Study on the Structure and Properties of Silk Fibers Obtained from Factory All-Age Artificial Diets
Abstract
:1. Introduction
2. Results and Discussion
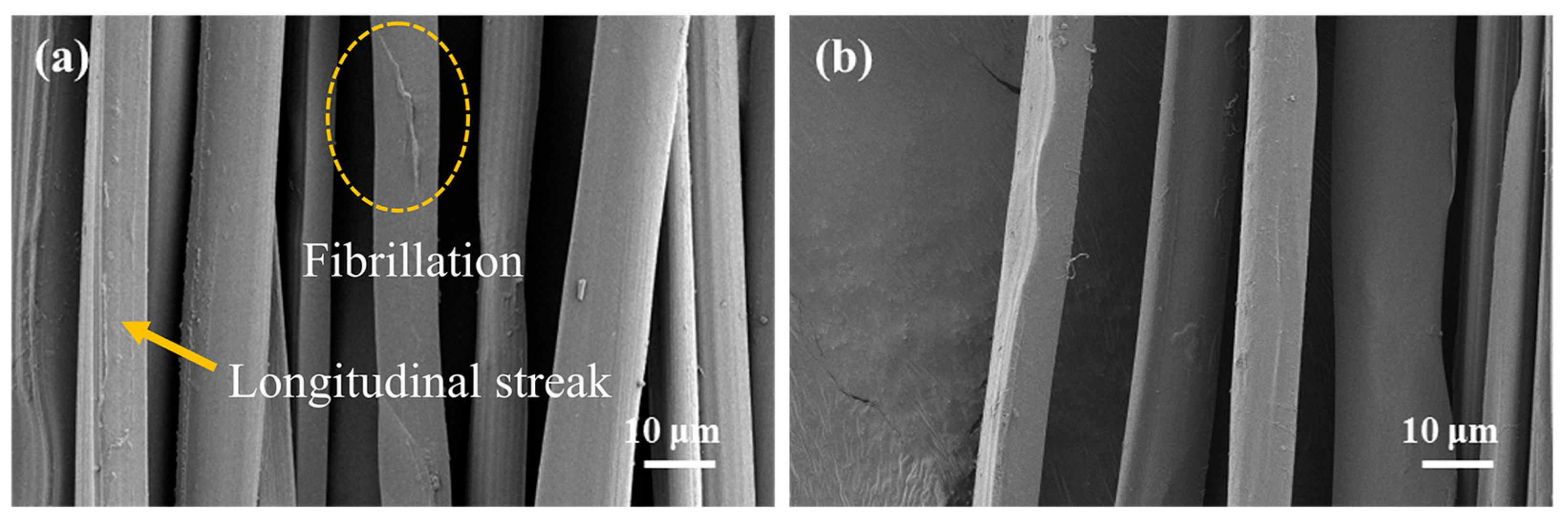
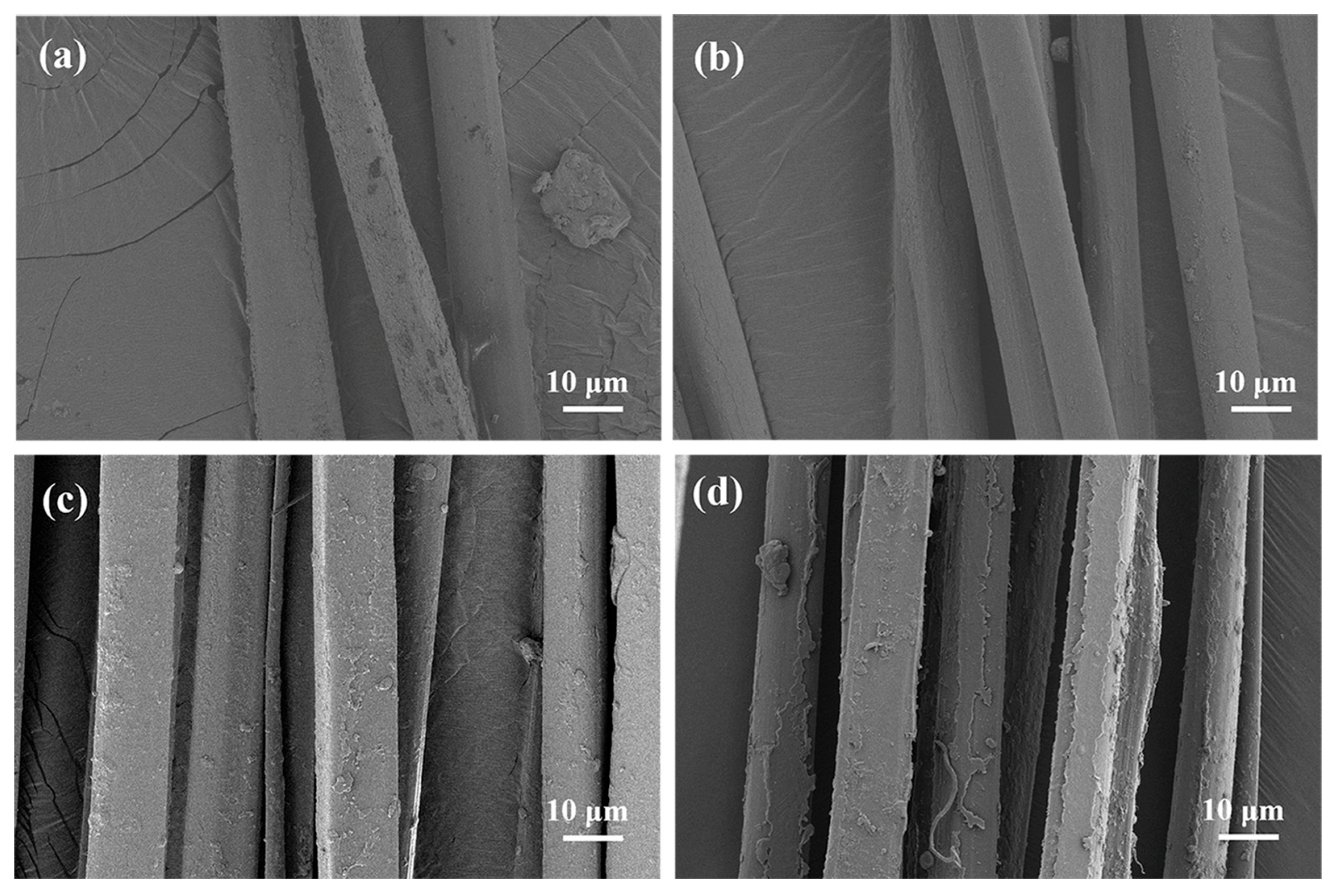
2.1. Morphology of Silk Fibers
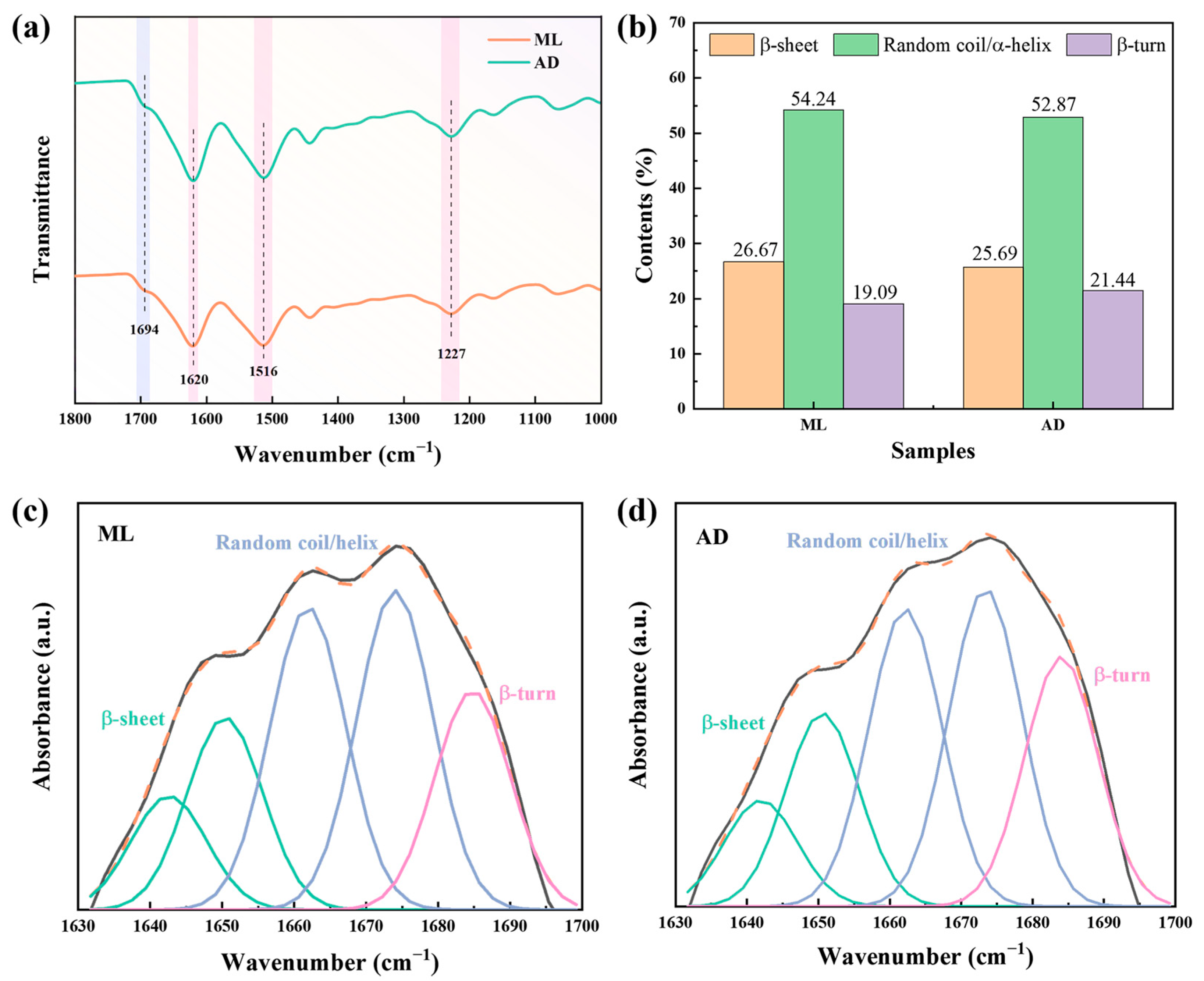
2.2. Effect of Factory All-Age Artificial Diet Feeding on the Structure of Silk Fibers
2.3. Effect of Factory All-Age Artificial Diet Feeding on the Amino Acid Composition of Silk Fibers
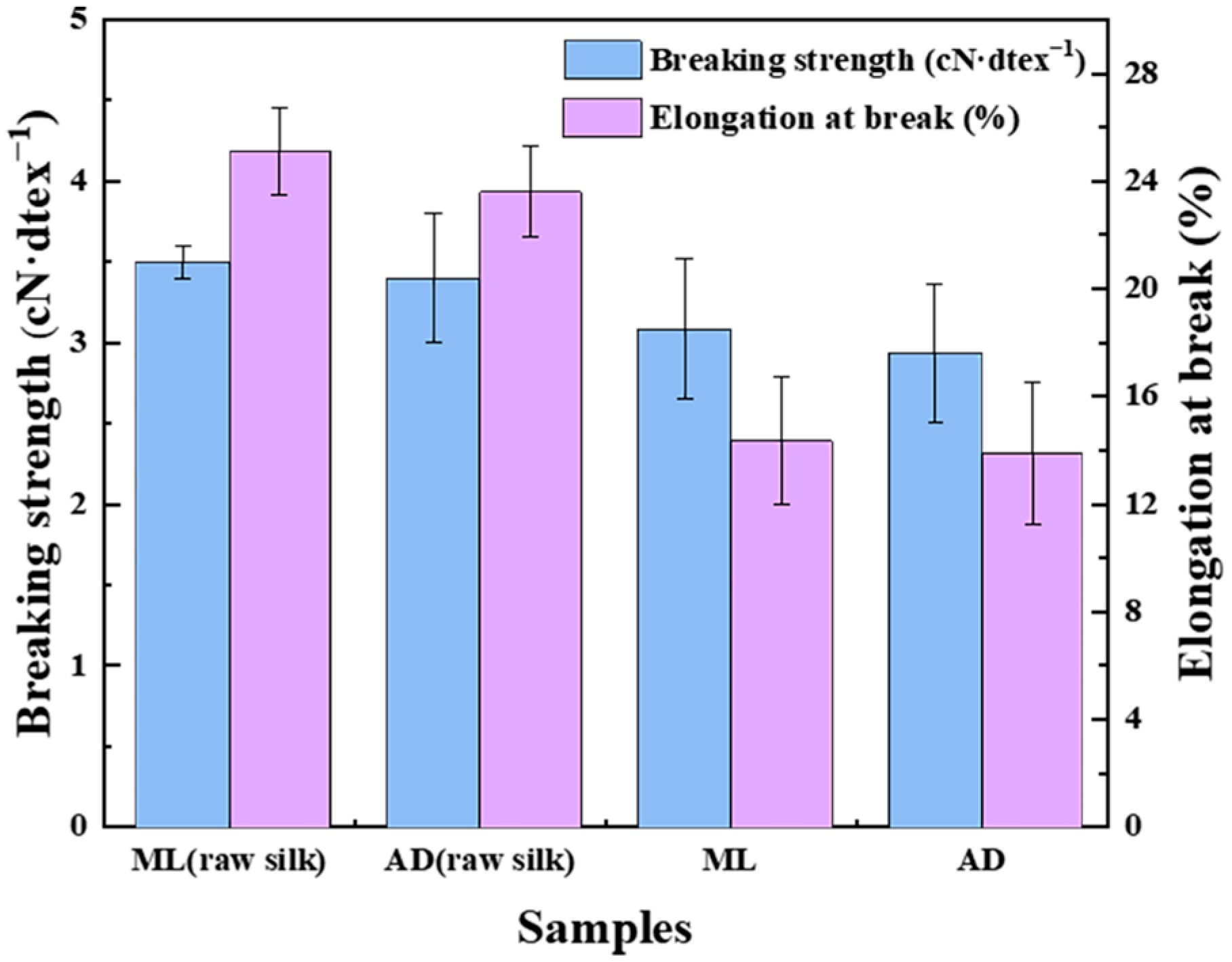
2.4. Effect of Factory All-Age Artificial Diet Feeding on the Mechanical Properties of Silk Fibers
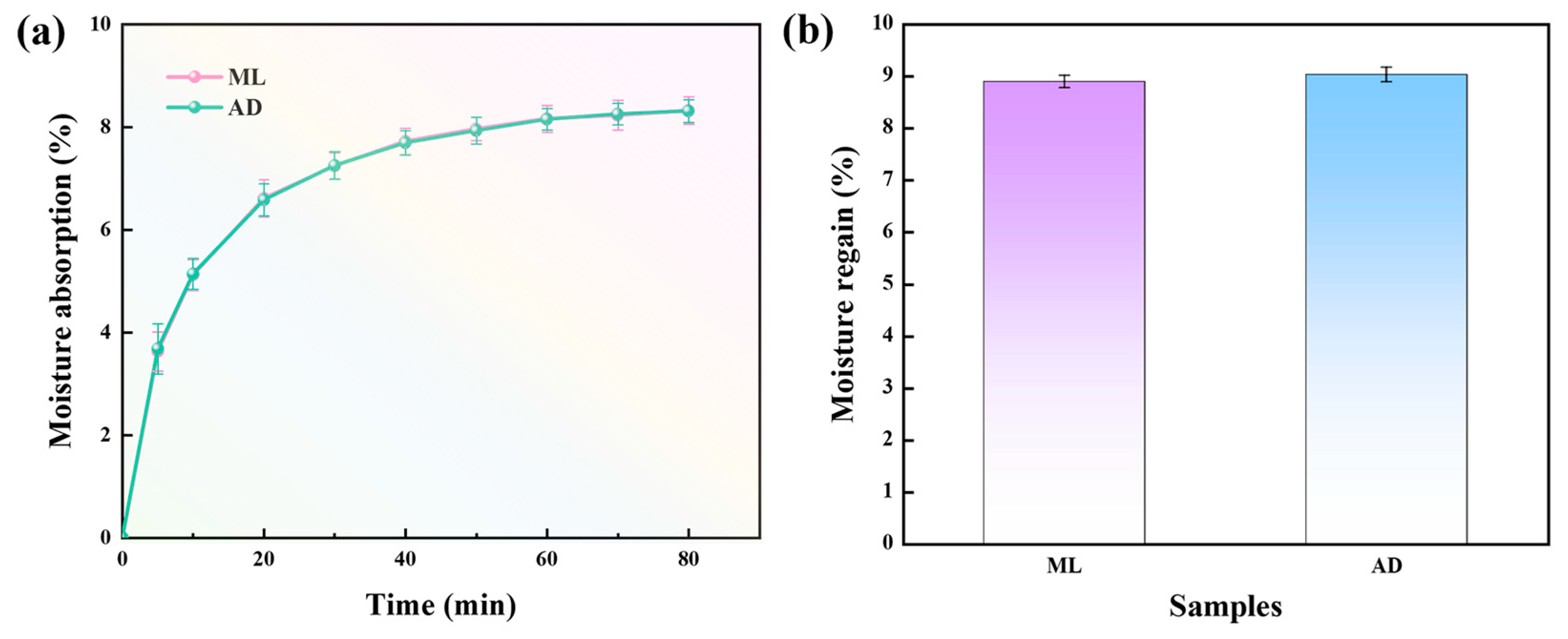
2.5. Effect of Factory All-Age Artificial Diet Feeding on Hygroscopic Properties of Silk Fibers
2.6. Effect of Factory All-Age Artificial Diet Feeding on Thermal Stability of Silk Fibers
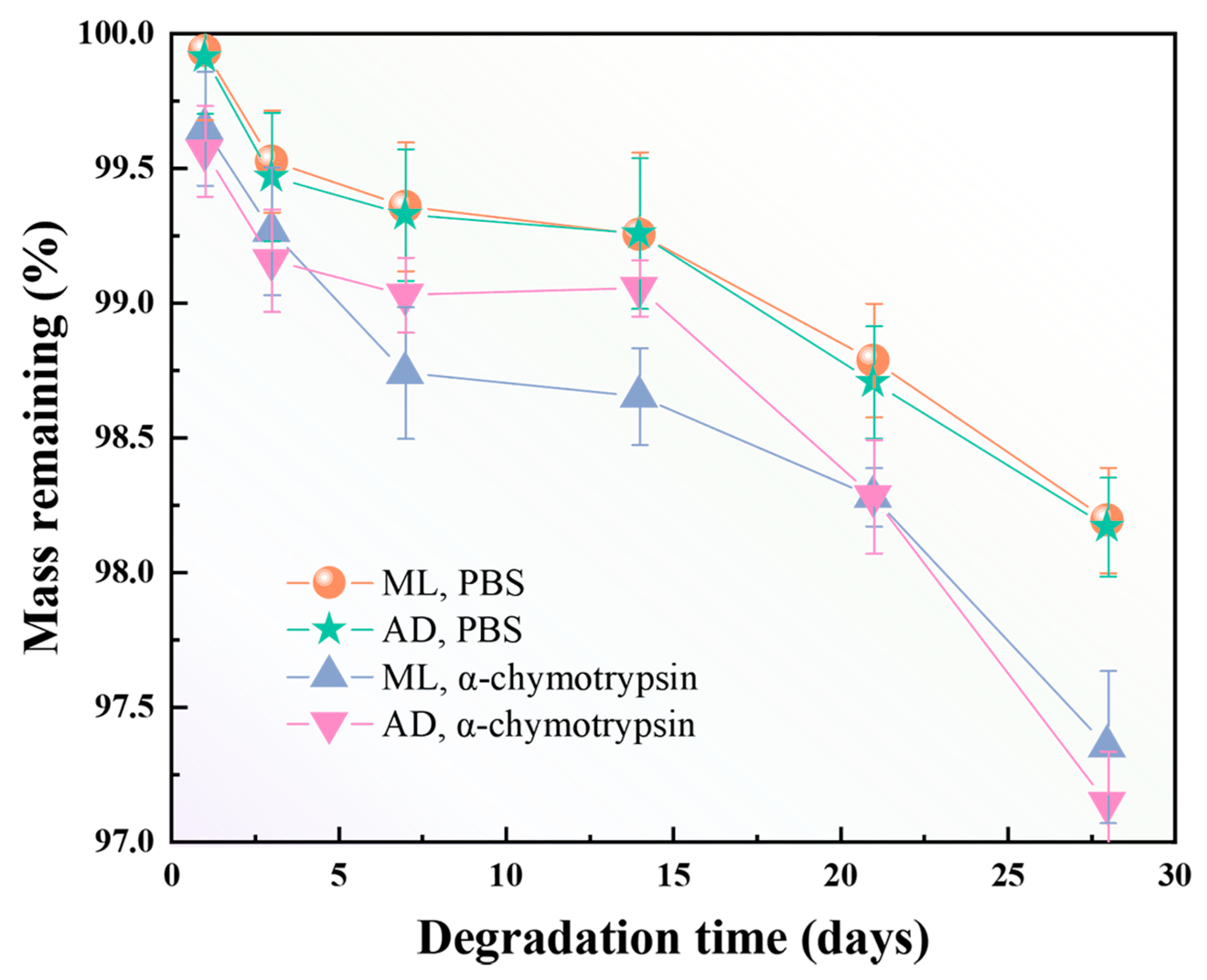
2.7. Effect of Factory All-Age Artificial Diet Feeding on In Vitro Degradation of Silk Fibers
3. Materials and Methods
3.1. Materials
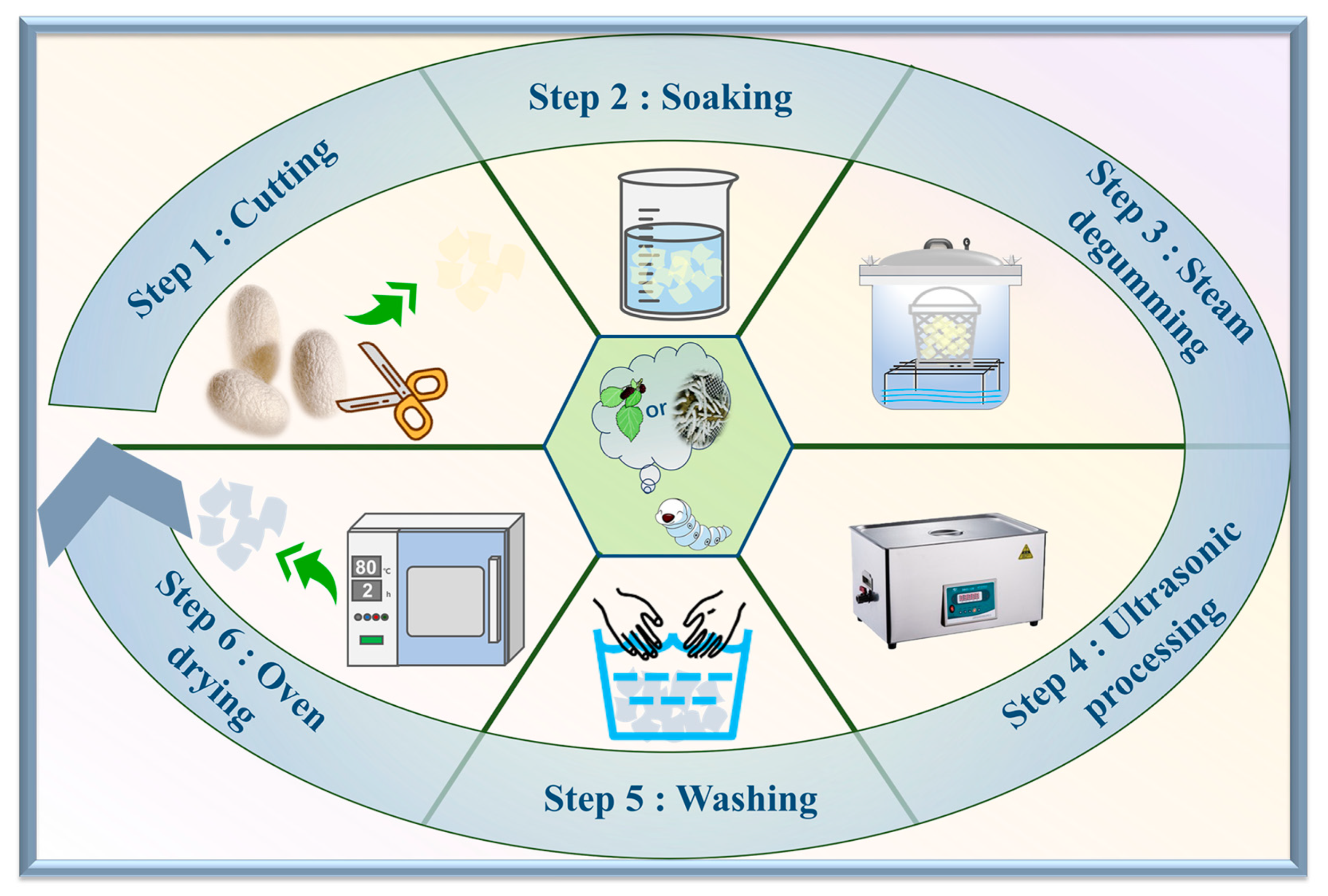
3.2. Preparation of Silk Fibroin Fibers
3.3. Measurement and Characterization
3.3.1. Morphological Observation
3.3.2. Fourier Transform Infrared Spectroscopy Test
3.3.3. X-ray Diffraction Test
3.3.4. Amino Acid Composition Test
3.3.5. Mechanical Property Test
3.3.6. Hygroscopic Test
3.3.7. Thermal Stability Test
3.3.8. In vitro Enzymatic Degradability Test
