. Free Radic. Res. 2021, 55, 853–864. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wang, C.; Cheng, K.K.; Xu, H.; Li, Q.; Hua, T.; Jiang, X.; Sheng, L.; Mao, J.; et al. miR-18a promotes glioblastoma development by down-regulating ALOXE3-mediated ferroptotic and anti-migration activities. Oncogenesis 2021, 10, 15. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Xue, P.; Huang, S.; Han, X.; Zhang, C.; Yang, L.; Xiao, W.; Fu, J.; Li, H.; Zhou, Y. Exosomal miR-101-3p and miR-423-5p inhibit medulloblastoma tumorigenesis through targeting FOXP4 and EZH2. Cell Death Differ. 2022, 29, 82–95. [Google Scholar] [CrossRef]
- Shang, S.; Wang, J.; Chen, S.; Tian, R.; Zeng, H.; Wang, L.; Xia, M.; Zhu, H.; Zuo, C. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. 2019, 8, 7728–7740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, L.; Chu, L.; Lan, J.; Wei, J.; Li, W.; Xue, C. Microscopic polyangiitis plasma-derived exosomal miR-1287-5p induces endothelial inflammatory injury and neutrophil adhesion by targeting CBL. PeerJ 2023, 11, e14579. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ji, J.; Yin, H.; Chen, X.; Zhao, P.; Lu, H.; Wang, T. Exosomes derived from microRNA-129-5p-modified tumor cells selectively enhanced suppressive effect in malignant behaviors of homologous colon cancer cells. Bioengineered 2021, 12, 12148–12156. [Google Scholar] [CrossRef] [PubMed]
- Plousiou, M.; De Vita, A.; Miserocchi, G.; Bandini, E.; Vannini, I.; Melloni, M.; Masalu, N.; Fabbri, F.; Serra, P. Growth inhibition of retinoblastoma cell line by exosome-mediated transfer of miR-142-3p. Cancer Manag. Res. 2022, 14, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Yao, Z.X.; Zheng, Z.; Yang, J.; Wang, R.; Fu, S.J.; Pan, X.F.; Liu, Z.H.; Wu, K. G-MDSCs-derived exosomal miRNA-143-3p promotes proliferation via targeting of ITM2B in lung cancer. Onco Targets Ther. 2020, 13, 9701–9719. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.; Wei, Y.; Lv, L.; Liu, N.; Lin, R.; Wang, X.; Shi, B. Human mesenchymal stem cell-derived exosomal microRNA-143 promotes apoptosis and suppresses cell growth in pancreatic cancer via target gene regulation. Front. Genet. 2021, 12, 581694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Gu, R.; Li, Z.; Wang, K.; Qi, Y.; Sun, X.; Xie, J.; Wang, L.; Xu, B.; et al. Circulating exosomal miR-144-3p inhibits the mobilization of endothelial progenitor cells post myocardial infarction via regulating the MMP9 pathway. Aging 2020, 12, 16294–16303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, W.; Wang, F.F.; Yang, S.Q.; Hu, J.Q.; Lu, B.J.; Pan, Z.M.; Ma, Y.; Zheng, M.Y.; Zhou, L.Y.