Abstract
Levosimendan’s calcium sensitizing effects in heart muscle cells are well established; yet, its potential impact on skeletal muscle cells has not been evidently determined. Despite controversial results, levosimendan is still expected to interact with skeletal muscle through off-target sites (further than troponin C). Adding to this debate, we investigated levosimendan’s acute impact on fast-twitch skeletal muscle biomechanics in a length-dependent activation study by submersing single muscle fibres in a levosimendan-supplemented solution. We employed our MyoRobot technology to investigate the calcium sensitivity of skinned single muscle fibres alongside their stress–strain response in the presence or absence of levosimendan (100 µM). While control data are in agreement with the theory of length-dependent activation, levosimendan appears to shift the onset of the ‘descending limb’ of active force generation to longer sarcomere lengths without notably improving myofibrillar calcium sensitivity. Passive stretches in the presence of levosimendan yielded over twice the amount of enlarged restoration stress and Young’s modulus in comparison to control single fibres. Both effects have not been described before and may point towards potential off-target sites of levosimendan.
1. Introduction
Levosimendan is a calcium sensitizing drug that has been used to treat heart failure for over a decade [1,2]. Its primary mechanism of action involves binding to the calcium-bound form of cardiac troponin C (cTnC) and enhances the stability of the cTnC–calcium complex. Thereby, levosimendan increases the affinity of cTnC and sensitizes the troponin complex to calcium ions leading to increased contractility and improved cardiac function [3]. In addition, levosimendan has vasodilative effects that reduce afterload, and it was shown to improve renal function and decrease inflammation [4,5]. In vivo, the parent drug (levosimendan) acts quickly but has a comparatively short half-time (∼1 h) as it is metabolized in the liver [6]. Yet, the long-term effects in vivo are mediated by the long-lasting metabolite OR-1896 (half-time ∼70–80 h) which has the same effect and similar mechanism of action as the parent drug [6,7,8]. While levosimendan is primarily used in the treatment of heart failure, recent studies suggest that the drug may also directly affect skeletal muscle contractility [9,10]. Regarding calcium sensitivity, cardiac muscle is reported to be slightly less calcium sensitive (e.g., murine cardiac myocytes pCa50 5.6 [11]) than skeletal muscle (ranging from pCa50 5.6 for both slow type-1, and fast type-2 twitch fibres [12] to pCa50 6.2 for slow-twitch fibres [13], and pCa50 5.9 for fast-twitch fibres [13] from rodents). This indicate a potentially larger amplification by administering the drug to cardiac muscle. Yet, levosimendan was also shown to significantly improve the calcium sensitivity of diaphragm muscle fibres in rats, which suggests that the drug may also affect skeletal muscle cells [14], potentially through off-target effects on proteins involved in calcium regulation other than TnC. For example, levosimendan was shown to interact with the ryanodine receptor [15,16], which is involved in the release of calcium from the sarcoplasmic reticulum (SR) in skeletal muscle, and an interaction of the drug with calmodulin is likewise supposed to explain the sensitizing effects [17]. However, the effects of levosimendan on skeletal muscle are not well understood and may be more complex than initially thought. Therefore, further experimental studies are needed to investigate this potential.
Skeletal muscle is a crucial tissue that enables movement and maintains posture. Muscle weakness presents a common symptom in many diseases, including neuromuscular disorders, heart failure, and ageing [18,19,20]. Levosimendan binds to cTnC, increasing its calcium affinity and thereby enhancing cardiac contractility [3,10,16]. It is possible that levosimendan has a similar effect on skeletal muscle troponin C (sTnC) or related proteins involved in calcium regulation, leading to improved calcium sensitivity and thus, muscle function [2,10]. This could have important implications for disease treatment, e.g., administering levosimendan to improve muscle function in patients with neuromuscular disorders [9,21] or to enhance recovery after muscle injury.
A study on skeletal muscle (diaphragm) suggested that the slow-twitch isoform of TnC is similar to the cardiac isoform, explaining levosimendan’s interaction [14]. Other mechanisms might include off-target interactions with the ryanodine receptor [15,16] or with titin, which influences muscle biomechanics and is calcium-sensitive [22,23,24]. Conclusively, the presently suspected protein targets of levosimendan are all involved in cellular calcium regulation (calcium-dependent force generation and sensitivity) and therefore, are responsive to changes in length-dependent activation (LDA), e.g., a higher calcium sensitivity at longer sarcomere lengths [25,26]. LDA is believed to arise from an optimal sarcomere spacing that facilitates actin–myosin binding and calcium docking to TnC, thus increasing muscular calcium sensitivity. In addition to actin and myosin, titin also seems to play a major role in LDA [27,28,29,30] by modifying muscle fibre stiffness in the A-band in active contraction and passive stretching [24]. As such, the potential involvement of titin in the mechanism of action of levosimendan may provide an additional explanation for the sensitizing effects seen in the diaphragm [14].
To investigate this, we employed our automated MyoRobot biomechatronics platform [31,32] to study the acute effects of both LDA and LDA+levosimendan on single muscle fibre active and passive biomechanics. To rule out the possibility that similar isoforms of cTnC and slow-twitch sTnC [21,33] contribute to our results, we investigated single fibres from fast-twitch M. extensor digtorum longus (EDL, >90% type II fibres [34]). With our MyoRobot technology, we carried out automated recordings of muscle contractility (calcium-saturated induced force), calcium sensitivity (pCa-force curves), and stress–strain relationships at different sarcomere lengths (SLs) in the presence (supplemented at 100 µM in each respective bioactive solution) or absence of levosimendan, thus providing insights into the functional implications of the drug on skeletal muscle. Overall, the potential effects of levosimendan on skeletal muscle are an intriguing area of research that could have important clinical implications.