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, Q.; Zhao, P.; Wang, X.; Zou, Y.; Zhong, X.; Wang, C.; Xiang, Z.; Xia, Q. Shotgun proteomic analysis of the Bombyx mori anterior silk gland: An insight into the biosynthetic fiber spinning process. Proteomics 2013, 13, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.; Le, T.; Huynh, V.Q.N.; Vo, D.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Shao, Z.; Frid, V. Surprising strength of silkworm silk. Nature 2002, 418, 6899. [Google Scholar] [CrossRef]
- Teramoto, H.; Kojima, K.; Iga, M.; Yoshioka, T. Unique Material Properties of Bombyx mori Silk Fiber Incorporated with 3-Azidotyrosine. Biomacromolecules 2023, 24, 4208–4217. [Google Scholar] [CrossRef]
- Jin, K.Y.; Wan, K.S.; Young, K.K.; Seok, K.C.; Chul, U.I. Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains. Int. J. Mol. Sci. 2023, 24, 4965. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef]
- Debari, M.K.; King, C.I.; Altgold, T.A.; Abbott, R.D. Silk Fibroin as a Green Material. ACS Biomater. Sci. Eng. 2021, 7, 3530–3544. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Bio-Technol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, Y.; Rajinikanth, P.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef]
- Sina, A.S.; Rouhollah, M.A.; Parisa, A.; Asieh, H.T. Amniotic membrane/silk fibroin-alginate nanofibrous scaffolds containing Cu-based metal organic framework for wound dressing. Int. J. Polym. Mater. Polym. Biomat. 2022, 73, 33–44. [Google Scholar]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef]