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Li, Y.; Liu, L.; Zhu, Y.; Ni, Y.; Huang, H.; Liu, Z.; Miao, Z.; Zhang, L. Apelin enhances biological functions in lung cancer A549 cells by downregulating exosomal miR-15a-5p. Carcinogenesis 2021, 42, 243–253. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Yu, T.; Yan, C.; Zhou, W.; Cao, F.; You, X.; Zhang, Y.; Sun, Y.; Liu, J.; et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging 2020, 12, 8968–8986. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, K.; Zhi, Y.; Wu, Y.; Chen, B.; Bai, J.; Wang, X. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 2021, 11, e478. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; He, D.; Shao, Y.; Qu, Y.; Ye, K.; Memet, O.; Zhang, L.; Shen, J. Lung-derived exosomes in phosgene-induced acute lung injury regulate the functions of mesenchymal stem cells partially via miR-28-5p. Biomed. Pharmacother. 2020, 121, 109603. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Y.; Li, L.; Wang, G.; Xing, L. Exosomal microRNA-302a promotes trophoblast migration and proliferation, and represses angiogenesis by regulating the expression levels of VEGFA in preeclampsia. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Y.; Liu, Y.; Lin, M.; Ma, J.; Zhang, W.; Dai, J.; Li, J.; Guo, Q.; Chen, H.; et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin alpha2beta1. Signal Transduct. Target. Ther. 2020, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Si, L.; Dai, X.; Dong, H.; Ma, Z.; Sun, Z.; Li, N.; Sha, H.; Chen, Y.; Qian, Y.; et al. Exosomal miR-409-3p secreted from activated mast cells promotes microglial migration, activation and neuroinflammation by targeting Nr4a2 to activate the NF-kappaB pathway. J. Neuroinflammation 2021, 18, 68. [Google Scholar] [CrossRef]
- Luo, X.; Wang, W.; Li, D.; Xu, C.; Liao, B.; Li, F.; Zhou, X.; Qin, W.; Liu, J. Plasma exosomal miR-450b-5p as a possible biomarker and therapeutic target for transient ischaemic attacks in rats. J. Mol. Neurosci. 2019, 69, 516–526. [Google Scholar] [CrossRef]
- Wang, D.D.; Xue, H.; Tan, J.F.; Liu, P.L.; Qiao, C.X.; Pang, C.J.; Zhang, L.Z. Bone marrow mesenchymal stem cells-derived exosomes containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1 signalling to alleviate inflammatory bowel disease. Inflamm. Res. 2022, 71, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zhao, C.; Wang, R.; Ren, L.; Qiu, H.; Zou, Z.; Ding, H.; Sun, Z.; Li, J.; Dong, S. Antagonizing exosomal miR-18a-5p derived from prostate cancer cells ameliorates metastasis-induced osteoblastic lesions by targeting Hist1h2bc and activating Wnt/beta-catenin pathway. Genes Dis. 2023, 10, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Gollmann-Tepekoylu, C.; Polzl, L.; Graber, M.; Hirsch, J.; Nagele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, H.; Shi, X.; Li, X. Exosomal microRNA-23a-3p contributes to the progression of cholangiocarcinoma by interaction with Dynamin3. Bioengineered 2022, 13, 6208–6221. