Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications
Abstract
:1. Introduction
2. Role of Eosinophils in Mucosal Immune Responses
3. Recruitment of Eosinophils to the Tumor Microenvironment in Colorectal Cancer
4. Eosinophils as Effectors in Anti-Tumoral Immune Responses in Colorectal Cancer
5. The Cytotoxic Arsenal of Eosinophils
6. Eosinophils as Modulators in Anti-Tumoral Immune Responses in Colorectal Cancer
| Cell Type | Clinical Significance | Modulation | Refs. |
|---|---|---|---|
| Eosinophils | Good prognosis. Circulating eosinopihls are a potential biomarker for improved response to immunotherapy. | Release of cytotoxic proteins, cytokines linked to Th1 responses, ROS. Normalization of the vasculature. | [33,34,35,36,42,50,52,53] |
| Neutrophils | Poor prognosis | Pro-tumoral: extracellular matrix remodeling, aberrant angiogenesis, and immune suppression. | [68,69,70] |
| Basophils | Circulating basophils indicate good prognosis | Unclear | [71] |
| Mast cells | Unclear | Pro-tumoral: extracellular matrix remodeling, aberrant angiogenesis, and immune suppression. Anti-tumoral: release of cytokines linked to Th1 responses, ROS and histamine. | [72,73] |
7. Microbiome as a Modulator of Eosinophil Responses
8. Evasion from Eosinophil Control
9. Potential Clinical Application of Eosinophil Evaluation in Colorectal Cancer
10. Future Directions
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez-Perez, D.; Redruello-Romero, A.; Garcia-Rubio, J.; Arana, C.; Garcia-Escudero, L.A.; Tamayo, F.; Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Salmeron, J.; Blanco, A.; et al. In Patients with Obesity, the Number of Adipose Tissue Mast Cells Is Significantly Lower in Subjects with Type 2 Diabetes. Front. Immunol. 2021, 12, 664576. [Google Scholar] [CrossRef]
- Lopez-Perez, D.; Redruello-Romero, A.; Garcia-Rubio, J.; Arana, C.; Garcia-Escudero, L.A.; Tamayo, F.; Salmeron, J.; Galvez, J.; Leon, J.; Carazo, Á. In Obese Patients With Type 2 Diabetes, Mast Cells in Omental Adipose Tissue Decrease the Surface Expression of CD45, CD117, CD203c, and FcϵRI. Front. Endocrinol. 2022, 13, 818388. [Google Scholar] [CrossRef]
- García-Rubio, J.; León, J.; Redruello-Romero, A.; Pavón, E.; Cozar, A.; Tamayo, F.; Caba-Molina, M.; Salmerón, J.; Carazo, Á. Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci. Rep. 2018, 8, 15203. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.T.; Beller, A.; Rausch, S.; Strandmark, J.; Zänker, M.; Arbach, O.; Kruglov, A.; Berek, C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 2014, 40, 582–593. [Google Scholar] [CrossRef]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.; Rothenberg, M.E. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015, 8, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wågsäter, D.; Löfgren, S.; Hugander, A.; Dienus, O.; Dimberg, J. Analysis of single nucleotide polymorphism in the promoter and protein expression of the chemokine eotaxin-1 in colorectal cancer patients. World J. Surg. Oncol. 2007, 5, 84. [Google Scholar] [CrossRef]