- Ding, Z.; Cheng, W.; Mia, M.S.; Lu, Q. Silk Biomaterials for Bone Tissue Engineering. Macromol. Biosci. 2021, 21, 2100153. [Google Scholar] [CrossRef] [PubMed]
- Mina, A.; Atefeh, S.; Somaye, A.; Mohammad, M. A hydrogel-fiber-hydrogel composite scaffold based on silk fibroin with the dual-delivery of oxygen and quercetin. Biotechnol. Bioeng. 2022, 120, 297–311. [Google Scholar]
- Lu, L.; Liu, X.; Sun, Y.; Wang, S.; Liu, J.; Ge, S.; Wei, T.; Zhang, H.; Su, J.; Zhang, Y.; et al. Silk-Fabric Reinforced Silk for Artificial Bones. Adv. Mater. 2024, 2308748. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, Y. Food Selection by Silkworm Larvæ. Nature 1959, 183, 1746–1747. [Google Scholar] [CrossRef]
- Qin, D.; Wang, G.; Dong, Z.; Xia, Q.; Zhao, P. Comparative Fecal Metabolomes of Silkworms Being Fed Mulberry Leaf and Artificial Diet. Insects 2020, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Deng, X.; Liu, L.; Zha, X. Metabonomic Analysis of Silkworm Midgut Reveals Differences between the Physiological Effects of an Artificial and Mulberry Leaf Diet. Insects 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, H.; Chen, M.; Lou, C.; Zhang, Y.; Chen, K.; Wang, Y.; Yu, M.; Fang, Y.; Li, J.; et al. Comparative proteomic analysis between the domesticated silkworm (Bombyx mori) reared on fresh mulberry leaves