2. Results
2.1. Levosimendan Improves Maximum Ca2+-Saturated Force Generation at High Sarcomere Lengths, without a Sensitizing Effect
Our experiments showed that the pCa50 value gradually increases with the SL in EDL single muscle fibre (Figure 1C). We observed an increase from ∼5.8 to ∼6.0 in both control single fibres and single fibres exposed to levosimendan-enriched solutions, yet without a significant difference between both groups. Interestingly, when exposing the skinned fibre preparation to a Ca2+-rich solution (HA), we detected a significantly improved active stress (force per area) generation for fibres at 3.0 µm SL in the levosimendan-enriched solution and an overall tendency towards higher stress generation (Figure 1D). While control fibres reproduced the descending limb of LDA at 3.0 µm SL, fibres activated in the levosimendan-containing solution maintained a similar tension as for shorter SLs.
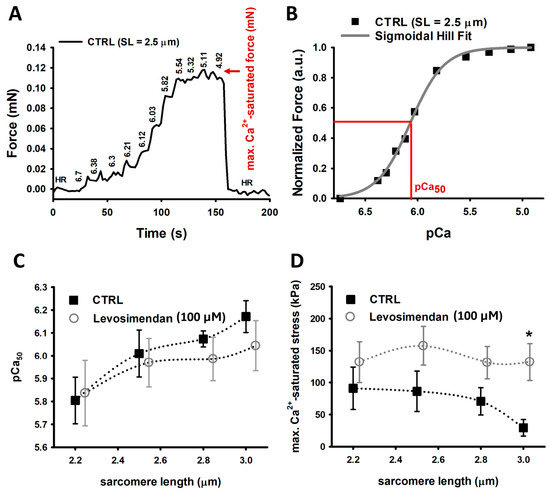
Figure 1.
Ca2+ sensitivity and maximum Ca2+-saturated force recordings. (A), Representative force–pCa recording of a CTRL single muscle fibre at 2.5 µm SL. (B), by plotting the steady-state force levels against their respective pCa value, a Hill fit’s inflection point represents the pCa50 value. Parameters: b (Hill coefficient) = 2.118 and c = 8.385 × 10−7 with —log10(c) = 6.07 = pCa50. (C), Ca2+ sensitivity recordings yield a gradually increasing pCa50 value in agreement with LDA. Both groups display a similar Ca2+ sensitivity. (D), Maximum Ca2+-saturated force is slightly enhanced in single fibres exposed to 100 µM levosimendan vs. control fibres for SLs from 2.2 µm to 2.8 µm. At 3.0 µm SL, single fibres in levosimendan-enriched solution produce significantly more force than untreated fibres. The latter reproduced a force decline in agreement with the descending limb of LDA. Data points were slightly shifted around each other at same SL for visualisation purposes only. The dotted curves connecting the data visualize potential trends but do not explicitly result from any fit procedure. Error bars represent standard error. n: 3–9, valid for both panels and study conditions. * p ≤ 0.05.
2.2. Passive Restoration Stress and Young’s Modulus Are Enhanced in Single Fibres Stretched in Levosimendan-Supplemented Solution
Stress–strain relationships were assessed to determine the maximum restoration stress at 140% elongation (40% strain). Maximum restoration stress and the Young’s modulus of untreated fibres slowly but gradually increased with greater SL, according to the passive behaviour of LDA, except for very large SLs of 4.2 µm (Figure 2A,B). This increase was more pronounced in single fibres stretched in a levosimendan-supplemented solution. They seem to display a somewhat exponential increase in maximum restoration stress and Young’s modulus with significantly larger values, particularly at SLs beyond 3.8 µm. As such, single EDL fibres appear to be stiffer in the presence of levosimendan.
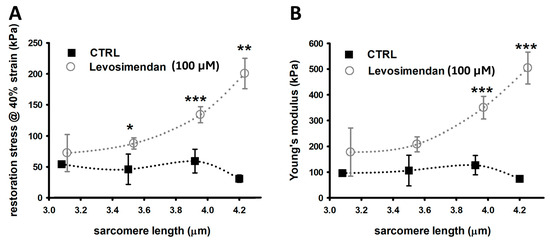
Figure 2.
Stress–strain relationship recordings yield an over twice enlarged axial stiffness in single fibres in the presence of levosimendan. (A), Maximum passive restoration force was measured at 40% strain. Single EDL fibres exposed to levosimendan solution produce notably more strain over controls, which was significant for larger SLs. (B), Similarly, the Young’s moduli of levosimendan-exposed single fibres are much larger. Untreated control fibres are more compliant and display a slow but gradual increase in Young’s modulus with larger SLs, in agreement with passive LDA. This effect is much more pronounced for single fibres in the levosimendan-enriched solution. Data points were slightly shifted at the same SL for visualisation purposes only. The dotted curves connecting the data visualize potential trends but do not explicitly result from any fit procedure. Error bars represent standard error. n: 3–9, valid for both panels and study conditions. * p ≤ 0.05. ** p ≤ 0.01, *** p ≤ 0.005.