- Rehman, M.Q.; Beal, D.; Liang, Y.; Noronha, A.; Winter, H.; Farraye, F.A.; Ganley-Leal, L. B cells secrete eotaxin-1 in human inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 922–933. [Google Scholar] [CrossRef]
- Ahrens, R.; Waddell, A.; Seidu, L.; Blanchard, C.; Carey, R.; Forbes, E.; Lampinen, M.; Wilson, T.; Cohen, E.; Stringer, K.; et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 2008, 181, 7390–7399. [Google Scholar] [CrossRef]
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. 2017, 4, 101. [Google Scholar] [CrossRef]
- Jung, Y.; Wen, T.; Mingler, M.K.; Caldwell, J.M.; Wang, Y.H.; Chaplin, D.D.; Lee, E.H.; Jang, M.H.; Woo, S.Y.; Seoh, J.Y.; et al. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015, 8, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Appleton, J.A. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016, 32, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Um, H.N.; Jang, J.; Bae, Y.A.; Park, W.J.; Kim, H.J.; Yoon, M.S.; Chung, I.Y.; Jung, Y. Eosinophil Activation by Toll-Like Receptor 4 Ligands Regulates Macrophage Polarization. Front. Cell. Dev. Biol. 2019, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Zaffran, I.; George, T.; Rahimli Alekberli, F.; Ben-Zimra, M.; Levi-Schaffer, F. The regulatory role of eosinophils in viral, bacterial, and fungal infections. Clin. Exp. Immunol. 2022, 209, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Padigel, U.M.; Lee, J.J.; Nolan, T.J.; Schad, G.A.; Abraham, D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect. Immun. 2006, 74, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, P.; Wang, H.; Weller, P.F. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 14–19. [Google Scholar] [CrossRef]
- Jia, S.; Li, W.; Liu, P.; Xu, L.X. A role of eosinophils in mediating the anti-tumour effect of cryo-thermal treatment. Sci. Rep. 2019, 9, 13214. [Google Scholar] [CrossRef]
- Qin, M.; Wang, L.; Li, F.; Yang, M.; Song, L.; Tian, F.; Yukht, A.; Shah, P.K.; Rothenberg, M.E.; Sharifi, B.G. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis 2017, 263, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Strandmark, J.; Steinfelder, S.; Berek, C.; Kühl, A.A.; Rausch, S.; Hartmann, S. Eosinophils are required to suppress Th2 responses in Peyer’s patches during intestinal infection by nematodes. Mucosal Immunol. 2017, 10, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Weller, P.F. Unraveling the complexity of lipid body organelles in human eosinophils. J. Leukoc. Biol. 2014, 96, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Zheng, X.; Jin, L.; Xiang, N.; Zhang, M.; Chen, Z. Eosinophils attenuate arthritis by inducing M2 macrophage polarization via inhibiting the IκB/P38 MAPK signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 894–901. [Google Scholar] [CrossRef]
