Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model
Abstract
1. Introduction
2. Results
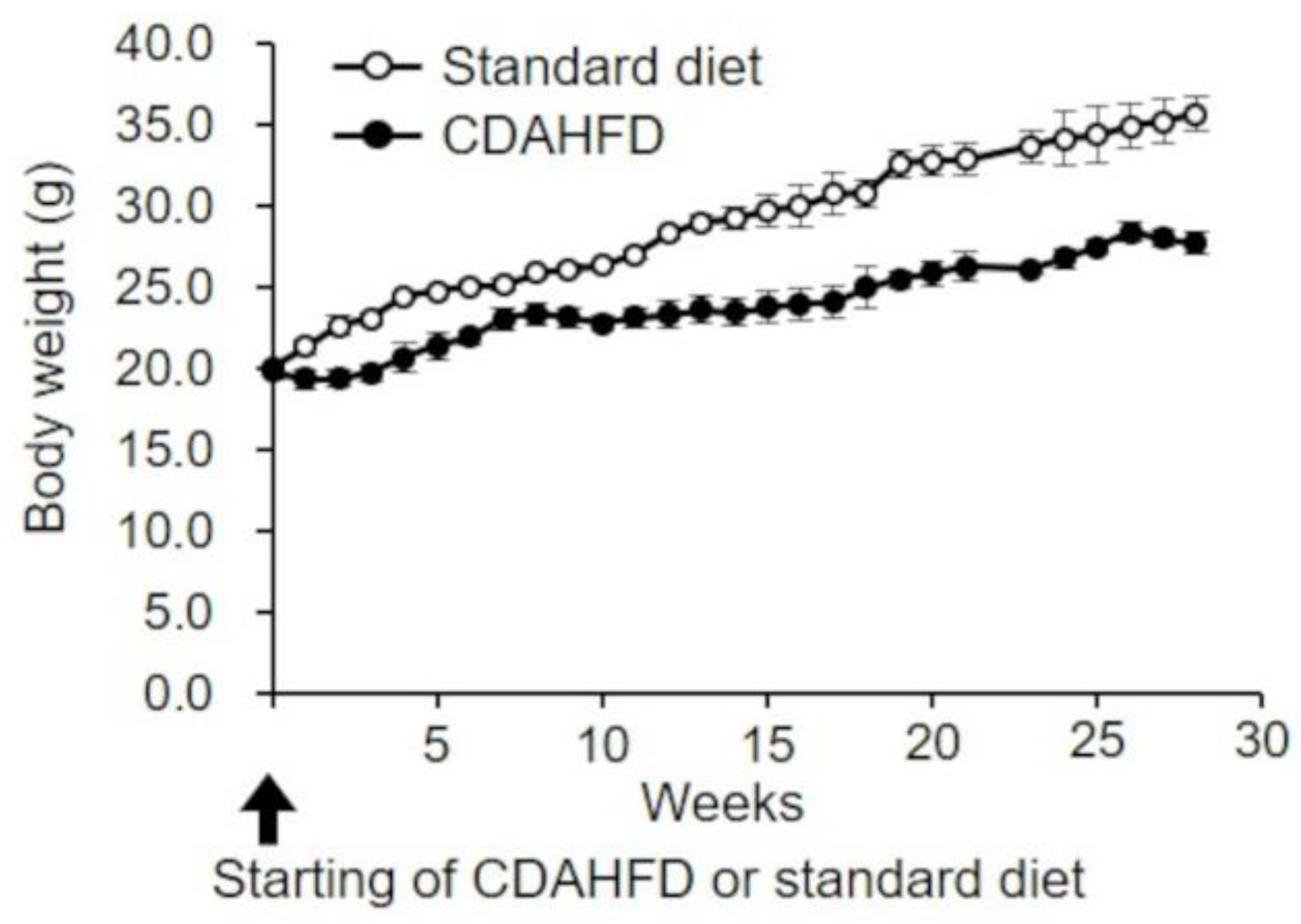
2.1. Changes in Body Weight in CDAHFD-Fed Mice
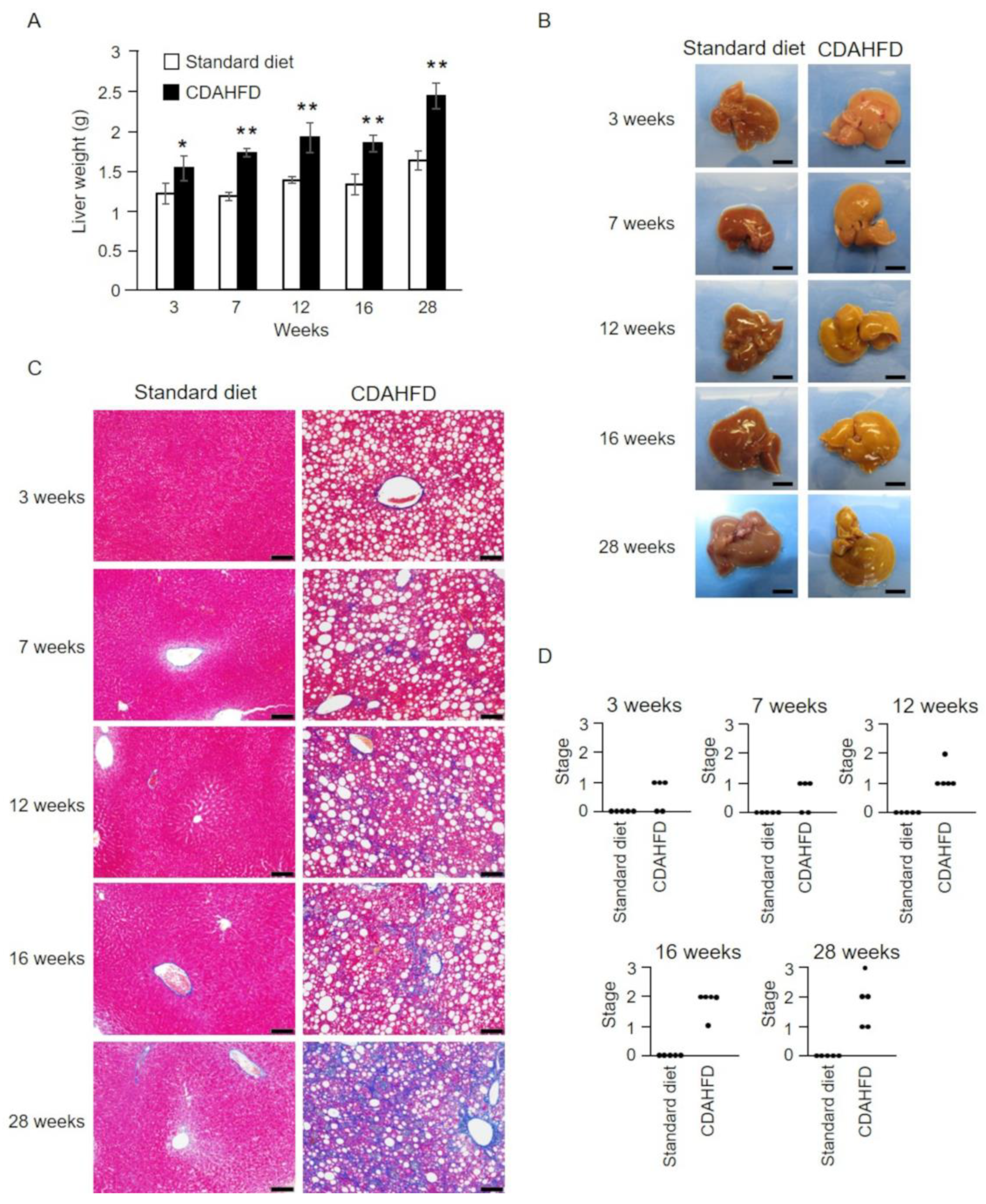
2.2. Pathophysiological Analysis of the Livers of CDAHFD-Fed Mice
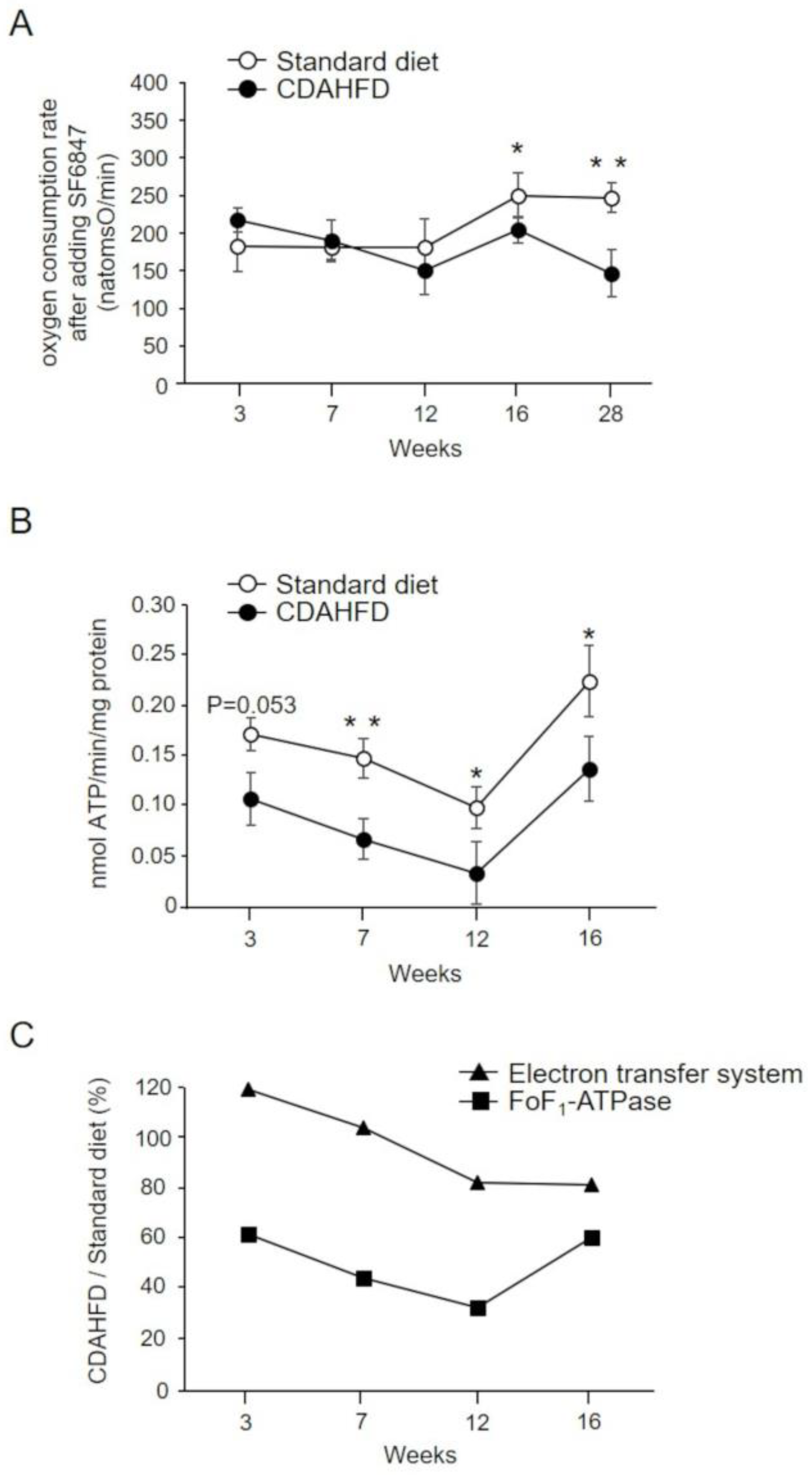
2.3. Analysis of Oxidative Phosphorylation in the Liver Mitochondria of CDAHFD-Fed Mice
2.4. Analysis of FoF1–ATPase Activity in Liver Mitochondria Isolated from CDAHFD-Fed Mice
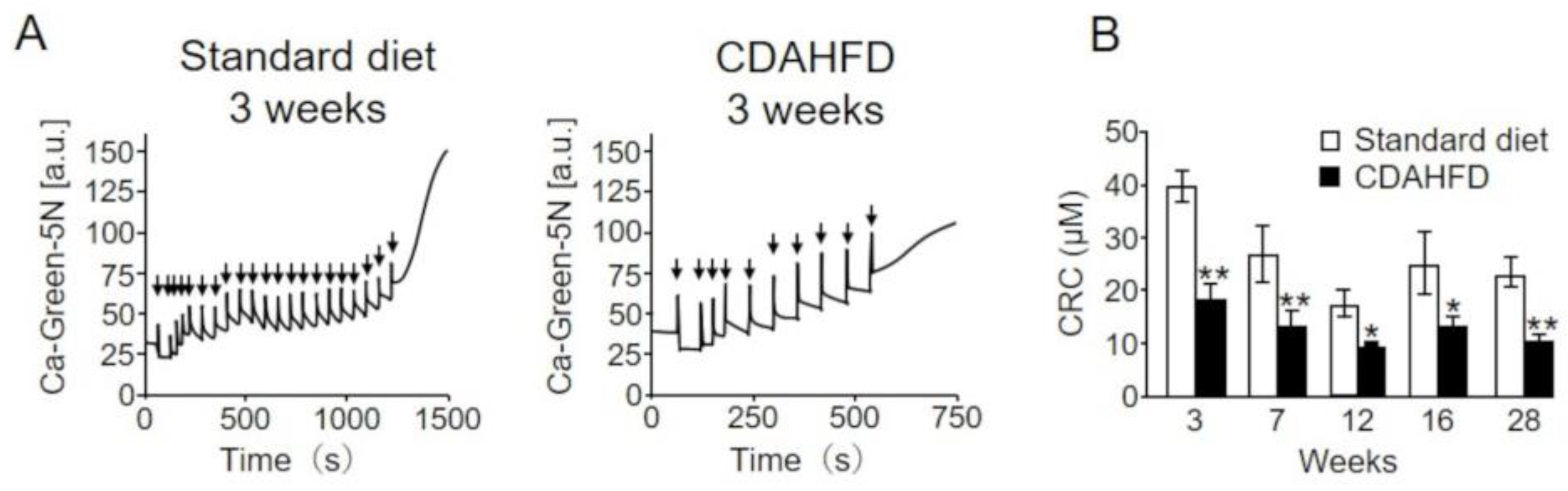
2.5. Analysis of the Calcium Retention Capacity (CRC) of Liver Mitochondria in CDAHFD-Fed Mice
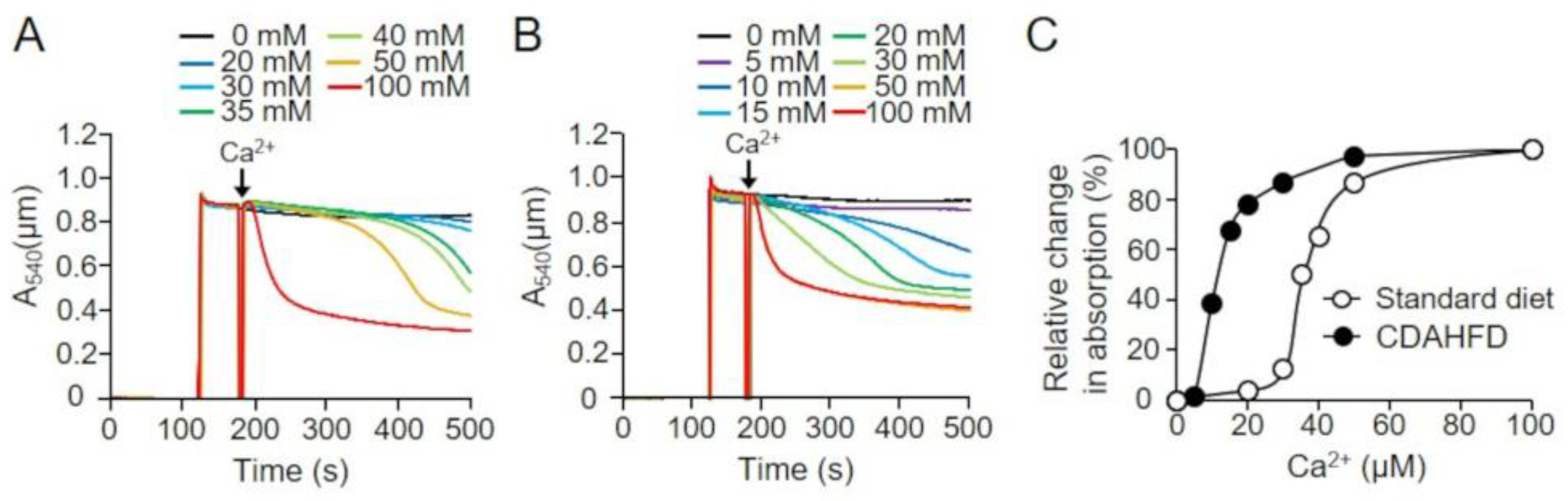
2.6. Effects of Short-Term CDAHFD Feeding on the Absorbance of Mitochondrial Suspensions
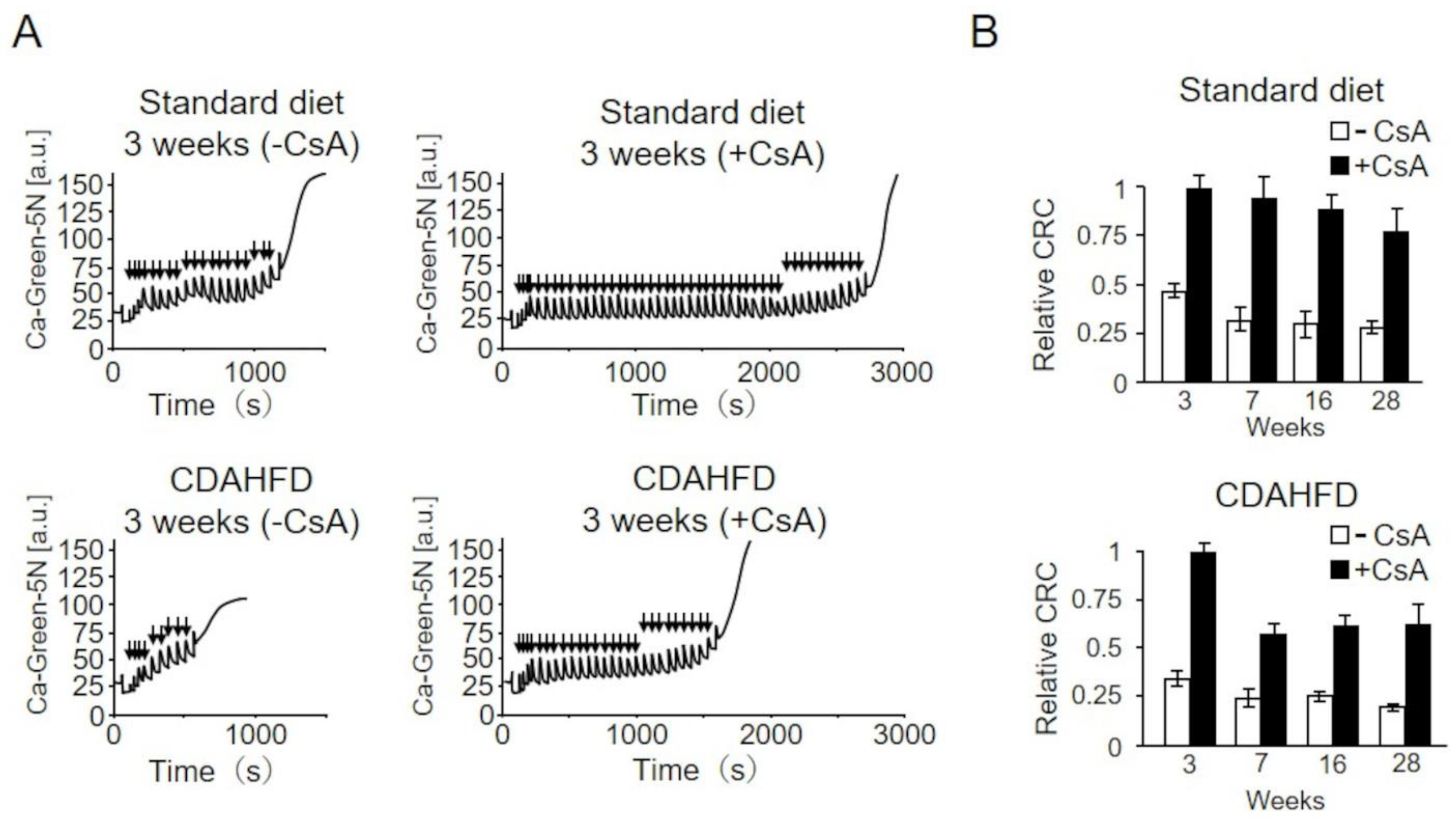
2.7. Analysis of the Effects of Cyclosporin A (CsA) on the CRC of Mitochondria from CDAHFD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurement of Body Weight
4.3. Anatomical and Histopathological Analyses of Liver Tissue
4.4. Isolation of Mouse Liver Mitochondria
4.5. Measurement of Mitochondrial Oxygen Consumption Rate
4.6. Measurement of FoF1–ATPase Activity
4.7. Mitochondrial Ca2+ Uptake Assay
4.8. Measuring the Absorbance of Mitochondrial Suspensions