3. Discussion
In this study, we investigated the effects of LDA [35] at the single-cell level in combination with levosimendan, a common cardiac Ca2+ sensitizer. While usually interacting with cTnC, its effects on skeletal muscle fibres are still being debated. Statements in the literature vary, with different authors reporting no effect on the slow-twitch muscle of nemaline myopathy patients [21], an improved Ca2+ sensitivity in rat diaphragms [14], enhanced contractility in the human diaphragm [9], an increased grip strength in mice [10], no effects on skeletal muscle at all [21], and that there remains indications that an effect on slow-twitch muscle still exists [2,36]. While in the heart, levosimendan was shown to improve health, continued research suggests potential off-target effects on other calcium-regulating proteins that could also translate to effects in skeletal muscle (e.g., ryanodine 1 receptor [15,16], calmodulin [17]. While other sarcomere proteins like nebulin also contribute to the calcium sensitivity of skeletal muscle [37] and may present potential off-target sites, studies indicate no change in force generation between fibres with intact or mutated nebulin in the presence/absence of levosimendan [21]. Until the present, most levosimendan studies on skeletal muscle focused on active contractility in slow-twitch muscle; therefore, we investigated potential effects on active and passive biomechanics of fast-twitch muscle in an LDA study.
In active contractility, LDA is expressed by, e.g., a higher Ca2+ sensitivity at a longer SL [25,38]. The increased sarcomere spacing is thought to facilitate Ca2+–TnC docking and actin–myosin interaction. Both untreated control EDL fibres and those incubated in the levosimendan solution display a gradual increase in pCa50, with no major difference between both groups. The values ranging from 5.8 to 6.2 compare well with previously published data [20,39,40]. Although in LDA Ca2+ sensitivity increases with SL, the amount of generated force follows a bell-shaped relationship, peaking at an optimum SL between 2.3 µm to 2.5 µm in mice [41]. Beyond this SL, maximum contractile force decreases; this is described as the ‘descending limb’ [42,43]. The onset of such decrease is seen in the actively generated stress upon Ca2+ exposure in skinned control muscle fibres at 3.0 µm SL. Interestingly, this is not the case for EDL fibres immersed in a levosimendan-supplemented Ca2+-rich solution: the fibres display a significant increase in force and appear to shift the descending limb towards higher SLs. While in heart muscle, levosimendan causes an overall improvement of contractile function due to Ca2+ sensitization [44], such correlation seems implausible for skeletal single muscle fibres based on our data. To our knowledge, no indication of levosimendan shifting the onset of the descending limb of active contractility has been described in the literature. Yet, to identify whether this is a systematic effect, extended investigations including even larger SLs are needed.
The passive biomechanics properties of single fibres in the presence of levosimendan yield an axial fibre stiffening and enlarged restoration stress at 40% strain. Usually, the response to stress in LDA is largely determined by titin [27,28,29,30] that modifies fibre stiffness, particularly within the A-band related proteins [24]. The restoration force/stress in response to stretch will therefore, consistently increase with SL up to rupture [45]. Myofibrils, single fibres, and whole EDL muscle typically produce between ∼40 to 70 kPa passive restoration stress from 3.0 µm to 4.0 µm [40,46,47,48] which is in agreement with data from our control group; the axial Young’s modulus can easily reveal values that are twice of those (100–200 kPa). This seems plausible given the fact that the Young’s modulus of soleus single muscle fibres scales between 400–600 kPa [48] and are considered twice as stiff as EDL single muscle fibres [49]. Interestingly, in the presence of levosimendan, EDL single muscle fibres produced over twice the restoration stress and display a significantly higher Young’s modulus. During passive stretch, sarcomeres are thought to somehow ‘sense’ titin stiffness by structural rearrangements or conformational changes of proteins within the actin–myosin overlap zone [30]. Although titin’s intrinsic stiffness also depends on Ca2+ levels [23,50], a sensitizing effect of levosimendan on titin’s potential Ca2+ binding sites is theoretically possible, but can be ruled out here, since the entire experiment was performed in the absence of Ca2+. It remains speculative as to whether the stiffening originates from a direct interaction with sTnC and an associated stiffening of its interaction with actin; yet, it raises the important question of whether levosimendan would potentiate the stiffening effects of Ca2+ ions on titin [51], which were shown to increase titin’s rupture forces within the N2A region and Ig domains [23]. This will require the execution of active stretches in the presence of levosimendan and will present an interesting future research question. So far, considering the absence of Ca2+ ions in our stress–strain relationship experiments, we currently cannot explain the observed stiffness increase, and more detailed experiments may provide an answer.
It must be noted that there are limitations to this study which must be addressed in future investigations. First, levosimendan (as in this study) is commonly diluted in a DMSO medium, which was suggested to depress muscle contractility [52]. As such, it is plausible that the effects of 100 µM levosimendan on maximum Ca2+-saturated stress might be underestimated due to potential remains of the DMSO vehicle, and a dilution in, e.g., glucose solution should be employed as cross-reference. In addition, 100 µM levosimendan represents the top end of externally applied concentrations to skeletal muscle in the literature [14]. In patients with heart failure, levosimendan is usually administered in doses up to 0.2 µg/kg/min, translating to roughly 0.4 nM [53] in the blood serum. Whether concentrations in the µM range are factually present in patient’s muscle tissue remains to be investigated and must be related to experiments where levosimendan is diluted, e.g., in glucose solution to avoid an underestimation of its effects and thus define a dosage at which effects can be expected in the body. Lastly, at the time of initiating this study, we expected the parameter ‘sarcomere length’ to play the predominant role in our LDA assessment, for which we decided on an experiment layout as in Figure 3D. With this design, we were able to conduct paired statistics considering the parameter ‘sarcomere length’. With knowledge of the here-presented data, a follow up study should include investigating the parameter ‘levosimendan presence/absence/concentration’ at a single predefined SL to elucidate the effects exclusive to levosimendan.