- Arnold, I.C.; Artola-Borán, M.; Tallón de Lara, P.; Kyburz, A.; Taube, C.; Ottemann, K.; van den Broek, M.; Yousefi, S.; Simon, H.U.; Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 2018, 215, 2055–2072. [Google Scholar] [CrossRef]
- Chu, D.K.; Jimenez-Saiz, R.; Verschoor, C.P.; Walker, T.D.; Goncharova, S.; Llop-Guevara, A.; Shen, P.; Gordon, M.E.; Barra, N.G.; Bassett, J.D.; et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J. Exp. Med. 2014, 211, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.S.; de Oliveira, L.M.; Amorim, C.C.O.; Gazzinelli-Guimarães, A.C.; Barbosa, F.S.; Oliveira, F.M.S.; Kraemer, L.; Mattos, M.; Cardoso, M.S.; Resende, N.M.; et al. Eosinophils mediate SIgA production triggered by TLR2 and TLR4 to control Ascaris suum infection in mice. PLoS Pathog. 2021, 17, e1010067. [Google Scholar] [CrossRef]
- Forman, R.; Bramhall, M.; Logunova, L.; Svensson-Frej, M.; Cruickshank, S.M.; Else, K.J. Eosinophils may play regionally disparate roles in influencing IgA(+) plasma cell numbers during large and small intestinal inflammation. BMC Immunol. 2016, 17, 12. [Google Scholar] [CrossRef]
- Berek, C. Eosinophils: Important players in humoral immunity. Clin. Exp. Immunol. 2016, 183, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Coden, M.E.; Berdnikovs, S. Eosinophils in wound healing and epithelial remodeling: Is coagulation a missing link? J. Leukoc. Biol. 2020, 108, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Bokemeyer, C.; Langner, C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod. Pathol. 2015, 28, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2017, 7, e1393134. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Saka, B.; Yarikkaya, E.; Bilici, A.; Oncel, M. The potential prognostic role of peritumoral eosinophils within whole tumor-associated inflammatory cells and stromal histological characteristics in colorectal cancer. Pol. J. Pathol. 2020, 71, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Moezzi, J.; Gopalswamy, N.; Haas, R.J., Jr.; Markert, R.J.; Suryaprasad, S.; Bhutani, M.S. Stromal eosinophilia in colonic epithelial neoplasms. Am. J. Gastroenterol. 2000, 95, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Pretlow, T.P.; Keith, E.F.; Cryar, A.K.; Bartolucci, A.A.; Pitts, A.M.; Pretlow, T.G., 2nd; Kimball, P.M.; Boohaker, E.A. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983, 43, 2997–3000. [Google Scholar]
- Saraiva, A.L.; Carneiro, F. New Insights Into the Role of Tissue Eosinophils in the Progression of Colorectal Cancer: A Literature Review. Acta Med. Port. 2018, 31, 329–337. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. Eotaxins and Their Receptor in Colorectal Cancer-A Literature Review. Cancers 2020, 12, 1383. [Google Scholar] [CrossRef]
