Chitosan/Polyvinyl Alcohol-Based Biofilms Using Ternary Deep Eutectic Solvents towards Innovative Color-Stabilizing Systems for Anthocyanins
Abstract
:1. Introduction
2. Results and Discussion
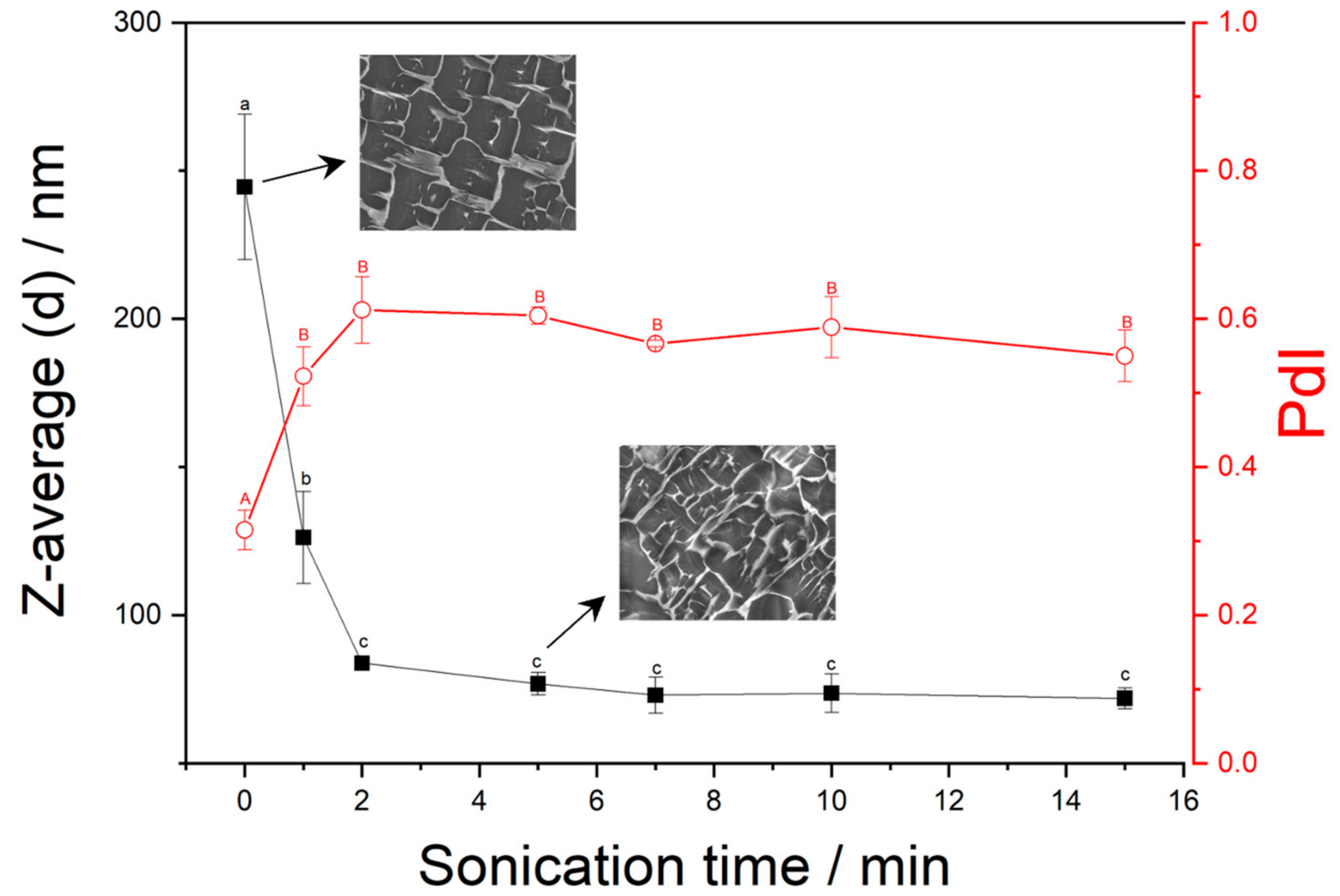
2.1. DLS
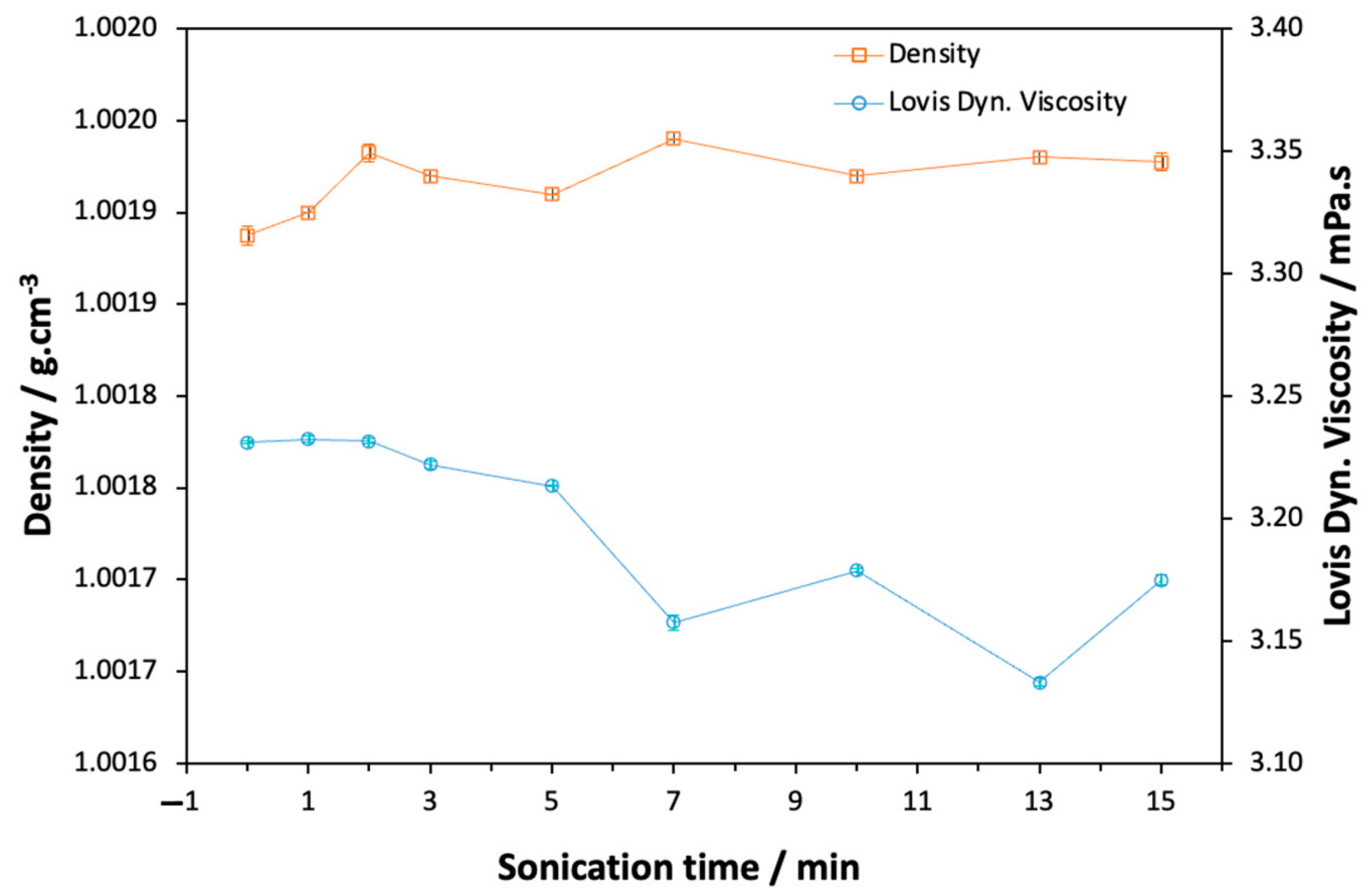
2.2. Density and Viscosity
2.3. Refractive Index
2.4. Chitosan Intrinsic Viscosity
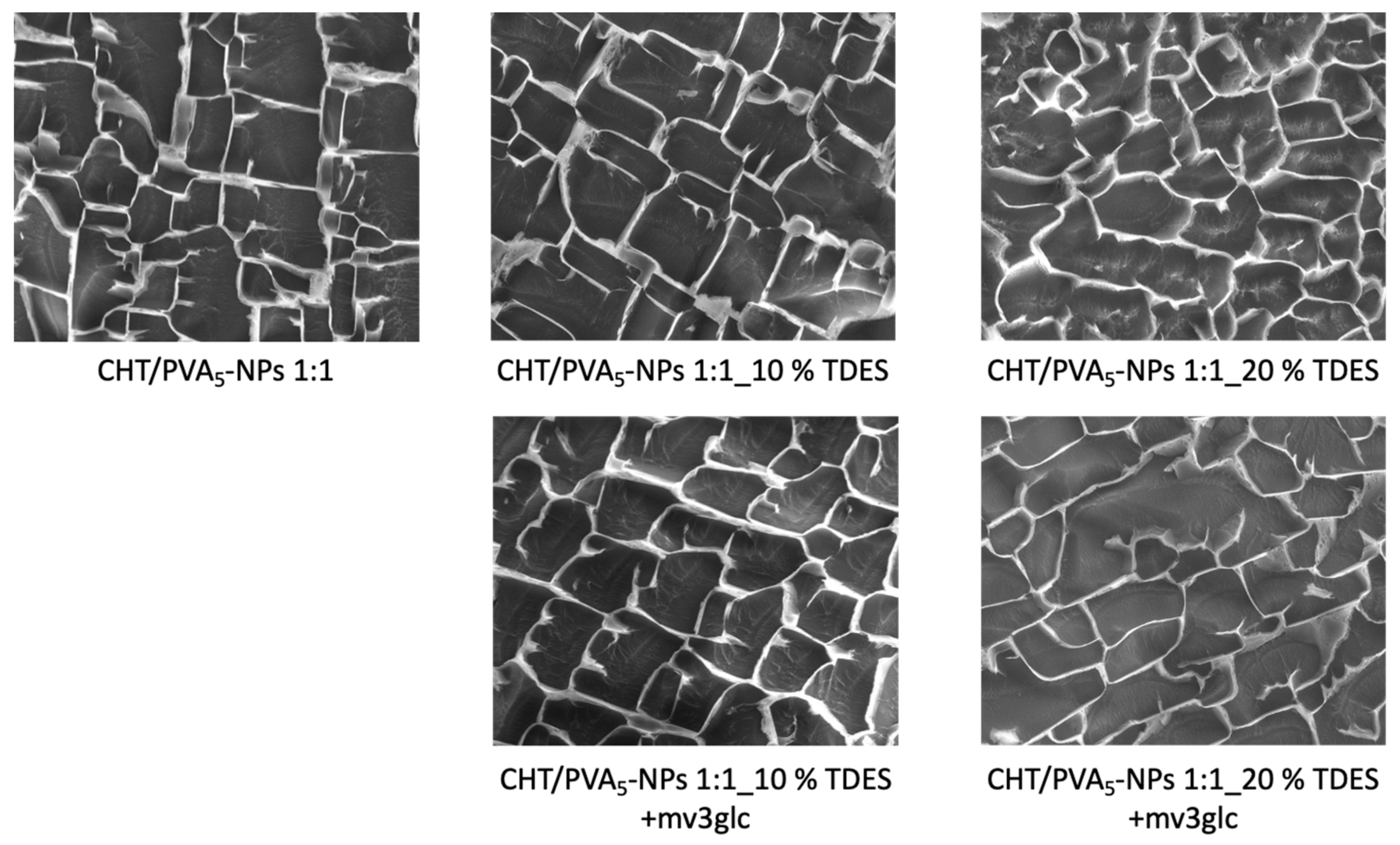
2.5. Cryo-SEM
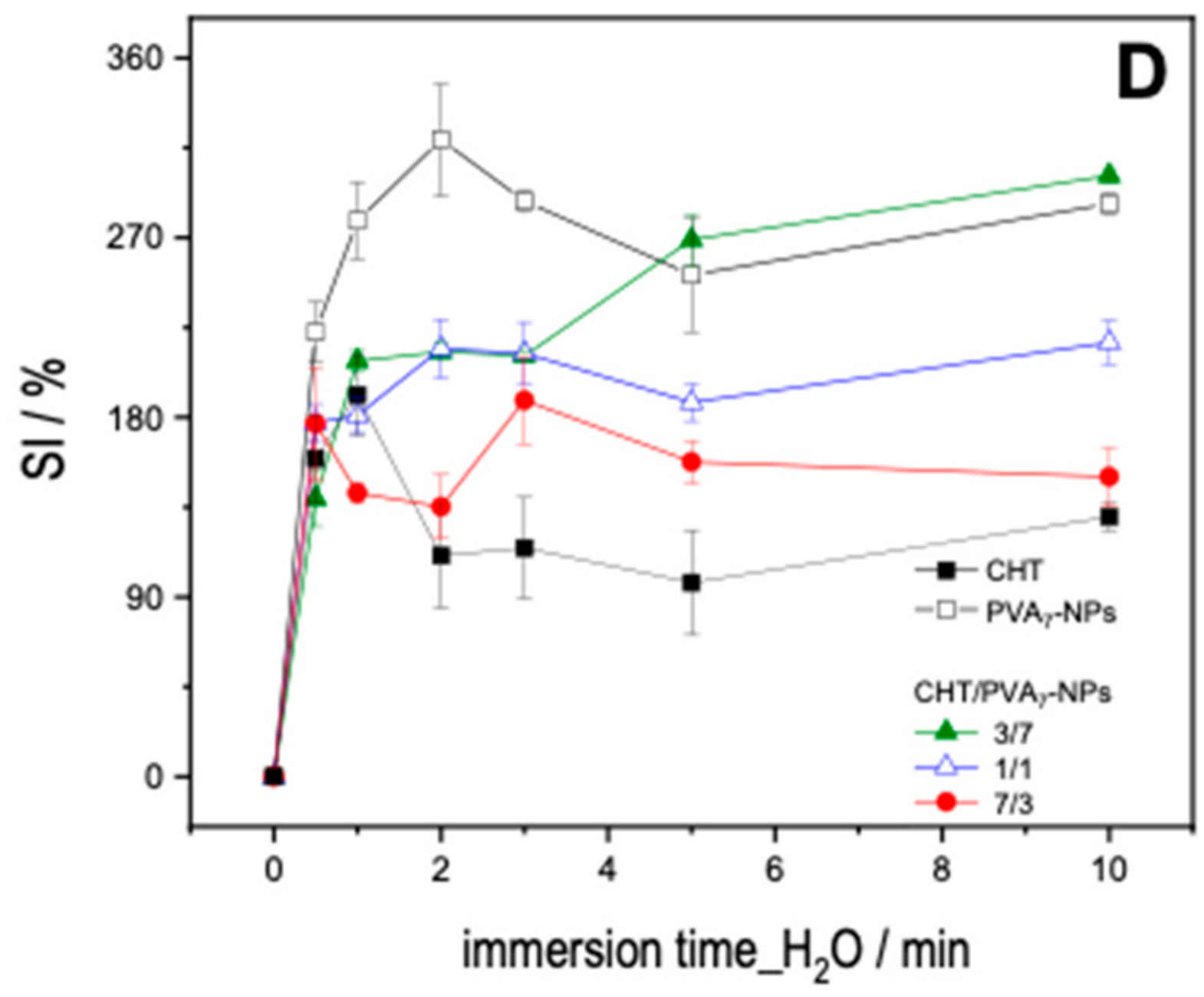
2.6. SI
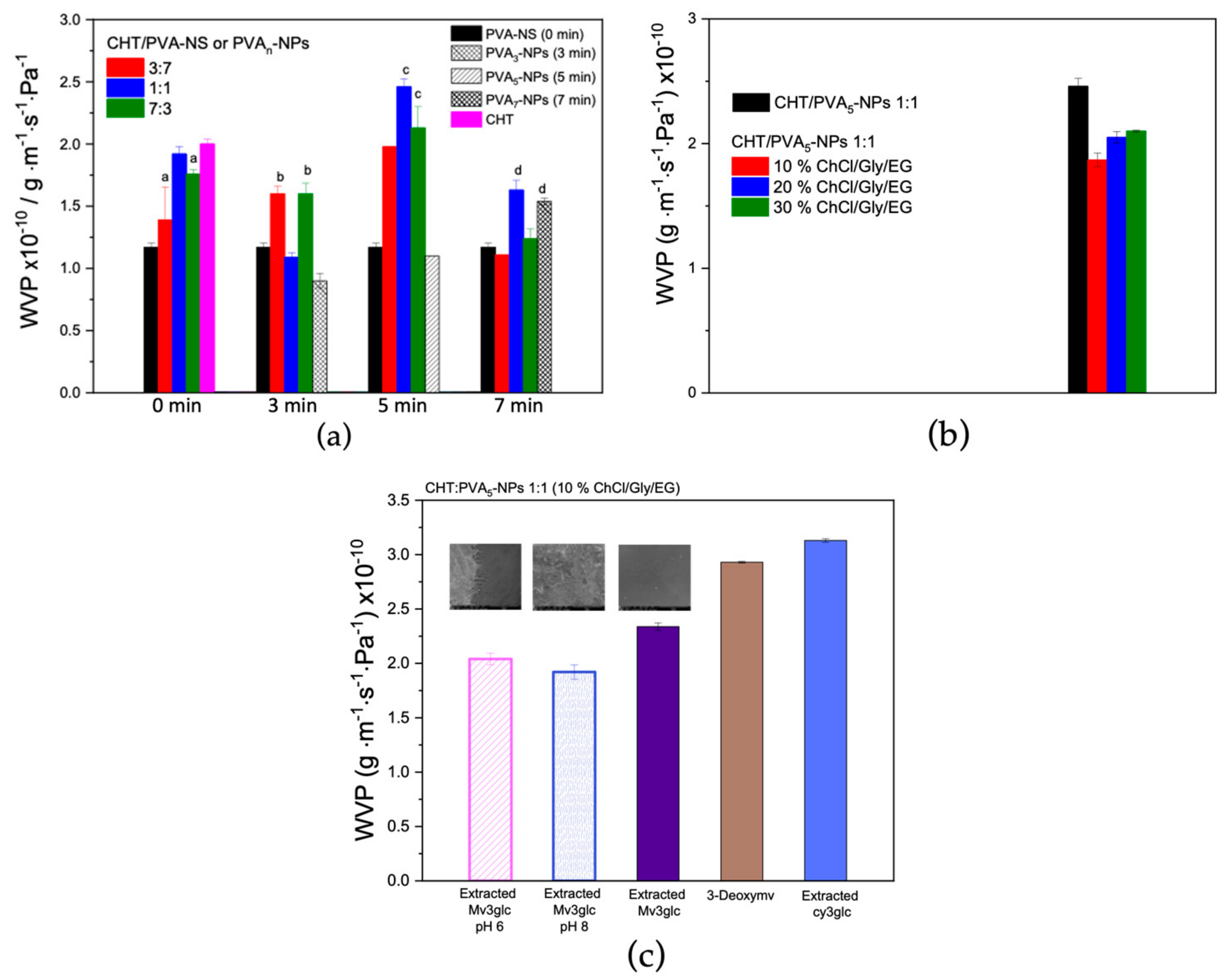
2.7. WVP
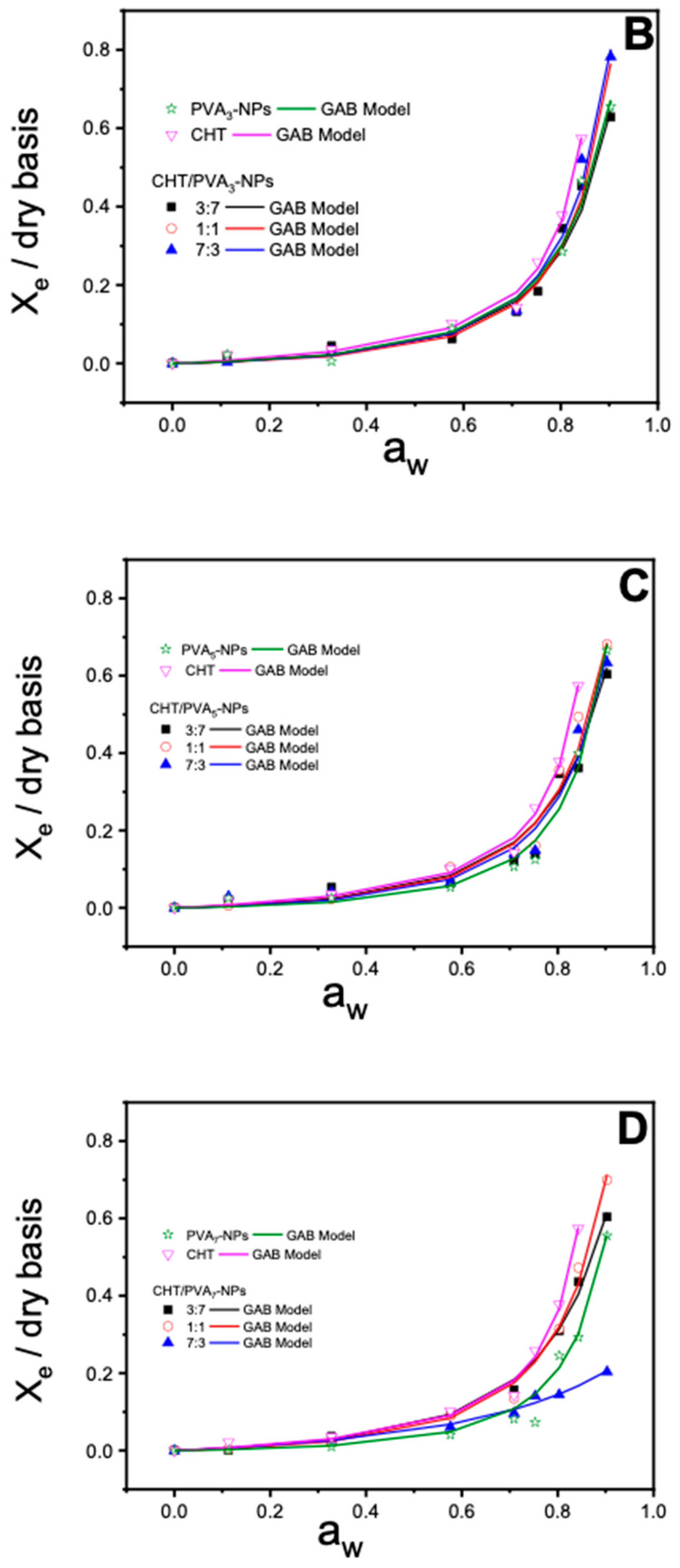
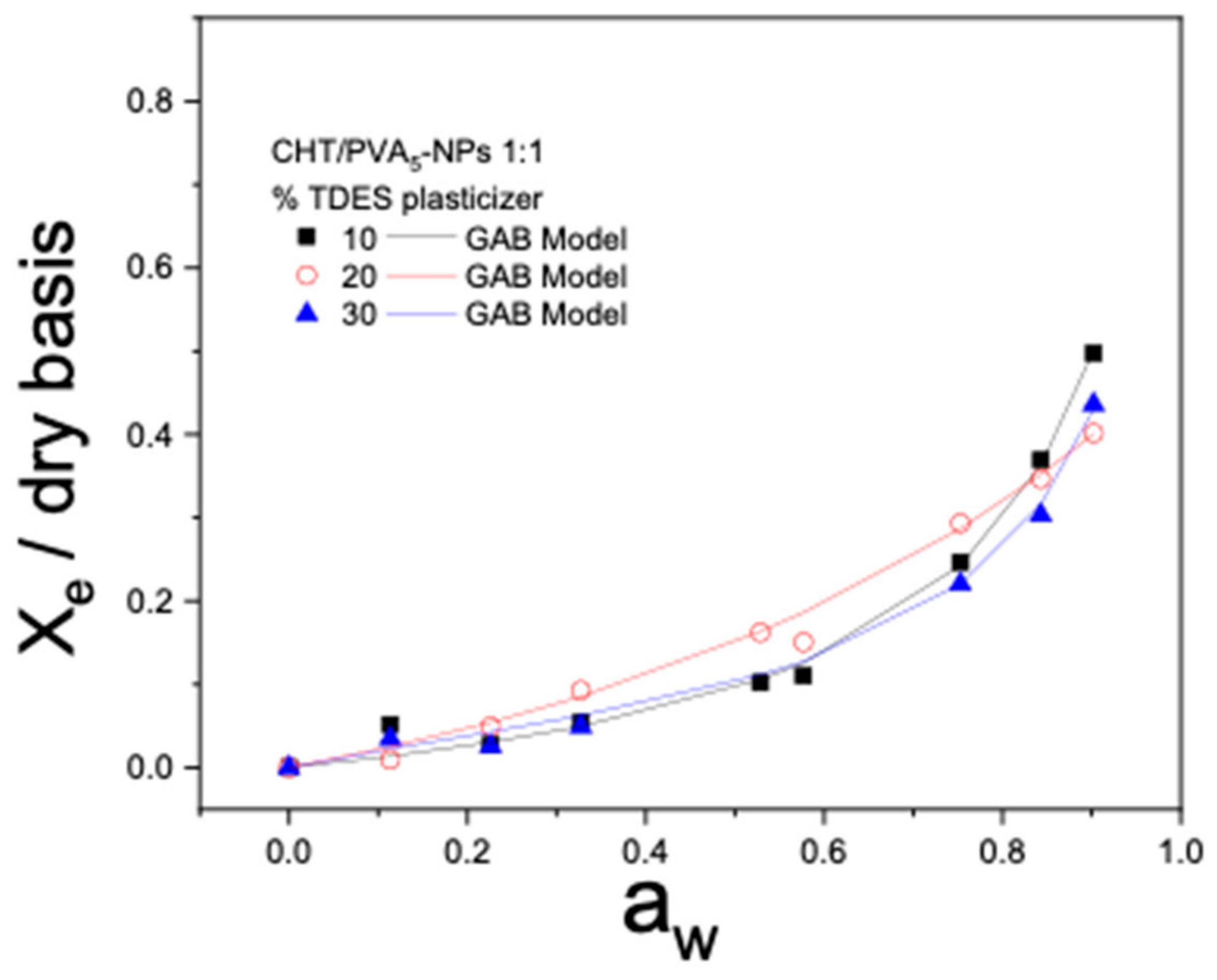
2.8. Moisture Sorption Isotherms
| pH | d (mm) × 10−2 | C | k | X0 | |
|---|---|---|---|---|---|
| CHT/PVA5-NPs | neat | 2.59 ± 0.20 | 0.007 | 1.002 | 0.823 |
| 8 | 3.28 ± 0.21 | 0.037 | 0.889 | 0.368 | |
| 6 | 3.37 ± 0.18 | 0.009 | 0.950 | 1.881 |
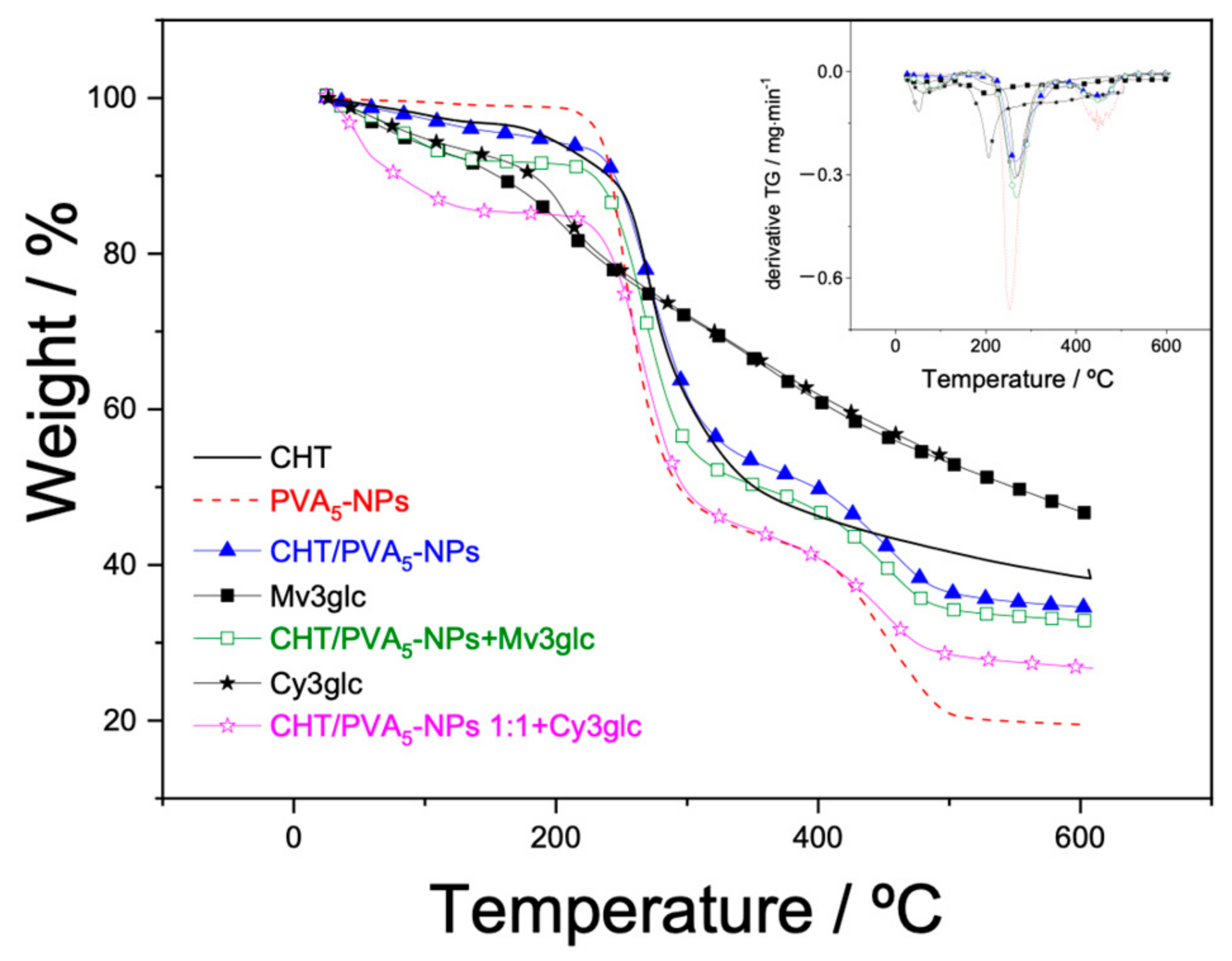
2.9. TGA
2.10. SEM and pH-Dependent Chromatic Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of PVA and CHT Solutions
3.3. Preparation of PVA Nanoparticles