and on artificial diet. J. Proteome Res. 2008, 7, 5103–5111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, A.; Wu, X.; Qu, Z.; Xu, S.; Sima, Y.; Wang, Y.; He, R.; Jin, F.; Zhan, P.; et al. Combined analysis of silk synthesis and hemolymph amino acid metabolism reveal key roles for glycine in increasing silkworm silk yields. Int. J. Biol. Macromol. 2022, 209, 1760–1770. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, S.; Tao, H.; Chen, Z.; Li, X.; Qiu, J.; Cui, W.; Sima, Y.; Cui, W.; Xu, S. Metabolomics differences between silkworms (Bombyx mori) reared on fresh mulberry (Morus) leaves or artificial diets. Sci Rep 2017, 7, 10972. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Q.; Gu, H.; Feng, P.; Dai, M.; Zhu, Q.; Liu, W.; Dai, Y.; Li, F.; Li, B. Effects of different diets on the growth and development of young silkworms. J. Asia-Pac. Entomol. 2023, 26, 102134. [Google Scholar] [CrossRef]
- Shu, Q.; Wang, Y.; Gu, H.; Zhu, Q.; Liu, W.; Dai, Y.; Li, F.; Li, B. Effects of artificial diet breeding on intestinal microbial populations at the young stage of silkworm (Bombyx mori). Arch. Insect Biochem. Physiol. 2023, 113, e22019. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zha, X. Midgut and Head Transcriptomic Analysis of Silkworms Reveals the Physiological Effects of Artificial Diets. Insects 2022, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, X.; Ye, A.; Cao, J.; He, R.; Pan, M.; Jin, F.; Ma, H.; Zhou, W. Multi-tissue metabolomic profiling reveals po-tential mechanisms of cocoon yield in silkworms (Bombyx mori) fed formula feed versus mulberry leaves. Front. Mol. Biosci. 