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, H.; Wang, Y.; Chen, Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020, 333, 113411. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Calin, G.A.; Steele, V.E.; Cartiglia, C.; Longobardi, M.; Croce, C.M.; De Flora, S. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev. Res. 2010, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Wolffram, S.; Rimbach, G. Effect of quercetin on inflammatory gene expression in mice liver in vivo—Role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 2012, 65, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bahlakeh, G.; Gorji, A.; Soltani, H.; Ghadiri, T. MicroRNA alterations in neuropathologic cognitive disorders with an emphasis on dementia: Lessons from animal models. J. Cell. Physiol. 2021, 236, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Luo, X.; Gu, C.; Li, Y.; Zhu, X.; Fei, J. Systematic analysis of berberine-induced signaling pathway between miRNA clusters and mRNAs and identification of mir-99a approximately 125b cluster function by seed-targeting inhibitors in multiple myeloma cells. RNA Biol. 2015, 12, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Qu, Y.; Ding, Y. Curcumin alleviates oxygen-glucose-deprivation/reperfusion-induced oxidative damage by regulating miR-1287-5p/LONP2 axis in SH-SY5Y cells. Anal. Cell. Pathol. 2021, 2021, 5548706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Peng, X.; Ma, Y.H.; Li, F.J.; Liao, Y.H. Matrine suppresses lipopolysaccharide-induced fibrosis in human peritoneal mesothelial cells by inhibiting the epithelial-mesenchymal transition. Chin. Med. J. 2019, 132, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.L.; Zhang, G.H.; Gao, Y.F. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz. J. Med. Biol. Res. 2019, 52, e7992. [Google Scholar] [CrossRef]
- MacKenzie, T.N.; Mujumdar, N.; Banerjee, S.; Sangwan, V.; Sarver, A.; Vickers, S.; Subramanian, S.; Saluja, A.K. Triptolide induces the expression of miR-142-3p: A negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther. 2013, 12, 1266–1275. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Curcumin inhibits proteasome activity in triple-negative breast cancer cells through regulating p300/miR-142-3p/PSMB5 axis. Phytomedicine 2020, 78, 153312. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Wang, Y.; Luo, J. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR-143 and miR-143 mediated autophagy inhibition. J. Drug Target. 