- Davis, B.P.; Rothenberg, M.E. Eosinophils and cancer. Cancer Immunol. Res. 2014, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Karo-Atar, D.; Munitz, A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer 2016, 2, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, Z.; Schill, E.M.; Bolock, A.M.; Good, M. IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine 2017, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 Promotes CD11b/CD18-Mediated Adhesion of Eosinophils to Cancer Cells and Synapse-Polarized Degranulation Leading to Tumor Cell Killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Kienzl, M.; Hasenoehrl, C.; Valadez-Cosmes, P.; Maitz, K.; Sarsembayeva, A.; Sturm, E.; Heinemann, A.; Kargl, J.; Schicho, R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology 2020, 9, 1776059. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Zhang, J.; Goleniewska, K.; Cephus, J.Y.; Rusznak, M.; Wu, L.; Van Kaer, L.; Zhou, B.; Newcomb, D.C.; Peebles, R.S., Jr. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J. Exp. Med. 2018, 215, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Ngo Thi Phuong, N.; Palmieri, V.; Adamczyk, A.; Klopfleisch, R.; Langhorst, J.; Hansen, W.; Westendorf, A.M.; Pastille, E. IL-33 Drives Expansion of Type 2 Innate Lymphoid Cells and Regulatory T Cells and Protects Mice From Severe, Acute Colitis. Front. Immunol. 2021, 12, 669787. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Rothenberg, M.E. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J. Immunol. 2014, 193, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Vogel, P.; Body-Malapel, M.; Lamkanfi, M.; Kanneganti, T.D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 2010, 185, 4912–4920. [Google Scholar] [CrossRef]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefèvre, G.; Capron, M. IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inf1ammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Mamtimin, M.; Pinarci, A.; Han, C.; Braun, A.; Anders, H.J.; Gudermann, T.; Mammadova-Bach, E. Extracellular DNA Traps: Origin, Function and Implications for Anti-Cancer Therapies. Front. Oncol. 2022, 12, 869706. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Hosseini, A.; Stojkov, D.; Oberson, K.; Claus, M.; Benarafa, C.; Calzavarini, S.; Angelillo-Scherrer, A.; Arnold, I.C.; Müller, A.; et al. ATG5 promotes eosinopoiesis but inhibits eosinophil effector functions. Blood 2021, 137, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and Cancer. Cancer Immunol. Res. 2017, 5, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Melo, R.C.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013, 121, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Artola-Boran, M.; Gurtner, A.; Bertram, K.; Bauer, M.; Frangez, Z.; Becher, B.; Kopf, M.; Yousefi, S.; Simon, H.U.; et al. The GM-CSF-IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. J. Exp. Med. 2020, 217, e20190706. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617, Erratum in Nat. Immunol. 2016, 17, 214. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.T.; Kowalski, L.P. The role of eosinophils and eosinophil cationic protein in oral cancer: A review. Arch. Oral Biol. 2011, 56, 353–358. [Google Scholar] [CrossRef]