2022, 9, 977047. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, T.; Liu, Q.; Zhong, S.; Shen, D.; Chen, A.; Zhao, Q. Transcriptome analysis of anorexic and preferred silkworms (Bombyx mori) on artificial diet. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101086. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, J.; Liu, M.; Sun, F.; Li, B.; Ye, C. Haemolymph metabolomic differences in silkworms (Bombyx mori L.) under mulberry leaf and two artificial diet rearing methods. Arch. Insect Biochem. Physiol. 2021, 109, e21851. [Google Scholar] [CrossRef]
- Qu, J.; Feng, P.; Zhu, Q.; Ren, Y.; Li, B. Study on the Effect of Stretching on the Strength of Natural Silk Based on Different Feeding Methods. ACS Biomater. Sci. Eng. 2021, 8, 100–108. [Google Scholar] [CrossRef]
- Xu, X.; Yao, X.; Jiang, K.; Zhou, Y.; Lu, W.; Jiang, W.; Wang, X. Novel ultrasonic-assisted cleaner technology for cocoon brushing at low temperature. J. Clean. Prod. 2022, 359, 132070. [Google Scholar] [CrossRef]
- Lee, K.I.; Wang, X.; Guo, X.; Yung, K.; Fei, B. Highly water-absorbing silk yarn with interpenetrating network via in situ polymerization. Int. J. Biol. Macromol. 2017, 95, 826–832. [Google Scholar] [CrossRef]