2017, 25, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Su, T.C.; Yang, M.J.; Chen, W.T.; Siao, A.C.; Huang, L.R.; Lin, Y.Y.; Kuo, Y.C.; Chung, J.F.; Cheng, C.F.; et al. Green tea epigallocatechin gallate suppresses 3T3-L1 cell growth via microRNA-143/MAPK7 pathways. Exp. Biol. Med. 2022, 247, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mandal, A.K.A. Role of epigallocatechin-3- gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front. Genet. 2022, 13, 995046. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Xie, Y.K.; Wu, Y.; Li, L.L.; Liu, Y.; Miao, X.B.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Sulforaphane inhibits cancer stem-like cell properties and cisplatin resistance through miR-214-mediated downregulation of c-MYC in non-small cell lung cancer. Oncotarget 2017, 8, 12067–12080. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Feng, Y.; Fang, J.; Yang, M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed. Pharmacother. 2015, 74, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Kim, H.E.; Mun, D.S.; Yun, N.R.; Joung, B.Y. Curcumin-loaded extracellular vesicles endowed with heart targeting properties facilitate treatment of myocardial infarction. Eur. Heart J. 2020, 41, 3609. [Google Scholar] [CrossRef]
- Gao, S.M.; Yang, J.J.; Chen, C.Q.; Chen, J.J.; Ye, L.P.; Wang, L.Y.; Wu, J.B.; Xing, C.Y.; Yu, K. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J. Exp. Clin. Cancer Res. 2012, 31, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Chen, W.; Liu, Y.; Zang, X. Baicalin protects against acute pancreatitis involving JNK signaling pathway via regulating miR-15a. Am. J. Chin. Med. 2021, 49, 147–161. [Google Scholar] [CrossRef]
- Shuaib, M.; Prajapati, K.S.; Gupta, S.; Kumar, S. Natural steroidal lactone induces G1/S phase cell cycle arrest and intrinsic apoptotic pathway by up-regulating tumor suppressive miRNA in triple-negative breast cancer cells. Metabolites 2023, 13, 29. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, Y.; Liu, W.; Wang, J. Cryptotanshinone inhibits lung cancer invasion via microRNA-133a/matrix metalloproteinase 14 regulation. Oncol. Lett. 2019, 18, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Derry, M.M.; Raina, K.; Balaiya, V.; Jain, A.K.; Shrotriya, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R.; Agarwal, C. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: Interlinking miRNA with cytokine signaling and inflammation. Cancer Prev. Res. 2013, 6, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Dahmke, I.N.; Backes, C.; Rudzitis-Auth, J.; Laschke, M.W.; Leidinger, P.; Menger, M.D.; Meese, E.; Mahlknecht, U. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS ONE 2013, 8, e81122. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.J. Overexpression of microRNA-25 by withaferin A induces cyclooxygenase-2 expression in rabbit articular chondrocytes. J. Pharmacol. Sci. 2014, 125, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Geng, J.; Liu, L.; Xu, L. beta-Elemene inhibits oxygen-induced retinal neovascularization via promoting miR-27a and reducing VEGF expression. Mol. Med. Rep. 2019, 19, 2307–2316. [Google Scholar] [PubMed]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Sun, W.L.; Lu, X.F.; Wang, X.L.; Jiang, L. MiR-28-5p mediates the anti-proliferative and pro-apoptotic effects of curcumin on human diffuse large B-cell lymphoma cells. J. Int. Med. Res. 2020, 48, 300060520943792. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, Y.; Yu, H. Curcumin suppresses colorectal cancer development with epithelial-mesenchymal transition via modulating circular RNA HN1/miR-302a-3p/PIK3R3 axis. J. Physiol. Pharmacol. 2022, 73, 219–231. [Google Scholar]
- Kuo, S.Z.; Blair, K.J.; Rahimy, E.; Kiang, A.; Abhold, E.; Fan, J.B.; Wang-Rodriguez, J.; Altuna, X.; Ongkeko, W.M. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer 2012, 12, 556. [Google Scholar] [CrossRef]
- Yin, L.; Xiao, X.; Georgikou, C.; Yin, Y.; Liu, L.; Karakhanova, S.; Luo, Y.; Gladkich, J.; Fellenberg, J.; Sticht, C.; et al. MicroRNA-365a-3p inhibits c-Rel-mediated NF-kappaB signaling and the progression of pancreatic cancer. Cancer Lett. 2019, 452, 203–212. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Tang, X.; Tang, Q.; Lou, L.; Yu, Y.; Zheng, F.; Wu, J.; Yang, X.B.; Wang, W.; et al. The reciprocal interaction between lncRNA CCAT1 and miR-375-3p contribute to the downregulation of IRF5 gene expression by solasonine in HepG2 human hepatocellular carcinoma cells. Front. Oncol. 2019, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yin, H.; Ma, H.; Wang, Y.; Kong, D.; Fan, Z. Curcumin regulates ERCC1 expression and enhances oxaliplatin sensitivity in resistant colorectal cancer cells through its effects on miR-409-3p. Evid. Based Complement. Altern. Med. 2020, 2020, 8394574. [Google Scholar] [CrossRef]
- Tsunekuni, K.; Konno, M.; Haraguchi, N.; Koseki, J.; Asai, A.; Matsuoka, K.; Kobunai, T.; Takechi, T.; Doki, Y.; Mori, M.; et al. CD44/CD133-positive colorectal cancer stem cells are sensitive to trifluridine exposure. Sci. Rep. 2019, 9, 14861. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, Z. Berberine inhibits the proliferation, invasion and migration of endometrial stromal cells by downregulating miR-429. Mol. Med. Rep. 2021, 23, 416. [Google Scholar] [CrossRef]
- Liu, H.; Huang, C.; Wu, L.; Wen, B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016, 9, 4121–4127. [Google Scholar] [PubMed]