- Rosenberg, H.F. Eosinophil-derived neurotoxin / RNase 2: Connecting the past, the present and the future. Curr. Pharm. Biotechnol. 2008, 9, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J. Granzyme A activates another way to die. Immunol. Rev. 2010, 235, 93–104. [Google Scholar] [CrossRef]
- Siemińska, I.; Poljańska, E.; Baran, J. Granulocytes and Cells of Granulocyte Origin-The Relevant Players in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 3801. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcázar, J.F.; Ataide, M.A.; Engels, G.; Schmitt-Mabmunyo, C.; Garbi, N.; Kastenmüller, W.; Latz, E.; Franklin, B.S. Charcot-Leyden Crystals Activate the NLRP3 Inflammasome and Cause IL-1β Inflammation in Human Macrophages. J. Immunol. 2019, 202, 550–558. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef]
- Rottmann, B.G.; Patel, N.; Ahmed, M.; Deng, Y.; Ciarleglio, M.; Vyas, M.; Jain, D.; Zhang, X. Clinicopathological significance of neutrophil-rich colorectal carcinoma. J. Clin. Pathol. 2023, 76, 34–39. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, J.; Peng, Y.; Sun, J.; Cheng, P.; Huang, Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Paolini, R. The Controversial Role of Intestinal Mast Cells in Colon Cancer. Cells 2023, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Wei, H.; Liu, Y.; Li, N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front. Immunol. 2023, 14, 1209056. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Cheung, P.F.; Ip, W.K.; Lam, C.W. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am. J. Respir. Cell. Mol. Biol. 2007, 37, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Takanaski, S.; Nonaka, R.; Xing, Z.; O’Byrne, P.; Dolovich, J.; Jordana, M. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J. Exp. Med. 1994, 180, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, C.; Irrazabal, T.; Martin, A.; Girardin, S.E.; Philpott, D.J. The impact of the gut microbiome on colorectal cancer. Annu. Rev. Cancer Biol. 2018, 2, 229–249. [Google Scholar] [CrossRef]
- Coleman, O.I.; Nunes, T. Role of the Microbiota in Colorectal Cancer: Updates on Microbial Associations and Therapeutic Implications. Biores. Open Access 2016, 5, 279–288. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Anipindi, V.C.; Galipeau, H.; Ellenbogen, Y.; Chaudhary, R.; Koenig, J.F.; Gordon, M.E.; Walker, T.D.; Mandur, T.S.; Abed, S.; et al. Microbial Regulation of Enteric Eosinophils and Its Impact on Tissue Remodeling and Th2 Immunity. Front. Immunol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Buonomo, E.L.; Cowardin, C.A.; Wilson, M.G.; Saleh, M.M.; Pramoonjago, P.; Petri, W.A., Jr. Microbiota-Regulated IL-25 Increases Eosinophil Number to Provide Protection during Clostridium difficile Infection. Cell Rep. 2016, 16, 432–443. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Toyama, S.; Motomura, K.; Matsuda, A.; Suto, H.; Okumura, K.; Sudo, K.; Takahashi, T.; et al. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS ONE 2015, 10, e0134226. [Google Scholar] [CrossRef]