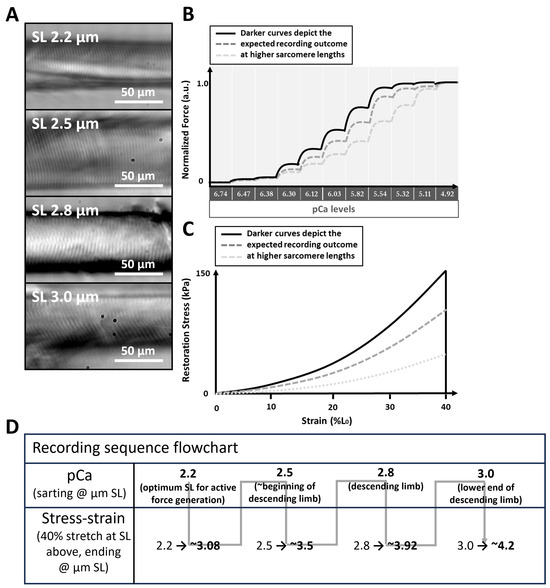
Figure 3.
LDA study design. (A), single muscle fibres were analysed at distinct SLs starting at 2.2 µm and iteratively increasing SL up to 3.0 µm. The images were obtained with the MyoRobot’s integrated optics system. The experimental procedures shown in (B) (force–pCa recording) and (C) (stress–strain relationship) were carried out in the presence or absence of 100 µM levosimendan. In force–pCa recordings, an increasing SL leads to the sensitization of the myofibrillar contractile apparatus. In stress–strain curves, an increasing SL results in a steeper increase in restoration stress. (D), flow chart of the recording sequence.
4. Materials and Methods
This study was conducted in accordance with the approved animal experimentation guidelines at the Friedrich-Alexander-University Erlangen-Nürnberg (TS06/2016). The German regulations for the care of laboratory animals and the guidelines of the Federation of European Laboratory Animal Sciences Associations apply.
4.1. Animal Handling and Muscle Fibre Preparation
All animals for this study were obtained from a tissue sharing collaboration with local institutes and were male C57BL/6 mice of approx. 30 g body weight and aged between 15–21 weeks. Five mice were assigned to each study cohort (CTRL group or in vitro exposition to levosimendan). After sedating the animal with 5% isoflurane and performing cervical dislocation, the hind limbs were cut off. Samples were transferred into Ringer’s solution (see Section 4.3 Bioactive Solutions.), and the M. extensor digitorum longus (EDL, >90% type II fibres [34]) was manually isolated (scissors and tweezers (Dumont #5): FST, Foster City, CA, USA) under a stereomicroscope (Olympus SZ-X7, Olympus, Shinjuku-ku, Japan). The muscle was pinned onto a Polydimethylsiloxane (PDMS, Sylgard, Dow Corning, Midland, MI, USA)-coated Petri dish under slight pre-stretch. Ringer’s solution was replaced by a high-potassium solution (HKS, see Section 4.3 ‘Bioactive solutions’), and the preparation was allowed to rest and equilibrate for 30 min. Whenever the preparation was allowed to rest, it was bubbled with atmospheric air. HKS permanently depolarizes the membrane and inactivates sodium channels after an initial contraction. Single muscle fibre segments were isolated and tied to two silk micro knots. The fibre was transferred in HKS to the MyoRobot and mounted between the pins of a force transducer (FT) and a voice coil (VC) actuator (a linear actuator working with the principle of Lorentz force) by tightening the knots and lowering it in the idle well underneath (the idle well contains a 0.01 v/v ratio of HR in LR solution to buffer Ca2+ ions and keep the preparation relaxed, see Section 4.3 Bioactive Solutions). Prior to any physiological recording, the sarcolemma was permeabilized by exposure to a 0.01% (w/v) saponin solution (20 s) for diffusional access to the myoplasm.
4.2. Study Design
To study LDA, the sarcomere length (SL) must be precisely controlled; for this purpose, the MyoRobot’s integrated optics system was used. Initially, SL was adjusted to 2.2 µm (considered as the optimum SL overlap in active LDA [54]) by driving the VC at 1 µm/s to elongate the fibre (Figure 3A). Once reached, the fibre was subjected to a force–pCa recording (active biomechanics, Figure 3B) and a stress–strain relationship recording (passive biomechanics, Figure 3C). Subsequently, the same single fibre was stretched to 2.5 µm SL (considered as the beginning of the descending limb [41]), and the procedure repeated until an SL of 3.0 µm (considered as end of the descending limb of active LDA [41]) was reached. In case of fibre rupture, which may occur when the fibre is stretched beyond 4.0 µm SL due to titin disintegration [55], the fibre was excluded from analysis and the process was re-initiated at 2.0 µm SL with a new muscle fibre. A single experiment day either investigated muscle fibre exposed to bioactive solutions without or with levosimendan, meaning that experiments with or without levosimendan took place at different days with different single muscle fibres (unpaired data). The recording sequence carried out on each respective day is displayed in Figure 3D. This design allowed us to conduct a complete pCa–force recording followed by a stress–strain recording on the same single fibre covering four distinct sarcomere lengths before moving to the next single fibre. This allowed for self-control experiments that consider the parameter ‘sarcomere length’.