- Fu, X.; Zhang, J.; Huang, X.; Mo, Z.; Sang, Z.; Duan, W.; Huang, W. Curcumin antagonizes glucose fluctuation-induced renal injury by inhibiting aerobic glycolysis via the miR-489/LDHA pathway. Mediat. Inflamm. 2021, 2021, 6104529. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Bak, K.M.; Nam, Y.K.; Park, H.T. Upregulation of microRNA 344a-3p is involved in curcumin induced apoptosis in RT4 schwannoma cells. Cancer Cell Int. 2018, 18, 199. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Shi, J.M.; Ding, Z.; Xia, Q.; Zheng, T.S.; Ren, Y.B.; Li, M.; Fan, L.H. Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag. Res. 2019, 11, 9005–9015. [Google Scholar] [CrossRef]
- Yang, W.N.; Kang, Q.; Li, C.L.; Bo, S.C.; Wang, Y. Matrine promotes trophoblast invasion and reduces inflammation via miR-19a-3p to prevent preeclampsia. Mol. Cell. Toxicol. 2023, 19, 591–599. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Chen, Y.; Li, X.; Jiang, Y.; Yang, X.; Li, Y.; Wang, X.; Meng, Y.; Zhu, M.; et al. miR-19 targeting of GSK3beta mediates sulforaphane suppression of lung cancer stem cells. J. Nutr. Biochem. 2017, 44, 80–91. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, M.; Wang, X.; Tan, H.Y.; Tsao, S.W.; Feng, Y. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in hepatocellular carcinoma. Biochim. Biophys. Acta 2014, 1839, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.C.; Chun, E.; Farrell, A.N.; Hur, E.Y.; Caroti, C.M.; Iuvone, P.M.; Haque, R. Global microRNA expression profiling: Curcumin (diferuloylmethane) alters oxidative stress-responsive microRNAs in human ARPE-19 cells. Mol. Vis. 2013, 19, 544–560. [Google Scholar] [PubMed]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef] [PubMed]

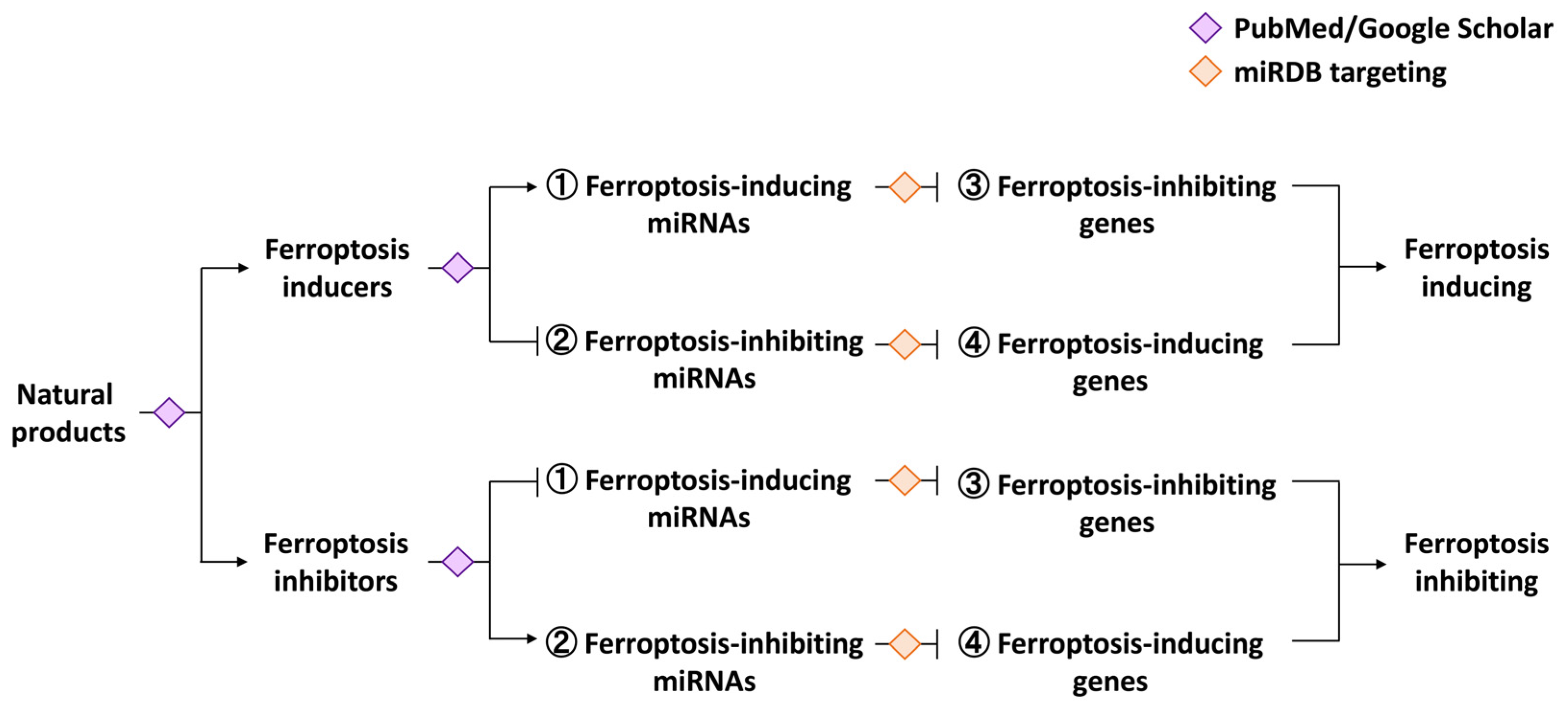
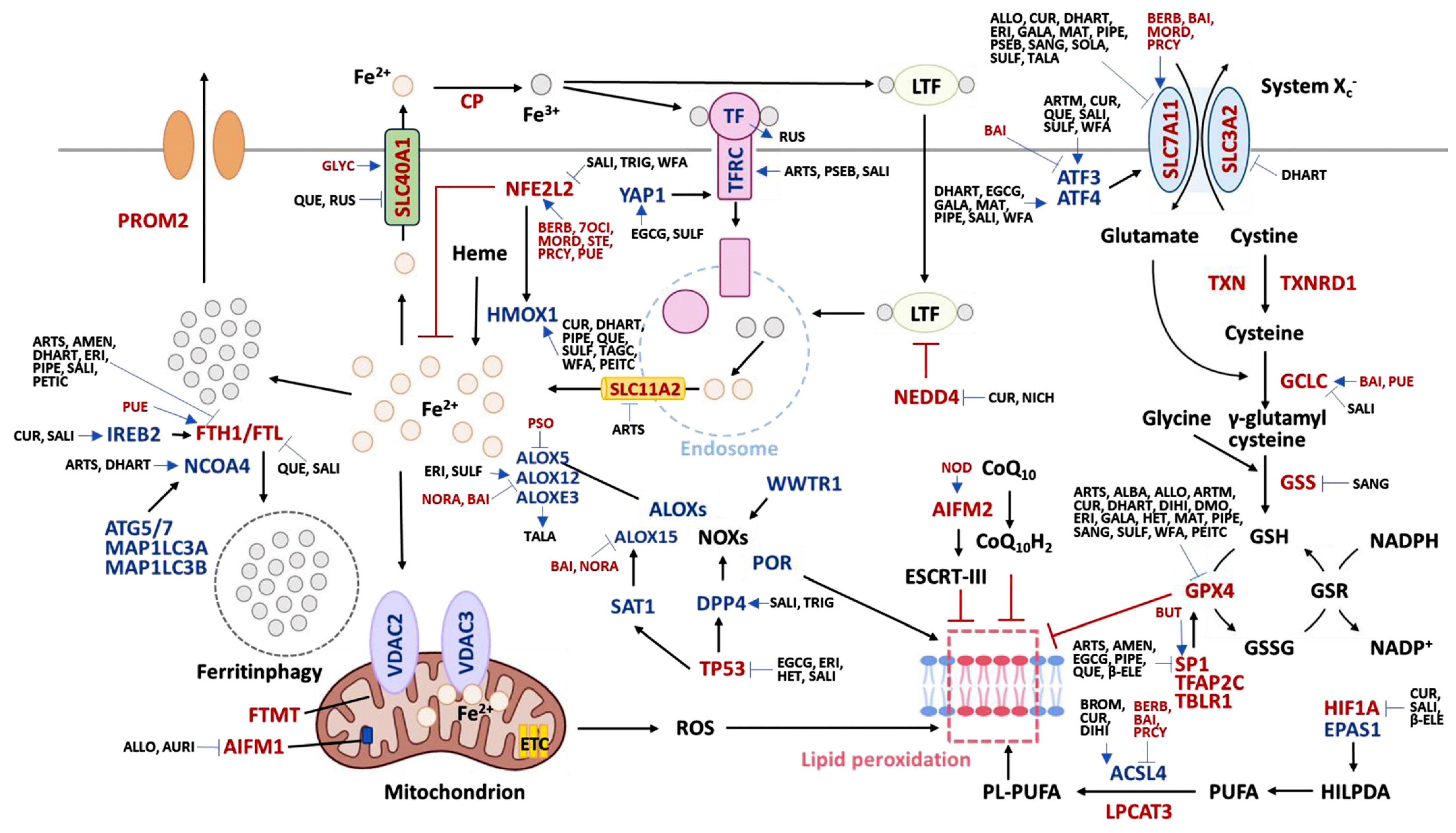
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, Y.-T.; Yen, C.-Y.; Chien, T.-M.; Chang, F.-R.; Tsai, Y.-H.; Wu, K.-C.; Tang, J.-Y.; Chang, H.-W. Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis. Int. J. Mol. Sci. 2024, 25, 6083. https://doi.org/10.3390/ijms25116083
Chuang Y-T, Yen C-Y, Chien T-M, Chang F-R, Tsai Y-H, Wu K-C, Tang J-Y, Chang H-W. Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis. International Journal of Molecular Sciences. 2024; 25(11):6083. https://doi.org/10.3390/ijms25116083
Chicago/Turabian StyleChuang, Ya-Ting, Ching-Yu Yen, Tsu-Ming Chien, Fang-Rong Chang, Yi-Hong Tsai, Kuo-Chuan Wu, Jen-Yang Tang, and Hsueh-Wei Chang. 2024. "Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis" International Journal of Molecular Sciences 25, no. 11: 6083. https://doi.org/10.3390/ijms25116083
APA StyleChuang, Y.-T., Yen, C.-Y., Chien, T.-M., Chang, F.-R., Tsai, Y.-H., Wu, K.-C., Tang, J.-Y., & Chang, H.-W. (2024). Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis. International Journal of Molecular Sciences, 25(11), 6083. https://doi.org/10.3390/ijms25116083