3.4. Preparation of Solutions
3.5. Fabrication of Bioplastic Films
3.6. Characterization of PVA Nanoparticles by DLS
3.7. Viscosity, Refraction Index, and Density
3.8. Cryo-SEM
3.9. Films Characterization
3.9.1. SEM
3.9.2. WVP
3.9.3. SI
3.9.4. TGA
3.9.5. Moisture Sorption Isotherm
3.10. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Voss, D.M.; Miyagusuku-Cruzado, G.; Giusti, M.M. Thermal stability comparison between 10-catechyl-pyranoanthocyanins and anthocyanins derived from pelargonidin, cyanidin, and malvidin. Food Chem. 2023, 403, 134305. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.L.; Cruz, H.; Oliveira, J.; Araújo, P.; Cruz, L.; Gomes, V.; Silva, C.P.; Silva, G.T.M.; Mateus, T.; Calogero, G.; et al. Dye-sensitized solar cells based on dimethylamino-π-bridge-pyranoanthocyanin dyes. Sol. Energy 2020, 206, 188–199. [Google Scholar] [CrossRef]
- Pinto, A.L.; Máximo, P.; Pina, J.; Calogero, G.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Exploring the impact of structural rigidification of amino-substituted bio-inspired flavylium dyes in DSSCs. Dyes Pigment. 