- Miyazawa, T.; Blout, E.R.J. The Infrared Spectra of Polypeptides in Various Conformations: Amide I and II Bands. J. Am. Chem. Soc. 1961, 83, 712–719. [Google Scholar] [CrossRef]
- Paquet-Mercier, F.; Lefevre, T.; Auger, M.; Pezolet, M. Evidence by infrared spectroscopy of the presence of two types of β-sheets in major ampullate spider silk and silkworm silk. Soft Matter 2013, 9, 208–215. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; David, P.K.; Shao, Z.; Chen, X. Synchrotron FTIR microspectroscopy of single natural silk fibers. Biomacromolecules 2011, 12, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Yeon-su, B.; In-chul, U. Effects of Fabrication Conditions on Structure and Properties of Mechanically Prepared Natural Silk Web and Non-Woven Fabrics. Polymers 2021, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- Yeong, C.Y.; Jin, J.M.; Byung-Dae, P.; Chul, U.I. Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers. Int. J. Mol. Sci. 2023, 24, 11485. [Google Scholar] [CrossRef]
- Zhao, M.; Qi, Z.; Tao, X.; Chad, N.; Hu, X.; Lu, S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef]
- Sen, K.; Babu, K.M. Studies on Indian silk. I. Macrocharacterization and analysis of amino acid composition. J. Appl. Polym. Sci. 2004, 92, 1080–1097. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, H.; Chen, S.; Wang, W.; Dai, F.; Zhao, H. Characterization of silkworm larvae growth and properties of silk fibres after direct feeding of copper or silver nanoparticles. Mater. Des. 2017, 129, 125–134. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Investigation of the Structure and Properties of Silk Fibers Produced by Actias lunas. J. Polym. Environ. 2012, 20, 659–664. [Google Scholar] [CrossRef]
- Fang, G.; Tang, Y.; Qi, Z.; Yao, J.; Shao, Z.; Chen, X. Precise correlation of macroscopic mechanical properties and microscopic structures of animal silks-using Antheraea pernyi silkworm silk as an example. J. Polym. Environ. 2017, 5, 6042–6048. [Google Scholar] [CrossRef]
- Koperska, M.; Pawcenis, D.; Bagniuk, J.; Zaid, M.; Missori, M.; Łojewski, T.; Łojewska, J. Degradation markers of fibroin in silk through infrared spectroscopy. Polym. Degrad. Stabil. 2014, 105, 185–196. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Hu, X.; Tsuzuki, T.; Kaplan, D.L.; Wang, X. Structure and Biodegradation Mechanism of Milled Bombyx mori Silk Particles. Biomacromolecules 2012, 13, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Thidarat, W.; Johnston, B.F.; Philipp, S.F. Degradation Behavior of Silk Nanoparticles—Enzyme Responsiveness. ACS Biomater. Sci. Eng. 2018, 4, 942–951. [Google Scholar]
- Wang, R.; Zhu, Y.; Shi, Z.; Jiang, W.; Liu, X.; Ni, Q. Degumming of raw silk via steam treatment. J. Clean. Prod. 2018, 203, 492–497. [Google Scholar] [CrossRef]


| Types of Amino Acids | Relative Content of 17 AA (%) | Change (ML-AD) | |
|---|---|---|---|
| ML Group | AD Group | ||