- Davoine, F.; Lacy, P. Eosinophil cytokines, chemokines, and growth factors: Emerging roles in immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef]
- Varyani, F.; Löser, S.; Filbey, K.J.; Harcus, Y.; Drurey, C.; Poveda, M.C.; Rasid, O.; White, M.P.J.; Smyth, D.J.; Gerbe, F.; et al. The IL-25-dependent tuft cell circuit driven by intestinal helminths requires macrophage migration inhibitory factor (MIF). Mucosal Immunol. 2022, 15, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lim, S.J.; Won, K.Y.; Bae, G.E.; Kim, G.Y.; Min, J.W.; Noh, B.J. Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24. J. Pathol. Transl. Med. 2016, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Davani, F.; Mojtahedi, Z.; Hosseini, S.V.; Bananzadeh, A.; Ghaderi, A. Dual association of serum interleukin-10 levels with colorectal cancer. J. Cancer Res. Ther. 2017, 13, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am. J. Cancer Res. 2022, 12, 138–151. [Google Scholar] [PubMed]
- Kottyan, L.C.; Collier, A.R.; Cao, K.H.; Niese, K.A.; Hedgebeth, M.; Radu, C.G.; Witte, O.N.; Khurana Hershey, G.K.; Rothenberg, M.E.; Zimmermann, N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 2009, 114, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Lee, T.D.; Hoskin, D.W. Adhesion of tumoricidal eosinophils to MCA-38 colon adenocarcinoma cells involves protein tyrosine kinase activation and is diminished by elevated cyclic AMP in the effector cell. Int. J. Oncol. 1998, 13, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Pryczynicz, A.; Guzińska-Ustymowicz, K.; Kemona, A. Fas/FasL expression in colorectal cancer. An immunohistochemical study. Folia Histochem. Cytobiol. 2010, 48, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, P.; Moilanen, E.; Kankaanranta, H. Regulation of spontaneous eosinophil apoptosis-a neglected area of importance. J. Cell Death 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Malka, D.; Lièvre, A.; André, T.; Taïeb, J.; Ducreux, M.; Bibeau, F. Immune scores in colorectal cancer: Where are we? Eur. J. Cancer 2020, 140, 105–118. [Google Scholar] [CrossRef]
- Galon, J.; Lanzi, A. Immunoscore and its introduction in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, D.S.; Schoof, D.D.; Rodrick, M.L.; Tai, P.C.; Spry, C.J.; David, J.R.; Eberlein, T.J. Activation of eosinophils in cancer patients treated with IL-2 and IL-2-generated lymphokine-activated killer cells. J. Immunol. 1989, 142, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Rivoltini, L.; Viggiano, V.; Spinazzè, S.; Santoro, A.; Colombo, M.P.; Takatsu, K.; Parmiani, G. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int. J. Cancer 1993, 54, 8–15, Erratum in Int. J. Cancer 1993, 54, 887. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Bartemes, K.; Offord, K.P.; Kita, H.; Fisher, S.G.; Kefer, C.; Ellis, T.A.; Fisher, R.I.; Higgins, T.J.; Gleich, G.J. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: Effects of interleukin-4 alone and following interleukin-2 administration. Clin. Cancer Res. 1995, 1, 805–812. [Google Scholar] [PubMed]