2023, 218, 111495. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Branco, L.C.; Cruz, L. Microwave-Assisted Synthesis and Ionic Liquids: Green and Sustainable Alternatives toward Enzymatic Lipophilization of Anthocyanin Monoglucosides. J. Agric. Food Chem. 2020, 68, 7387–7392. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.K.S.; Mateus, N.; de Freitas, V.; Gonçalves, M.P.; Cruz, L. Chemical/Color Stability and Rheological Properties of Cyanidin-3-Glucoside in Deep Eutectic Solvents as a Gateway to Design Task-Specific Bioactive Compounds. ACS Sustain. Chem. Eng. 2020, 8, 16184–16196. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Pedrosa, L.d.F.; Nascimento, K.R.; Fioroto, A.M.; Toniazzo, T.; Tadini, C.C.; Purgatto, E.; Hassimotto, N.M.A.; Fabi, J.P. Nanoencapsulated anthocyanins: A new technological approach to increase physical-chemical stability and bioaccessibility. Food Hydrocoll. 2023, 139, 108516. [Google Scholar] [CrossRef]
- Basílio, N.; Cabrita, L.; Pina, F. Mimicking Positive and Negative Copigmentation Effects in Anthocyanin Analogues by Host–Guest Interaction with Cucurbit[7]uril and β-Cyclodextrins. J. Agric. Food Chem. 2015, 63, 7624–7629. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Gallori, S.; Giaccherini, C.; Vincieri, F.F. Stabilisation of natural anthocyanins by micellar Systems. Int. J. Pharm. 2001, 216, 23–31. [Google Scholar] [CrossRef]
- Tan, C.; Celli, G.B.; Abbaspourrad, A. Copigment-polyelectrolyte complexes (PECs) composite systems for anthocyanin stabilization. Food Hydrocoll. 2018, 81, 371–379. [Google Scholar] [CrossRef]
- Xu, M.; Fang, D.; Kimatu, B.M.; Lyu, L.; Wu, W.; Cao, F.; Li, W. Recent advances in anthocyanin-based films and its application in sustainable intelligent food packaging: A review. Food Control 2024, 162, 110431. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Xie, J.; Cui, H.; Mozafari, M.R.; Chen, W. Potential micro-/nano-encapsulation systems for improving stability and bioavailability of anthocyanins: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 63, 3362–3385. [Google Scholar] [CrossRef] [PubMed]