4.3. Bioactive Solutions
Solutions for fibre manipulation (calcium activation or relaxation) were used and composed as described in [39]. Their summarized physiological purposes are the following:
- Ringer’s solution [56]: isotonic electrolyte solution for tissue dissection, storage, and transfer (before single-fibre isolation);
- High-potassium solution (HKS): permanently depolarises the membrane potential and keeps the muscle preparation in a relaxed, inexcitable state;
- High-activating solution (HA): EGTA-buffered Ca2+-rich solution to evoke skinned single muscle fibre maximum contraction at pCa = 4.92;
- High-relaxing solution (HR): calcium-free, EGTA-buffered solution to relax the permeabilized muscle fibre (pCa = 9);
- Low-relaxing solution (LR): calcium-free, HDTA-buffered solution applied after HR exposure to exchange the strong EGTA calcium buffer by the mild buffering agent HDTA.
For calcium sensitivity recordings, HA and HR solutions were mixed to achieve defined total and free calcium concentrations. Mixing ratios were calculated in MAXCHELATOR (developed by Chriss Paton, somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/) to achieve the distinct pCa steps shown in Figure 3. All solutions were supplemented with 10 µL of creatine kinase to a total of 1 mL in the rack’s wells. On experiment days investigating the potential effects of levosimendan, the drug was added to each well to a total concentration of 100 µM.
4.4. Recording Procedure and Data Analysis
All recordings were performed with our robotized MyoRobot biomechatronics system [32] at 23 °C room temperature with no external oxygenation in the MyoRobot’s wells. Data were sampled at 100 Hz while two linear stages sequentially immersed the single muscle fibre in the bioactive solutions of the rack. A VC was used to stretch the preparation to 140% of its resting length (typically: L0 2 mm) at 1 µm/s. Data were stored in a LabVIEW (Laboratory Virtual Instrument Engineering Workbench, National Instruments, TX, USA) internal data format (TDMS) and text format for automated processing using scripts written in RStudio (RStudio Inc., rstudio.com, Boston, MA, USA [57], RRID:SCR_000432) as described in [31].
To assess the calcium sensitivity of the contractile apparatus in the absence of levosimendan (CTRL study group), a single muscle fibre was sequentially exposed to solutions with increasing calcium concentration (decreasing pCa, pCa = —log10[Ca2+]). The same recording was also carried out on another preparation in the same solutions but supplemented with 100 µM levosimendan (in vitro levosimendan exposed study group). The exact values are displayed in Figure 3B. For each pCa level, the plateau force was analysed, while the steady-state force at pCa = 4.92 was considered as maximum calcium-saturated force (Figure 1A). Normalizing this to the fibre’s cross-sectional area yielded the maximum calcium-saturated stress. To compute the pCa50 value, a marker for calcium sensitivity, extracted plateau forces were normalized to maximum calcium-induced force. A Hill fit according to , with x being pCa, and y being force [58], was fitted to the data (Figure 1B). The optimized c value reflects the Ca50 value, and the —log10(c) equals the pCa50 value.
A stress–strain recording consisted of stretching the single muscle fibre to 140% of its resting length under relaxing and calcium-free conditions. Those experiments were also independently carried out in levosimendan-free (CTRL study group) and levosimendan supplemented solutions (in vitro levosimendan-exposed study group). Commencing at a median SL of 2.2 µm, a stretch by 40% resulted in a median SL of 3.08 at 140% L0. These values are associated with a margin of error (roughly ±0.1 µm) arising from a Gaussian distribution of all SLs within a field-of-view around the median SL. Such Gaussian distributions of SLs are thoroughly reported in the literature [41,59,60] and extensively discussed in a previous publication [61]. Once 140% resting length was reached, maximum passive axial restoration force was analysed and normalized to the single-fibre cross-sectional area to yield restoration stress. A linear fit applied to the resulting stress–strain curve allowed the extraction of the fibre’s passive axial elastic modulus (Young’s modulus: , with being the tensile stress at a specific elongation, and being the tensile strain).
Data were plotted using Sigma Plot (Systat Software Inc., www.systat.de/SigmaPlot_Produktseite, accessed on 10 April 2024, San Jose, CA, USA, RRID:SCR_003210). All data were analysed for normality by performing a Shapiro–Wilk test. If a normal distribution was not given, a rank-based ANOVA test was conducted and followed by a post-hoc analysis according to Dunn’s median comparison. If data were normally distributed, a one-way ANOVA test following post-hoc analysis according to Bonferroni’s method was applied. If statistical tests confirmed any significant difference, this was indicated by vertical bars in all plots, including an asterisk to indicate the magnitude as follows: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005. An absence of such indicators is equivalent to ‘no significant difference’.
5. Conclusions
Our investigation delved into the intricate interplay between LDA and levosimendan in the context of skeletal muscle biomechanics. Through a comprehensive investigation, we confirmed that levosimendan’s effects extend beyond its known impact on cardiac muscle. Our findings demonstrate that acute levosimendan exposition induces notable shifts in the descending limb of active contractility, a phenomenon previously unreported in skeletal muscle. Furthermore, we revealed a significant enhancement in axial fibre stiffness and restoration stress under passive stretch conditions in the presence of levosimendan, opening new avenues for understanding the roles of titin and sarcomere mechanics. The identification of levosimendan’s differential effects on cardiac and skeletal muscle underscores the intricate regulatory mechanisms governing contractility. This knowledge may have significant implications for therapeutic strategies targeting muscle-related disorders and conditions. The pronounced modifications in passive biomechanics suggest a potential role of levosimendan in influencing tissue stability and mechanical properties, prompting further investigation into its mechanotransduction pathways and implications for muscle health. However, it must be mentioned that this study involved acute exposure to levosimendan. As such, we cannot conclude on the potential effects of in vivo metabolism of the drug and its potential influence on regulating muscle homeostasis and morphology.