- Krishnan, T.; Tomita, Y.; Roberts-Thomson, R. A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Future Sci. OA 2020, 6, FSO608. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Santa Lucia, G.; Li, A.; Oberholtzer, N.; Plante, J.; Quinn, K.M.; Reuben, D.; Mehrotra, S.; Valdebran, M. Eosinophils and melanoma: Implications for immunotherapy. Pigment Cell Melanoma Res. 2022, 35, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Rezaei, N. Eosinophils in the tumor microenvironment: Implications for cancer immunotherapy. J. Transl. Med. 2023, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Elshenawy, M.A.; AlSayed, A.D.; Al-Tweigeri, T. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy 2022, 14, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Driss, V.; Hornez, N.; Abdallah, M.; Roumier, T.; Abboud, G.; Legrand, F.; Staumont-Sallé, D.; Quéant, S.; Bertout, J.; et al. Eosinophil-derived IFN-γ induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J. Allergy Clin. Immunol. 2009, 124, 573–582.e9. [Google Scholar] [CrossRef]

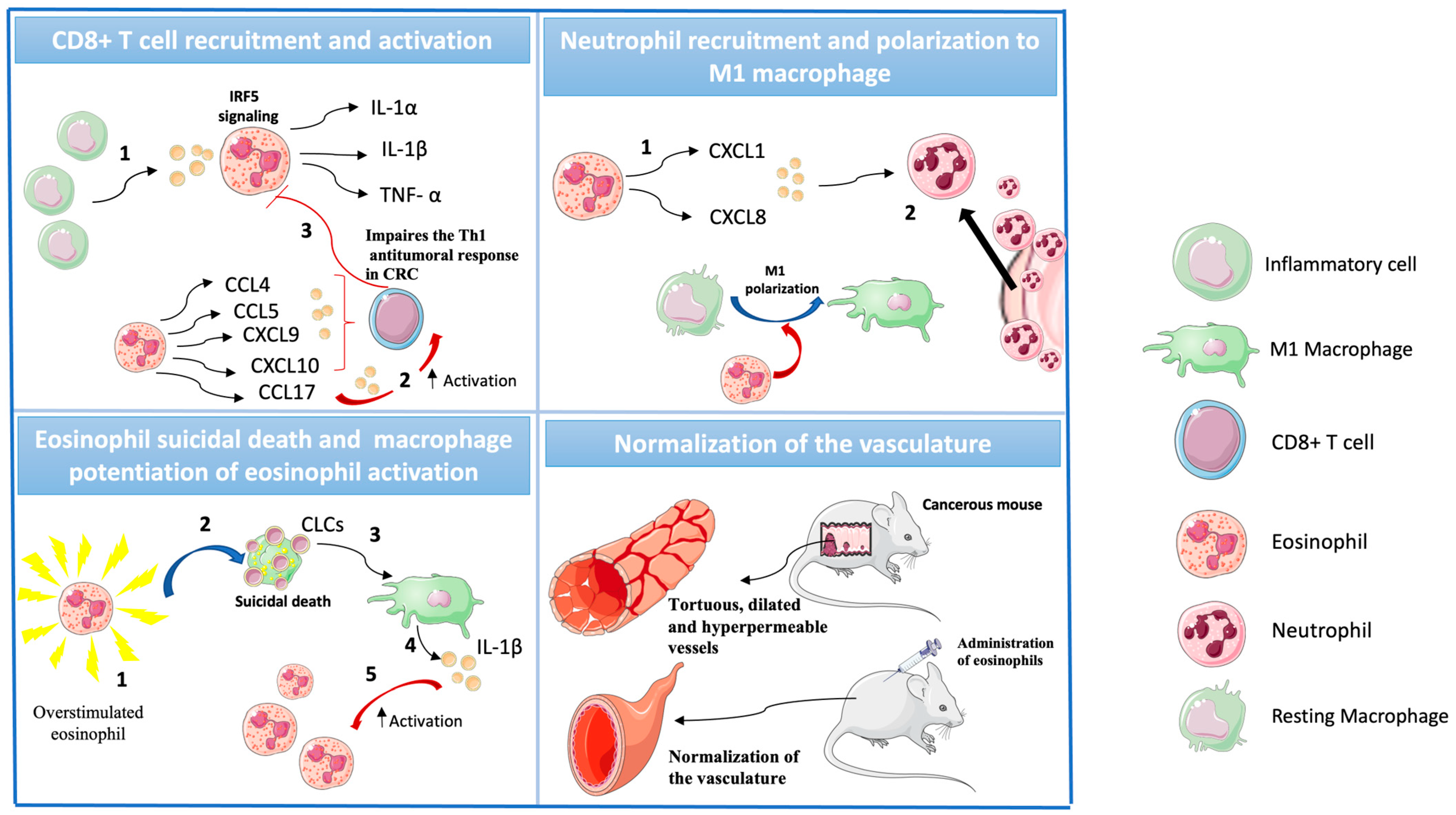
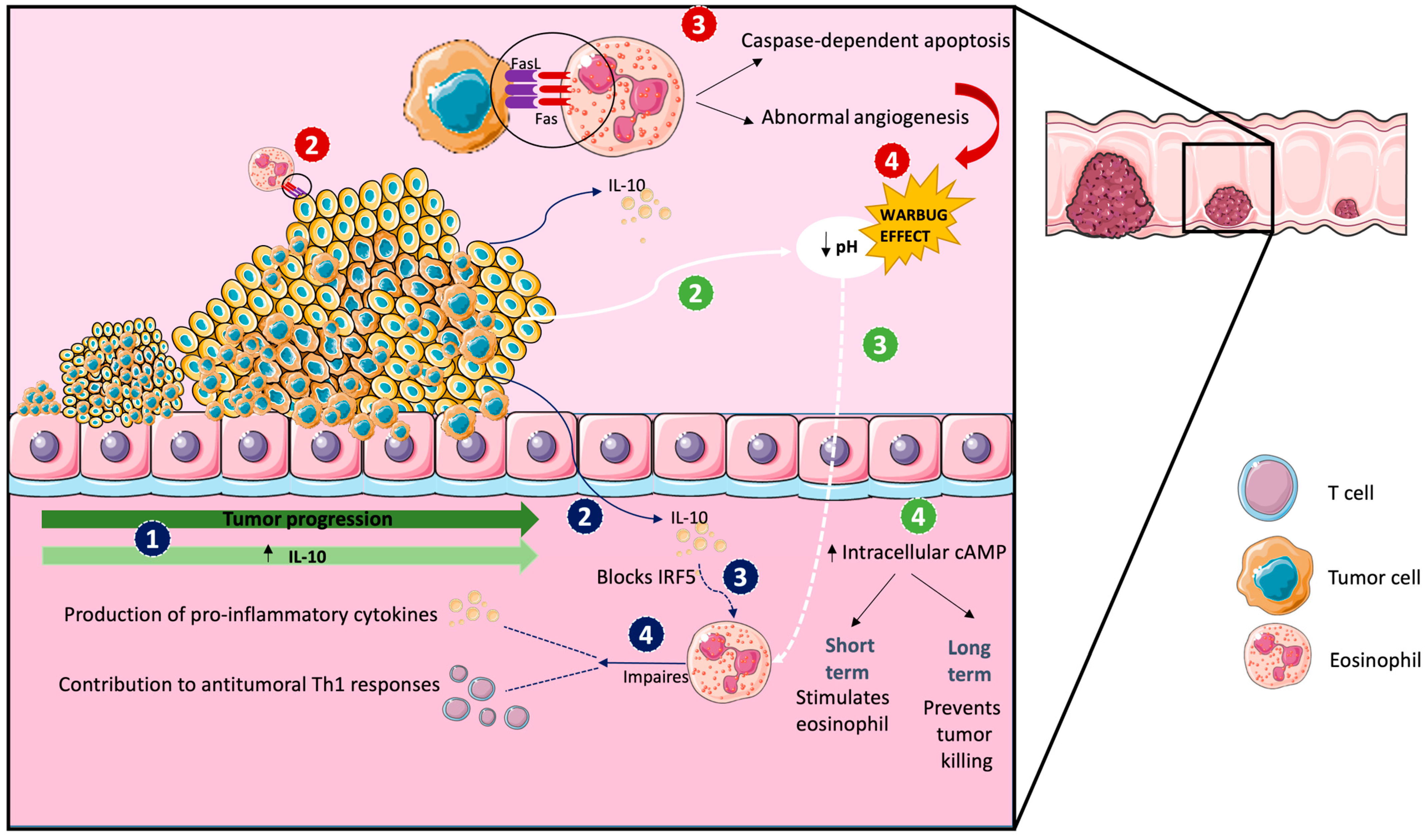
| Setup of CRC Treatment | Model | Treatment Parameters | Results | Ref. |
|---|---|---|---|---|
| C57BL/6 mice, IL-5–transgenic, eosinophil-deficient mice (PHIL), BALB/c. Eo-Cre × Irf5 fl/f, mice, and Eo- Cre × Csf2rbfl/fl were injected with MC38 CRC cells derived from B57BL/6 mice or with the CT26 cell line derived from BALB/c mice. ApcMin/+ mice were treated with anti-IL5. | - Syngenic ectopic murine models of colorectal cancer - 240 CRC patients | Tumor weight, volume, leukocyte infiltration, and RNA expression profile | Eosinophil activation and migration to the tumor site required GM-CSF signaling, with GM-CSF-activated eosinophils driving CD4+ and CD8+ T cells activation and infiltration, which inversely correlated with the tumor state. | [57] |
| C57BL/6 mice and CD3-IL5 transgenic mice and ApcMin/+ mice were injected intraperitoneally with azoxymethane (AOM) and dextran sodium sulfate (DSS) or were injected with MC38. | Murine model of inflammation-induced colorectal cancer and orthopic model | Status and number of tumors versus percentage of eosinophilic infiltrate, size and number of tumors, quantitative assessment of tumor load in adenoma and transcriptome, and proteomic analysis of intratumoral eosinophil | Intratumoral eosinophils had a phenotype, which was associated with IFN-γ signaling. IFN-γ potentiated the eosinophil-mediated killing of colorectal cancer (CRC) cells by the release of reactive oxygen species, mitochondrial DNA, and nitric oxide. | [58] |