- Dupeyrón, D.; Kawakami, M.; Rieumont, J.; Carvalho, J.C. Formulation and Characterization of Anthocyanins-Loaded Nanoparticles. Curr. Drug Deliv. 2017, 14, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, X.; Sun, Y.; Liang, J.; Yue, P.; Gao, X. Nanocomplexes derived from chitosan and whey protein isolate enhance the thermal stability and slow the release of anthocyanins in simulated digestion and prepared instant coffee. Food Chem. 2021, 336, 127707. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yue, X.; Wang, S.; Chi, J.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Nanocomplexes composed of chitosan derivatives and β-Lactoglobulin as a carrier for anthocyanins: Preparation, stability and bioavailability in vitro. Food Res. Int. 2019, 116, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nwabor, O.F.; Syukri, D.M.; Voravuthikunchai, S.P. Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 2021, 182, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Amaregouda, Y.; Kamanna, K.; Gasti, T. Fabrication of intelligent/active films based on chitosan/polyvinyl alcohol matrices containing Jacaranda cuspidifolia anthocyanin for real-time monitoring of fish freshness. Int. J. Biol. Macromol. 2022, 218, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Mero, A.; Mezzetta, A.; De Leo, M.; Braca, A.; Guazzelli, L. Sustainable valorization of cherry (Prunus avium L.) pomace waste via the combined use of (NA)DESs and bio-ILs. Green Chem. 2024; 26, 6109–6123. [Google Scholar] [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168. [Google Scholar] [CrossRef]
- Mero, A.; Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A.; Guazzelli, L. Comparison of physicochemical and thermal properties of choline chloride and betaine-based deep eutectic solvents: The influence of hydrogen bond acceptor and hydrogen bond donor nature and their molar ratios. J. Mol. Liq. 2023, 377, 121563. [Google Scholar] [CrossRef]
- ISO 22412:2017; Particle Size Analysis—Dynamic Light Scattering (DLS). ISO: Geneva, Switzerland, 2017.