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010, 26, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Rolo, A.P.; Duarte, F.V.; Simões, A.M.; Palmeira, C.M. Differential alterations in mitochondrial function induced by a choline-deficient diet: Understanding fatty liver disease progression. Mitochondrion 2008, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, Q.; Song, N.; Yan, Z.; Lin, R.; Wu, S.; Jiang, L.; Hong, S.; Xie, J.; Zhou, H.; et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020, 11, 5807. [Google Scholar] [CrossRef]
- Romestaing, C.; Piquet, M.A.; Letexier, D.; Rey, B.; Mourier, A.; Servais, S.; Belouze, M.; Rouleau, V.; Dautresme, M.; Ollivier, I.; et al. Mitochondrial adaptations to steatohepatitis induced by a methionine- and choline-deficient diet. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 110–119. [Google Scholar] [CrossRef]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Li, Y.; Zhao, G.; Guo, Z.; Luo, Y.; et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: A plausible role of adropin. Lab. Investig. 2021, 101, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Wang, H.; Xiao, F.; Ning, Q. Epoxyeicosatrienoic acids alleviate methionine-choline-deficient diet–induced non-alcoholic steatohepatitis in mice. Scand. J. Immunol. 2019, 90, e12791. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hada, N.; Sakamaki, Y.; Uno, A.; Shiga, T.; Tanaka, C.; Ito, T.; Katsume, A.; Sudoh, M. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 2013, 94, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ikawa-Yoshida, A.; Matsuo, S.; Kato, A.; Ohmori, Y.; Higashida, A.; Kaneko, E.; Matsumoto, M. Hepatocellular carcinoma in a mouse model fed a choline-deficient, L-amino acid-defined, high-fat diet. Int. J. Exp. Pathol. 2017, 98, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta Rev. Biomembr. 1995, 1241, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Bandou, S.; Kora, S.; Kitamura, S.; Inazumi, S.; Terada, H. Cationic uncouplers of oxidative phosphorylation are inducers of mitochondrial permeability transition. FEBS Lett. 1998, 428, 89–92. [Google Scholar] [CrossRef]
- Yamada, A.; Yamamoto, T.; Yoshimura, Y.; Gouda, S.; Kawashima, S.; Yamazaki, N.; Yamashita, K.; Kataoka, M.; Nagata, T.; Terada, H.; et al. Ca2+-induced permeability transition can be observed even in yeast mitochondria under optimized experimental conditions. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1486–1491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Ono, S.; Yonamine, M.; Fujita, S.