Author Contributions
Conceptualization, M.H. and L.K.; methodology, M.H., M.M. and L.K.; software, M.H.; validation, M.H.; formal analysis, M.H. and M.M.; investigation, M.H., M.M., L.K. and M.E.V.; resources, O.F.; data curation, M.H. and P.R.; writing—original draft preparation, M.H.; writing—review and editing, P.R., L.K., M.E.V. and O.F.; visualization, M.H.; supervision, M.H. and O.F.; project administration, M.M.; funding acquisition, P.R. and O.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Erlangen Graduate School in Advanced Optical Technologies (SAOT, graduate school GSC 80, Deutsche Forschungsgemeinschaft).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.H.
Acknowledgments
We acknowledge our neighbouring institutes and co-workers Susanne Sauer and Anette Kuhn for their efforts to reduce animal numbers by encouraging a tissue-sharing collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| cTnC | Cardiac troponin C |
| EGTA | Ethylene glycol-bis(-aminoethyl ether)-N,N,N’,N’-tetraacetic acid |
| EDL | M. extensor digitorum longus |
| FT | Force transducer |
| HA | High-activating solution |
| HDTA | Hexamethylene-diamine tetraacetate |
| HKS | High-potassium solution |
| HR | High-relaxing solution |
| L0 | Resting length |
| LDA | Length dependent activation |
| LR | Low-relaxing solution |
| PDMS | Polydimethylsiloxane |
| RyR1 | Ryanodine 1 Receptor |
| SR | Sarcoplasmic reticulum |
| sTnC | Skeletal muscle troponin C |
| SL | Sarcomere length |
| VC | Voice coil |
References
- Follath, F.; Cleland, J.G.F.; Just, H.; Papp, J.G.Y.; Scholz, H.; Peuhkurinen, K.; Harjola, V.P.; Mitrovic, V.; Abdalla, M.; Sandell, E.P.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet 2002, 360, 196–202. [Google Scholar] [CrossRef]
- Heringlake, M.; Alvarez, J.; Bettex, D.; Bouchez, S.; Fruhwald, S.; Girardis, M.; Grossini, E.; Guarracino, F.; Herpain, A.; Toller, W.; et al. An update on levosimendan in acute cardiac care: Applications and recommendations for optimal efficacy and safety. Expert Rev. Cardiovasc. Ther. 2021, 19, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Pollesello, P.; Ovaska, M.; Kaivola, J.; Tilgmann, C.; Lundström, K.; Kalkkinen, N.; Ulmanen, I.; Nissinen, E.; Taskinen, J. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J. Biol. Chem. 1994, 269, 28584–28590. [Google Scholar] [CrossRef] [PubMed]
- Toller, W.G.; Stranz, C. Levosimendan, a new inotropic and vasodilator agent. Anesthesiology 2006, 104, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Lebrin, M.; Vaccaro, A.; Senard, J.M.; Despas, F. Pharmacology of levosimendan: Inotropic, vasodilatory and cardioprotective effects. J. Clin. Pharm. Ther. 2013, 38, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Antila, S.; Sundberg, S.; Lehtonen, L.A. Clinical pharmacology of levosimendan. Clin. Pharmacokinet. 2007, 46, 535–552. [Google Scholar] [CrossRef]
- Segreti, J.A.; Marsh, K.C.; Polakowski, J.S.; Fryer, R.M. Evoked changes in cardiovascular function in rats by infusion of levosimendan, OR-1896 (R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)acetamide, OR-1855 (R)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one, dobutamine, and milrinone: Comparative effects on peripheral resistance, cardiac output, dP/dt, pulse rate, and blood pressure. J. Pharmacol. Exp. Ther. 2008, 325, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Louhelainen, M.; Merasto, S.; Finckenberg, P.; Vahtola, E.; Kaheinen, P.; Levijoki, J.; Mervaala, E. Effects of the calcium sensitizer OR-1896, a metabolite of levosimendan, on post-infarct heart failure and cardiac remodelling in diabetic Goto-Kakizaki rats. Br. J. Pharmacol. 2010, 160, 142–152. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Heunks, L.M.A.; Papp, Z.; Pollesello, P. Potential of the Cardiovascular Drug Levosimendan in the Management of Amyotrophic Lateral Sclerosis: An Overview of a Working Hypothesis. J. Cardiovasc. Pharmacol. 2019, 74, 389–399. [Google Scholar] [CrossRef]
- Wang, D.; Song, M.; Shen, L.F.; Han, L.; Zhu, P.; Jia, X.; Shang, G.K.; Cao, Y.; Zhang, W.; Zhong, M.; et al. Exercise Capacity Is Improved by Levosimendan in Heart Failure and Sarcopenia via Alleviation of Apoptosis of Skeletal Muscle. Front. Physiol. 2021, 12, 786895. [Google Scholar] [CrossRef]
- Fentzke, R.C.; Buck, S.H.; Patel, J.R.; Lin, H.; Wolska, B.M.; Stojanovic, M.O.; Martin, A.F.; Solaro, R.J.; Moss, R.L.; Leiden, J.M. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J. Physiol. 1999, 517 Pt 1, 143–157. [Google Scholar] [CrossRef]
- Kazmierczak, K.; Liang, J.; Yuan, C.C.; Yadav, S.; Sitbon, Y.H.; Walz, K.; Ma, W.; Irving, T.C.; Cheah, J.X.; Gomes, A.V.; et al. Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 3152–3166. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lamb, G.D.; Murphy, R.M. Changes in contractile and metabolic parameters of skeletal muscle as rats age from 3 to 12 months. J. Muscle Res. Cell Motil. 2017, 38, 405–420. [Google Scholar] [CrossRef]