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. 2020, 9, 2477–2486. J. Mater. Res. Technol. 2020; 9, 2477–2486. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Ghareeb, H.O.; Dannoun, E.M.A.; Hussen, S.A.; Hadi, J.M.; Ahmed, K.K.; Hussein, A.M. Characteristics of Poly(vinyl Alcohol) (PVA) Based Composites Integrated with Green Synthesized Al3+-Metal Complex: Structural, Optical, and Localized Density of State Analysis. Polymers 2021, 13, 1316. [Google Scholar] [CrossRef] [PubMed]
- Bonal, V.; Quintana, J.A.; Villalvilla, J.M.; Muñoz-Mármol, R.; Mira-Martínez, J.C.; Boj, P.G.; Cruz, M.E.; Castro, Y.; Díaz-García, M.A. Simultaneous Determination of Refractive Index and Thickness of Submicron Optical Polymer Films from Transmission Spectra. Polymers 2021, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture Sorption Isotherm Characteristics of Food Products: A Review. Food Bioprod. Process. 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Muangrat, R.; Nuankham, C. Moisture sorption isotherm and changes in physico-mechanical properties of films produced from waste flour and their application on preservation quality of fresh strawberry. Food Sci. Nutr. 2018, 6, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Heiba, Z.K.; Imam, N.G. Optical and thermogravimetric analysis of Zn1-xCuxS/PVA nanocomposite films. J. Mol. Struct. 2018, 1163, 442–448. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. A thermal degradation mechanism of polyvinyl alcohol/silica nanocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, I.; Guimaraes, M.; de Freitas, V.; Mateus, N. Enzymatic synthesis, structural characterization and antioxidant capacity assessment of a new lipophilic malvidin-3-glucoside-oleic acid conjugate. Food Funct. 2016, 7, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef] [PubMed]
- Al Bittar, S.; Mora, N.; Loonis, M.; Dangles, O. A simple synthesis of 3-deoxyanthocyanidins and their O-glucosides. Tetrahedron 2016, 72, 4294–4302. [Google Scholar] [CrossRef]
- Grala, D.; Biernacki, K.; Freire, C.; Kuźniarska-Biernacka, I.; Souza, H.K.S.; Gonçalves, M.P. Effect of natural deep eutectic solvent and chitosan nanoparticles on physicochemical properties of locust bean gum films. Food Hydrocoll. 2022, 126, 107460. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent–temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Alves, T.F.P.; Teixeira, N.; Vieira, J.; Vicente, A.A.; Mateus, N.; de Freitas, V.; Souza, H.K.S. Sustainable chitosan packaging films: Green tea polyphenolic extraction strategies using deep eutectic solvents. J. Clean. Prod. 2022, 372, 133589. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Sousa, A.M.M.; Hilliou, L.; Gonçalves, M.P.; Souza, H.K.S. Thermo-compression molding of chitosan with a deep eutectic mixture for biofilms development. Green Chem. 2016, 18, 1571–1580. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapour transmission of materials. Designation: E96-00. In ASTM, Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2000; pp. 907–914. [Google Scholar]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.d.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- GONTARD, N.; DUCHEZ, C.; CUQ, J.-L.; GUILBERT, S. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]





| PVA | Refractive Index |
|---|---|
| Sonication time (min) | nD |
| 0 | 1.33565 |
| 1 | 1.33564 |
| 2 | 1.33565 |
| 3 | 1.33564 |
| 5 | 1.33564 |
| 7 | 1.33564 |
| 10 | 1.33564 |
| 13 | 1.33564 |
| 15 | 1.33565 |
| CHT | 1.33717 |
| BDES (ChCl/Gly) | 1.46578 |
| TDES (ChCl/Gly/EG) | 1.47776 |
| Sample | [η] (dL·g−1) a | [η] (dL·g−1) b | Mv (KDa) |
|---|---|---|---|
| CHT | 4.35 | 4.29 | 258 |
| Ratio | d (mm) × 10−2 | Solubility | C | k | X0 | |
|---|---|---|---|---|---|---|
| CHT | - | 2.93 ± 0.21 | 26.92 ± 0.34 | 6.66 | 0.960 | 0.059 |
| CHT/PVA-NS (0 min) | 3:7 | 3.28 ± 0.14 | 20.76 ± 0.45 | 3.05 | 0.871 | 0.058 |
| 1:1 | 3.27 ± 0.33 | 19.16 ± 0.70 | 4.10 | 0.955 | 0.095 | |
| 7:3 | 3.23 ± 0.63 | 21.58 ± 2.32 | 6.28 | 0.931 | 0.075 | |
| PVA-NS (0 min) | - | 1.83 ± 0.26 | 20.74 ± 1.38 | 1.40 | 0.99 | 0.05 |
| CHT/PVA3-NPs (3 min) | 3:7 | 3.43 ± 0.05 | 21.04 ± 0.81 | 2.923 | 0.859 | 0.184 |
| 1:1 | 2.56 ± 0.14 | 16.84 ± 3.30 | 4.34 | 0.970 | 0.097 | |
| 7:3 | 3.35 ± 0.35 | 24.15 ± 2.58 | 1.38 | 0.959 | 0.118 | |
| PVA3-NPs (3 min) | - | 2.10 ± 0.03 | 19.99 ± 1.68 | 1.333 | 0.877 | 0.246 |
| CHT/PVA5-NPs (5 min) | 3:7 | 3.45 ± 0.42 | 14.87 ± 0.14 | 2.10 | 0.937 | 0.099 |
| 1:1 | 3.82 ± 0.05 | 12.31 ± 0.18 | 1.08 | 0.890 | 0.164 | |
| 7:3 | 3.05 ± 0.65 | 10.85 ± 0.04 | 3.11 | 0.983 | 0.076 | |
| PVA5-NPs (5 min) | - | 2.33 ± 0.14 | 17.47 ± 0.28 | 1.60 | 0.984 | 0.081 |
| CHT/PVA7-NPs (7 min) | 3:7 | 2.70 ± 0.65 | 26.80 ± 6.41 | 1.45 | 0.883 | 0.145 |
| 1:1 | 3.09 ± 0.22 | 23.64 ± 0.59 | 1.29 | 0.947 | 0.115 | |
| 7:3 | 2.40 ± 0.04 | 25.10 ± 1.01 | 3.20 | 0.69 | 0.09 | |
| PVA7-NPs (7 min) | - | 3.19 ± 0.06 | 1.82 | 1.00 | 0.059 |