-I.; Matsumoto, Y.; Aoki, K.; Nakano, T.; Tamai, S.; Yoshida, Y.; Kawakami, Y.; et al. One Week of CDAHFD Induces Steatohepatitis and Mitochondrial Dysfunction with Oxidative Stress in Liver. Int. J. Mol. Sci. 2021, 22, 5851. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Chen, X.; Boitano, A.; Swenson, L.; Opipari, A.W.; Glick, G.D. Identification and validation of the mitochondrial F1F 0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem. Biol. 2005, 12, 485–496. [Google Scholar] [CrossRef]
- Cleary, J.; Johnson, K.M.; Opipari, A.W.; Glick, G.D. Inhibition of the mitochondrial F1F0-ATPase by ligands of the peripheral benzodiazepine receptor. Bioorg. Med. Chem. Lett. 2007, 17, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, A.C.; Frazee, R.W.; Van Huis, C.; Cleary, J.; Opipari, A.W.; Glick, G.D.; Al-Hashimi, H.M. NMR studies of an immunomodulatory benzodiazepine binding to its molecular target on the mitochondrial F 1 F 0-ATPase. Biopolymers 2010, 93, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabo, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef] [PubMed]
- Guo, L. Mitochondrial ATP synthase inhibitory factor 1 interacts with the p53–cyclophilin D complex and promotes opening of the permeability transition pore. J. Biol. Chem. 2022, 298, 101858. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ford, H.C.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 3409–3414. [Google Scholar] [CrossRef] [PubMed]
- Pekson, R.; Liang, F.G.; Axelrod, J.L.; Lee, J.; Qin, D.; Wittig, A.J.H.; Paulino, V.M.; Zheng, M.; Peixoto, P.M.; Kitsis, R.N. The mitochondrial ATP synthase is a negative regulator of the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 2023, 120, e2303713120. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, F.; Liu, M.; Ning, L.; Yan, Y.; Zhang, S.; Huang, S.; Tu, C. Nobiletin mitigates hepatocytes death, liver inflammation, and fibrosis in a murine model of NASH through modulating hepatic oxidative stress and mitochondrial dysfunction. J. Nutr. Biochem. 2022, 100, 108888. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Markhard, A.L.; Kitami, T.; Kovács-Bogdán, E.; Kamer, K.J.; Udeshi, N.D.; Carr, S.A.; Chaudhuri, D.; Clapham, D.E.; Li, A.A.; et al. EMRE Is an Essential Component of the Mitochondrial Calcium Uniporter Complex. Science 2013, 342, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamagoshi, R.; Harada, K.; Kawano, M.; Minami, N.; Ido, Y.; Kuwahara, K.; Fujita, A.; Ozono, M.; Watanabe, A.; et al. Analysis of the structure and function of EMRE in a yeast expression system. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Broekemeier, K.M.; Dempsey, M.E.; Pfeiffer, D.R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989, 264, 7826–7830. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.J.; Mattiasson, G.; Månsson, R.; Karlsson, J.; Keep, M.F.; Waldmeier, P.; Ruegg, U.T.; Dumont, J.-M.; Besseghir, K.; Elmér, E. The Nonimmunosuppressive Cyclosporin Analogs NIM811 and UNIL025 Display Nanomolar Potencies on Permeability Transition in Brain-Derived Mitochondria. J. Bioenerg. Biomembr. 2004, 36, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Šileikytė, J.; Neuenswander, B.; Hedrick, M.P.; Chung, T.D.Y.; Aubé, J.; Schoenen, F.J.; Forte, M.A.; Bernardi, P. N -Phenylbenzamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem 2016, 11, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tachikawa, A.; Terauchi, S.; Yamashita, K.; Kataoka, M.; Terada, H.; Shinohara, Y. Multiple effects of DiS-C3(5) on mitochondrial structure and function. Eur. J. Biochem. 2004, 271, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Yamamoto, T.; Horiuchi, Y.; Fujiwara, K.; Gouda, S.; Yoshimura, Y.; Yamamoto, A.; Inotani, Y.; Yamashita, K.; Kitamura, S.; et al. S-15176 and its methylated