- van Hees, H.W.H.; Andrade Acuña, G.; Linkels, M.; Dekhuijzen, P.N.R.; Heunks, L.M.A. Levosimendan improves calcium sensitivity of diaphragm muscle fibres from a rat model of heart failure. Br. J. Pharmacol. 2011, 162, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Altenberger, J.; Gustafsson, F.; Harjola, V.P.; Karason, K.; Kindgen-Milles, D.; Kivikko, M.; Malfatto, G.; Papp, Z.; Parissis, J.; Pollesello, P.; et al. Levosimendan in Acute and Advanced Heart Failure: An Appraisal of the Clinical Database and Evaluation of Its Therapeutic Applications. J. Cardiovasc. Pharmacol. 2018, 71, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Farruggio, S.; Pierelli, D.; Bolzani, V.; Rossi, L.; Pollesello, P.; Monaco, C. Levosimendan Improves Oxidative Balance in Cardiogenic Shock/Low Cardiac Output Patients. J. Clin. Med. 2020, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Bowman, P.; Haikala, H.; Paul, R.J. Levosimendan, a calcium sensitizer in cardiac muscle, induces relaxation in coronary smooth muscle through calcium desensitization. J. Pharmacol. Exp. Ther. 1999, 288, 316–325. [Google Scholar] [PubMed]
- Friedrich, O.; Hund, E.; Weber, C.; Hacke, W.; Fink, R.H.A. Critical illness myopathy serum fractions affect membrane excitability and intracellular calcium release in mammalian skeletal muscle. J. Neurol. 2004, 251, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef]
- Pollmann, C.; Haug, M.; Reischl, B.; Prölß, G.; Pöschel, T.; Rupitsch, S.J.; Clemen, C.S.; Schröder, R.; Friedrich, O. Growing Old Too Early: Skeletal Muscle Single Fiber Biomechanics in Ageing R349P Desmin Knock-in Mice Using the MyoRobot Technology. Int. J. Mol. Sci. 2020, 21, 5501. [Google Scholar] [CrossRef]
- de Winter, J.M.; Joureau, B.; Sequeira, V.; Clarke, N.F.; van der Velden, J.; Stienen, G.J.; Granzier, H.; Beggs, A.H.; Ottenheijm, C.A. Effect of levosimendan on the contractility of muscle fibers from nemaline myopathy patients with mutations in the nebulin gene. Skelet. Muscle 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Tsiros, C.; Sundar, S.L.; Athar, H.; Moore, J.; Nelson, B.; Gage, M.J.; Nishikawa, K. Calcium increases titin N2A binding to F-actin and regulated thin filaments. Sci. Rep. 2018, 8, 14575. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Dutta, S.; DuVall, M.; Nelson, B.; Gage, M.J.; Monroy, J.A. Calcium-dependent titin-thin filament interactions in muscle: Observations and theory. J. Muscle Res. Cell Motil. 2020, 41, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A. Titin Gene and Protein Functions in Passive and Active Muscle. Annu. Rev. Physiol. 2018, 80, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Smith, S.H. Calcium, cross-bridges, and the Frank-Starling relationship. News in physiological sciences: An international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. Int. Union Physiol. 2001, 16, 5–10. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.S.; Moss, R.L. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ. Res. 1995, 77, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.; Wu, Y.; Bekyarova, T.; Farman, G.P.; Fukuda, N.; Granzier, H. Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys. J. 2011, 100, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Inoue, T.; Yamane, M.; Terui, T.; Kobirumaki, F.; Ohtsuki, I.; Ishiwata, S.; Kurihara, S. Sarcomere length-dependent Ca2+ activation in skinned rabbit psoas muscle fibers: Coordinated regulation of thin filament cooperative activation and passive force. J. Physiol. Sci. JPS 2011, 61, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, O.; Wu, Y.; Irving, T.C.; Granzier, H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ. Res. 2001, 88, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lang, P.; Linke, W.A. Titin stiffness modifies the force-generating region of muscle sarcomeres. Sci. Rep. 2016, 6, 24492. [Google Scholar] [CrossRef]
- Haug, M.; Reischl, B.; Prölß, G.; Pollmann, C.; Buckert, T.; Keidel, C.; Schürmann, S.; Hock, M.; Rupitsch, S.; Heckel, M.; et al. The MyoRobot: A novel automated biomechatronics system to assess voltage/Ca2+ biosensors and active/passive biomechanics in muscle and biomaterials. Biosens. Bioelectron. 2018, 102, 589–599. [Google Scholar] [CrossRef]
- Haug, M.; Meyer, C.; Reischl, B.; Prölß, G.; Nübler, S.; Schürmann, S.; Schneidereit, D.; Heckel, M.; Pöschel, T.; Rupitsch, S.J.; et al. MyoRobot 2.0: An advanced biomechatronics platform for automated, environmentally controlled skeletal muscle single fiber biomechanics assessment employing inbuilt real-time optical imaging. Biosens. Bioelectron. 2019, 138, 111284. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yamazaki, M.; Shen, Q.W.; Swartz, D.R. Differences between cardiac and skeletal troponin interaction with the thin filament probed by troponin exchange in skeletal myofibrils. Biophys. J. 2009, 97, 183–194. [Google Scholar] [CrossRef]
- Lynch, G.S.; Cuffe, S.A.; Plant, D.R.; Gregorevic, P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul. Disord. 2001, 11, 260–268. [Google Scholar] [CrossRef]