| % TDES | d (mm) × 10−2 | C | k | X0 | |
|---|---|---|---|---|---|
| CHT/PVA5-NPs 1:1 | 10 | 3.64 ± 0.46 | 1.12 | 0.908 | 0.109 |
| 20 | 4.09 ± 0.30 | 0.145 | 0.386 | 3.641 | |
| 30 | 3.49 ± 0.50 | 2.99 | 0.922 | 0.077 |
| pH | Cross-Section (5000×) | Surface (10,000×) | Aspect | CIELAB Parameters (First Day of Preparation) | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| 3 | solubilized | - | - | - | ||
| 4 |  |  | solubilized | - | - | - |
| 5 |  |  |  | 43.97 ± 1.2 | 26.25 ± 1.3 | −6.42 ± 1.4 |
| Water (5.7) |  |  |  | 30.23 ± 1.1 | 9.52 ± 0.3 | −25.26 ± 0.4 |
| 6 |  |  |  | 43.43 ± 2.1 | 20.65 ± 0.3 | −12.12 ± 0.8 |
| 7 |  |  |  | 45.49 ± 0.8 | 10.66 ± 0.1 | −16.92 ± 0.2 |
| 7.6 |  |  |  | 43.27 ± 1.5 | 7.00 ± 0.3 | −21.81 ± 0.2 |
| 8 |  |  |  | 43.50 ± 1.1 | 2.82 ± 0.3 | −25.36 ± 0.4 |
| 8.2 |  |  |  | 48.90 ± 0.5 | −6.70 ± 0.1 | −21.89 ± 0.2 |
| Film in Water (pH 5.7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aw | Exposed Time/Days | Visual Easy RGB | CIELAB Coordinates | Exposed Time/Days | Visual Easy RGB | CIELAB Coordinates | ||||
| L* | a* | b* | L* | a* | b* | |||||
| 0 |  | 30.23 ± 1.1 | 9.52 ± 0.3 | −25.26 ± 0.4 | ||||||
| 0.1135 | 19 |  | 28.54 ± 2.1 | 5.48 ± 1.0 | −23.05 ± 2.3 | |||||
| 0.0260 |  | 32.87 ± 1.8 | 2.38 ± 0.2 | −23.84 ± 1.0 | ||||||
| 0.3273 |  | 25.35 ± 1.0 | 8.47 ± 0.2 | −23.76 ± 0.7 | ||||||
| 0.5286 |  | 46.51 ± 2.5 | 5.32 ± 0.3 | −22.06 ± 0.5 | ||||||
| 0.5777 |  | 44.54 ± 3.4 | 6.11 ± 0.6 | −20.50 ± 0.6 | ||||||
| 0.7532 |  | 73.29 ± 0.4 | 1.47 ± 0.3 | 13.06 ± 0.6 | ||||||
| 0.8432 |  | 75.15 ± 0.3 | 1.57 ± 0.2 | 13.53 ± 0.6 | ||||||
| 0.9026 |  | 76.02 ± 0.3 | 1.59 ± 0.2 | 12.93 ± 0.5 | ||||||
| Film in Water (pH 6) | ||||||||||
| aw | Exposed Time/Days | Visual Easy RGB | CIELAB Coordinates | Exposed Time/Days | Visual Easy RGB | CIELAB Coordinates | ||||
| L* | a* | b* | L* | a* | b* | |||||
| 0 |  | 43.43 ± 2.1 | 20.65 ± 0.3 | −12.12 ± 0.8 | ||||||
| 0.1135 | 7 | brittle (dry) | - | - | - | 70 | - | - | - | - |
| 0.0260 | brittle (dry) | - | - | - | - | - | - | - | ||
| 0.3273 | brittle (dry) | - | - | - | - | - | - | - | ||
| 0.5286 |  | 50.10 ± 0.6 | 10.26 ± 0.2 | −15.37 ± 0.2 |  | 56.11 ± 1.3 | 4.06 ± 0.2 | −15.44 ± 0.2 | ||
| 0.5777 |  | 50.65 ± 1.8 | 8.44 ± 0.5 | −10.32 ± 0.3 |  | 54.65 ± 1.9 | 4.37 ± 1.2 | −13.61 ± 0.3 | ||
| 0.7532 |  | 35.52 ± 0.3 | 8.22 ± 0.3 | −8.62 ± 0.6 | shrunk | - | - | - | ||
| 0.8432 |  | 51.55 ± 4.4 | 7.15 ± 0.5 | −4.89 ± 0.6 | shrunk | - | - | - | ||
| 0.9026 |  | 56.41 ± 4.9 | 8.11 ± 0.4 | 0.88 ± 0.2 | shrunk | - | - | - | ||
| Film in Water (pH 8.2) | ||||||||||
| aw | Exposed Time/Days | Visual Easy RGB | CIELAB coordinates | Exposed Time/Days | Visual Easy RGB | CIELAB Coordinates | ||||
| L* | a* | b* | L* | a* | b* | |||||
| 0 |  | 48.52 ± 0.5 | −6.70 ± 0.1 | −21.89 ± 0.2 | ||||||
| 0.1135 | 7 |  | 51.49 ± 0.7 | −6.48 ± 0.1 | −20.83 ± 0.6 | 70 |  | 51.24 ± 0.8 | 0.65 ± 0.6 | −23.50 ± 0.2 |
| 0.0260 |  | 53.49 ± 0.9 | −4.94 ± 0.3 | −21.33 ± 1.2 |  | 55.65 ± 1.3 | −3.94 ± 0.5 | −20.94 ± 0.2 | ||
| 0.3273 |  | 36.70 ± 1.6 | 0.81 ± 0.1 | −25.23 ± 1.5 |  | 34.82 ± 0.2 | 0.65 ± 0.1 | −23.56 ± 0.4 | ||
| 0.5286 |  | 33.62 ± 0.3 | 1.74 ± 0.3 | −17.18 ± 2.3 |  | 34.37 ± 0.3 | 0.60 ± 0.5 | −19.24 ± 0.6 | ||
| 0.5777 |  | 51.09 ± 1.0 | −2.41 ± 0.3 | −17.05 ± 1.1 |  | 53.29 ± 1.7 | −5.58 ± 0.5 | −12.75 ± 0.2 | ||
| 0.7532 |  | 37.61 ± 1.3 | 0.04 ± 0.02 | −17.65 ± 1.0 |  | 49.69 ± 1.6 | −2.26 ± 0.4 | −6.48 ± 0.2 | ||
| 0.8432 |  | 58.05 ± 1.9 | −3.46 ± 0.5 | −6.13 ± 1.7 |  | 77.49 ± 0.5 | 0.11 ± 0.2 | 16.50 ± 1.5 | ||
| 0.9026 |  | 51.15 ± 1.3 | −4.87 ± 0.4 | −14.67 ± 0.2 |  | 74.27 ± 0.9 | −0.89 ± 0.2 | 15.22 ± 0.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, H.K.S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Chitosan/Polyvinyl Alcohol-Based Biofilms Using Ternary Deep Eutectic Solvents towards Innovative Color-Stabilizing Systems for Anthocyanins. Int. J. Mol. Sci. 2024, 25, 6154. https://doi.org/10.3390/ijms25116154
de Souza HKS, Guimarães M, Mateus N, de Freitas V, Cruz L. Chitosan/Polyvinyl Alcohol-Based Biofilms Using Ternary Deep Eutectic Solvents towards Innovative Color-Stabilizing Systems for Anthocyanins. International Journal of Molecular Sciences. 2024; 25(11):6154. https://doi.org/10.3390/ijms25116154
Chicago/Turabian Stylede Souza, Hiléia K. S., Marta Guimarães, Nuno Mateus, Victor de Freitas, and Luís Cruz. 2024. "Chitosan/Polyvinyl Alcohol-Based Biofilms Using Ternary Deep Eutectic Solvents towards Innovative Color-Stabilizing Systems for Anthocyanins" International Journal of Molecular Sciences 25, no. 11: 6154. https://doi.org/10.3390/ijms25116154
APA Stylede Souza, H. K. S., Guimarães, M., Mateus, N., de Freitas, V., & Cruz, L. (2024). Chitosan/Polyvinyl Alcohol-Based Biofilms Using Ternary Deep Eutectic Solvents towards Innovative Color-Stabilizing Systems for Anthocyanins. International Journal of Molecular Sciences, 25(11), 6154. https://doi.org/10.3390/ijms25116154