| Stage I and II patients did not receive adjuvant therapy, whereas stage III patients were given 5-fluorouracil/folinic acid-based chemotherapy. | 381 colorectal cancer patients | TNM classification, tumor cell differentiation, vascular invasion and tumor budding | Increasing peritumoral and intratumoral eosinophil counts were associated with favorable tumor parameters (lower T and N classification), progression-free and cancer-specific survival, and although the peritumoral eosinophil count correlated with the intensity of the overall inflammatory cell reaction, it was independently associated with the outcome. | [34] |
| For the heterotopic CRC model, CT26 cells were injected subcutaneously into the flank of BALB/c or ΔdblGATA-1 mice. When tumors were palpable, mice were treated with IL-33. For colitis-associated CRC, model mice were injected wirh AOM and DSS. | Heterotopic CRC tumor engraftment model and colitis-associated CRC model. | Tumor area, volume, weight, leukoyite infiltration, eosinophil infiltration, and cell viability | Reduction in tumor growth was significantly enhanced when eosinophils were activated by IL-33, and the degranulation of eosinophils seemed to be the mechanism that contributed to the IL-33 dependent anti-tumoral effects. | [45] |
| C57BL/6N mice and transgenic Foxp3.LuciDTR-4 BAC mice (DTR4) were injected with the MC38 aenocarcinoma cell line. For the depletion of Treg cells, transgenic Foxp3.LuciDTR-4 BAC mice received intraperitoneal injection of diphtheria toxin. For the depletion of eosinophils, anti Siglec-F was injected. | Heterotopic CRC tumor engraftment model | Tumor size, % survival, epsinophil infiltration, vessel normalitation, and % of migration | Eosinophils migrated preferently into tumors and les into other tissues such as the lymphoid organs or liver. Activated tumor-infiltrating eosinophils produced large amounts of chemokines, such as CCL5, CXCL9, and CXCL10, that recruited co-transferred CD8+ T cells to the tumor, which resulted in tumor rejection and prolonged survival. Eosinophil infiltration also normalized tumor vasculature and macrophage polarization. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Perez, D.; Prados-Lopez, B.; Galvez, J.; Leon, J.; Carazo, A. Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 6098. https://doi.org/10.3390/ijms25116098
Lopez-Perez D, Prados-Lopez B, Galvez J, Leon J, Carazo A. Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2024; 25(11):6098. https://doi.org/10.3390/ijms25116098
Chicago/Turabian StyleLopez-Perez, David, Belen Prados-Lopez, Julio Galvez, Josefa Leon, and Angel Carazo. 2024. "Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications" International Journal of Molecular Sciences 25, no. 11: 6098. https://doi.org/10.3390/ijms25116098
APA StyleLopez-Perez, D., Prados-Lopez, B., Galvez, J., Leon, J., & Carazo, A. (2024). Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 25(11), 6098. https://doi.org/10.3390/ijms25116098