- Cheng, C.S.; Davis, B.N.J.; Madden, L.; Bursac, N.; Truskey, G.A. Physiology and metabolism of tissue-engineered skeletal muscle. Exp. Biol. Med. 2014, 239, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.; Johannsen, S.; Isbary, S.; Türkmeneli, I.; Roewer, N. In vitro effects of levosimendan on muscle of malignant hyperthermia susceptible and non-susceptible swine. BMC Anesthesiol. 2018, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Mijailovich, S.M.; Stojanovic, B.; Nedic, D.; Svicevic, M.; Geeves, M.A.; Irving, T.C.; Granzier, H.L. Nebulin and titin modulate cross-bridge cycling and length-dependent calcium sensitivity. J. Gen. Physiol. 2019, 151, 680–704. [Google Scholar] [CrossRef]
- McDonald, K.S.; Field, L.J.; Parmacek, M.S.; Soonpaa, M.; Leiden, J.M.; Moss, R.L. Length dependence of Ca2+ sensitivity of tension in mouse cardiac myocytes expressing skeletal troponin C. J. Physiol. 1995, 483, 131–139. [Google Scholar] [CrossRef]
- Haug, M.; Meyer, C.; Reischl, B.; Prölß, G.; Vetter, K.; Iberl, J.; Nübler, S.; Schürmann, S.; Rupitsch, S.J.; Heckel, M.; et al. The MyoRobot technology discloses a premature biomechanical decay of skeletal muscle fiber bundles derived from R349P desminopathy mice. Sci. Rep. 2019, 9, 108. [Google Scholar] [CrossRef]
- Michael, M.; Kovbasyuk, L.; Ritter, P.; Reid, M.B.; Friedrich, O.; Haug, M. Redox Balance Differentially Affects Biomechanics in Permeabilized Single Muscle Fibres-Active and Passive Force Assessments with the Myorobot. Cells 2022, 11, 3715. [Google Scholar] [CrossRef]
- Moo, E.K.; Fortuna, R.; Sibole, S.C.; Abusara, Z.; Herzog, W. In vivo Sarcomere Lengths and Sarcomere Elongations Are Not Uniform across an Intact Muscle. Front. Physiol. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Rassier, D.E.; MacIntosh, B.R.; Herzog, W. Length dependence of active force production in skeletal muscle. J. Appl. Physiol. 1999, 86, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 221, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Masutani, S.; Cheng, H.J.; Tachibana, H.; Little, W.C.; Cheng, C.P. Levosimendan restores the positive force-frequency relation in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H488–H496. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W. Skeletal muscle mechanics: Questions, problems and possible solutions. J. Neuroeng. Rehabil. 2017, 14, 98. [Google Scholar] [CrossRef]
- Powers, K.; Nishikawa, K.; Joumaa, V.; Herzog, W. Decreased force enhancement in skeletal muscle sarcomeres with a deletion in titin. J. Exp. Biol. 2016, 219, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jang, H.C.; Koo, K.H.; Kim, H.Y.; Lim, J.Y. Mechanical Properties of Single Muscle Fibers: Understanding Poor Muscle Quality in Older Adults with Diabetes. Ann. Geriatr. Med. Res. 2020, 24, 267–273. [Google Scholar] [CrossRef]
- Noonan, A.M.; Mazara, N.; Zwambag, D.P.; Weersink, E.; Power, G.A.; Brown, S.H.M. Age-related changes in human single muscle fibre passive elastic properties are sarcomere length dependent. Exp. Gerontol. 2020, 137, 110968. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Ranatunga, K.W. The viscous, viscoelastic and elastic characteristics of resting fast and slow mammalian (rat) muscle fibres. J. Physiol. 1996, 496, 827–836. [Google Scholar] [CrossRef]
- Herzog, W. Why are muscles strong, and why do they require little energy in eccentric action? J. Sport Health Sci. 2018, 7, 255–264. [Google Scholar] [CrossRef]
- Leonard, T.R.; Herzog, W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am. J. Physiol. Cell Physiol. 2010, 299, C14–C20. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Moody, M.R. Dimethyl sulfoxide depresses skeletal muscle contractility. J. Appl. Physiol. 1994, 76, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Kipka, H.; Tomasi, R.; Hübner, M.; Liebchen, U.; Hagl, C.; Wanner, K.T.; Mannell, H.; Höfner, G. Simultaneous LC-ESI-MS/MS Quantification of Levosimendan and Its Metabolites for Therapeutic Drug Monitoring of Cardiac Surgery Patients. Pharmaceutics 2022, 14, 1454. [Google Scholar] [CrossRef]
- Rassier, D.E.; MacIntosh, B.R. Sarcomere length-dependence of activity-dependent twitch potentiation in mouse skeletal muscle. BMC Physiol. 2002, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. A Solution for the Heart: The Life of Sydney Ringer (1836–1910); The Physiological Society: London, UK, 2007. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its oxygen dissociation. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Telley, I.A.; Stehle, R.; Ranatunga, K.W.; Pfitzer, G.; Stüssi, E.; Denoth, J. Dynamic behaviour of half-sarcomeres during and after stretch in activated rabbit psoas myofibrils: Sarcomere asymmetry but no ‘sarcomere popping’. J. Physiol. 2006, 573, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Moo, E.K.; Herzog, W. Single sarcomere contraction dynamics in a whole muscle. Sci. Rep. 2018, 8, 15235. [Google Scholar] [CrossRef]
- Haug, M.; Ritter, P.; Michael, M.; Reischl, B.; Schurmann, S.; Prols, G.; Friedrich, O. Structure-Function Relationships in Muscle Fibres: MyoRobot Online Assessment of Muscle Fibre Elasticity and Sarcomere Length Distributions. IEEE Trans. Biomed. Eng. 2022, 69, 148–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).