| Aspartic acid | 2.46 | 2.47 | −0.01 |
| Threonine | 1.21 | 1.22 | −0.01 |
| Serine | 12.86 | 12.47 | 0.39 |
| Glutamic acid | 2.15 | 2.21 | −0.06 |
| Proline | 0.77 | 0.74 | 0.03 |
| Glycine | 35.65 | 36.20 | −0.55 |
| Alanine | 25.81 | 25.72 | 0.09 |
| Cystine | 0.96 | 1.00 | −0.04 |
| Valine | 2.95 | 2.95 | 0.00 |
| Methionine | 0.15 | 0.15 | 0.00 |
| Isoleucine | 0.88 | 0.91 | −0.03 |
| Leucine | 0.79 | 0.79 | 0.00 |
| Tyrosine | 10.16 | 9.98 | 0.18 |
| Phenylalanine | 1.21 | 1.23 | −0.02 |
| Lysine | 0.79 | 0.75 | 0.04 |
| Histidine | 0.24 | 0.25 | −0.01 |
| Arginine | 0.94 | 0.94 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Jiang, K.; Jin, Y.; Mao, Y.; Lu, W.; Jiang, W.; Chen, W. Study on the Structure and Properties of Silk Fibers Obtained from Factory All-Age Artificial Diets. Int. J. Mol. Sci. 2024, 25, 6129. https://doi.org/10.3390/ijms25116129
Pan M, Jiang K, Jin Y, Mao Y, Lu W, Jiang W, Chen W. Study on the Structure and Properties of Silk Fibers Obtained from Factory All-Age Artificial Diets. International Journal of Molecular Sciences. 2024; 25(11):6129. https://doi.org/10.3390/ijms25116129
Chicago/Turabian StylePan, Mengyao, Kexin Jiang, Yuwei Jin, Ying Mao, Wangyang Lu, Wenbin Jiang, and Wenxing Chen. 2024. "Study on the Structure and Properties of Silk Fibers Obtained from Factory All-Age Artificial Diets" International Journal of Molecular Sciences 25, no. 11: 6129. https://doi.org/10.3390/ijms25116129