derivative suppress the CsA-insensitive mitochondrial permeability transition and subsequent cytochrome c release induced by silver ion, and show weak protonophoric activity. Mol. Cell. Biochem. 2011, 358, 45–51. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Glazunov, V.V.; Korotkov, S.M. Cd2+-promoted mitochondrial permeability transition: A comparison with other heavy metals. Acta Biochim. Pol. 2004, 51, 545–551. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ito, M.; Kageyama, K.; Kuwahara, K.; Yamashita, K.; Takiguchi, Y.; Kitamura, S.; Terada, H.; Shinohara, Y. Mastoparan peptide causes mitochondrial permeability transition not by interacting with specific membrane proteins but by interacting with the phospholipid phase. FEBS J. 2014, 281, 3933–3944. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tsunoda, M.; Ozono, M.; Watanabe, A.; Kotake, K.; Hiroshima, Y.; Yamada, A.; Terada, H.; Shinohara, Y. Polyethyleneimine renders mitochondrial membranes permeable by interacting with negatively charged phospholipids in them. Arch. Biochem. Biophys. 2018, 652, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Ito, T.; Chance, B. Studies on bacterial photophosphorylation. III. A sensitive and rapid method of determination of photophosphorylation. Biochim. Biophys. Acta 1962, 59, 177–182. [Google Scholar] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, A.; Watanabe, A.; Nara, A.; Ishimaru, N.; Maeda, K.; Ido, Y.; Kotake, K.; Asano, M.; Shinohara, Y.; Yamamoto, T. Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model. Int. J. Mol. Sci. 2024, 25, 6193. https://doi.org/10.3390/ijms25116193
Yamada A, Watanabe A, Nara A, Ishimaru N, Maeda K, Ido Y, Kotake K, Asano M, Shinohara Y, Yamamoto T. Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model. International Journal of Molecular Sciences. 2024; 25(11):6193. https://doi.org/10.3390/ijms25116193
Chicago/Turabian StyleYamada, Akiko, Akira Watanabe, Atsushi Nara, Naozumi Ishimaru, Kosuke Maeda, Yusuke Ido, Kazumasa Kotake, Masatake Asano, Yasuo Shinohara, and Takenori Yamamoto. 2024. "Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model" International Journal of Molecular Sciences 25, no. 11: 6193. https://doi.org/10.3390/ijms25116193
APA StyleYamada, A., Watanabe, A., Nara, A., Ishimaru, N., Maeda, K., Ido, Y., Kotake, K., Asano, M., Shinohara, Y., & Yamamoto, T. (2024). Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model. International Journal of Molecular Sciences, 25(11), 6193. https://doi.org/10.3390/ijms25116193






