The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities
Abstract
1. Introduction
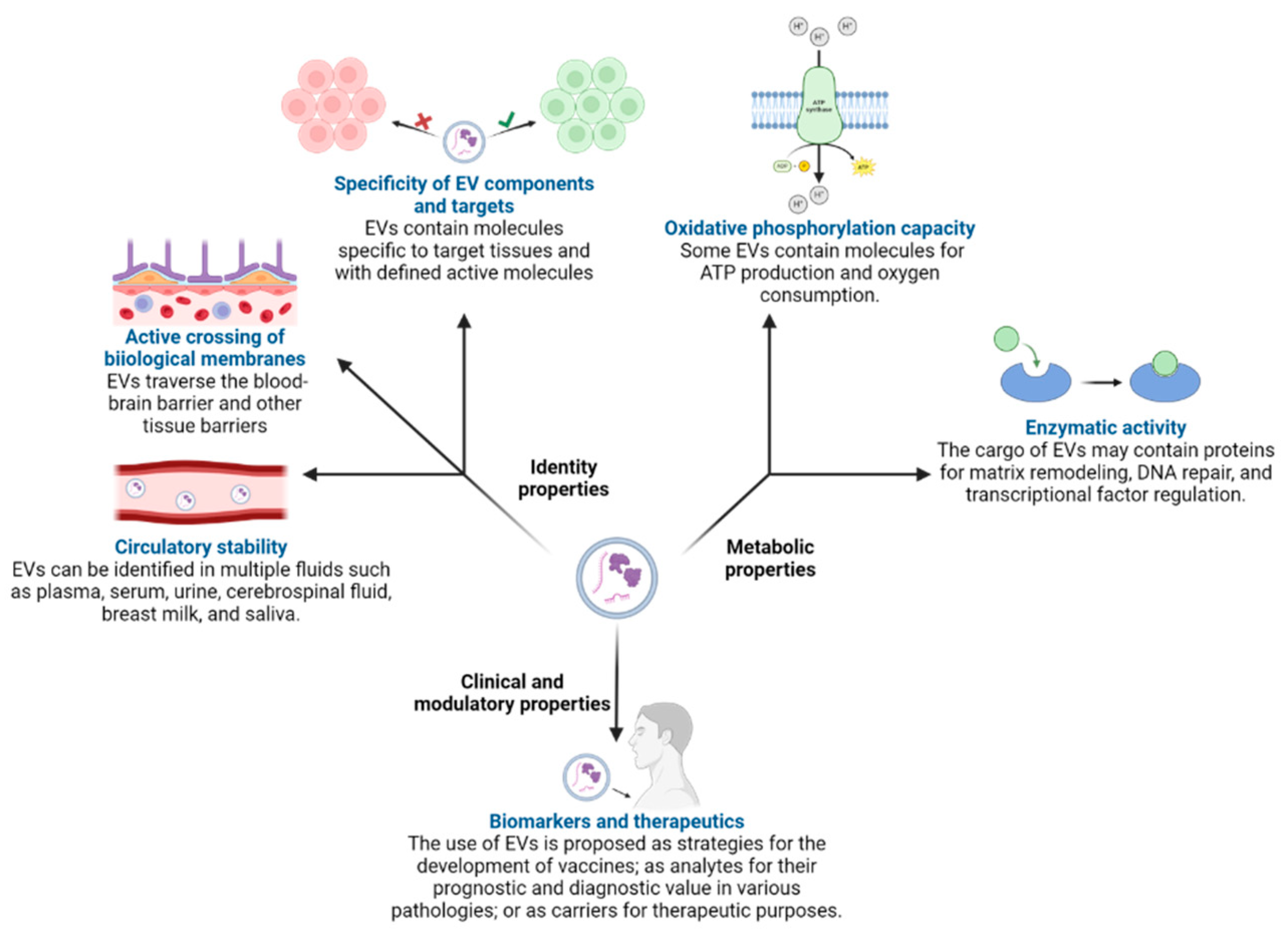
2. Extracellular Vesicles
2.1. Extracellular Vesicles Produced by Human Cells
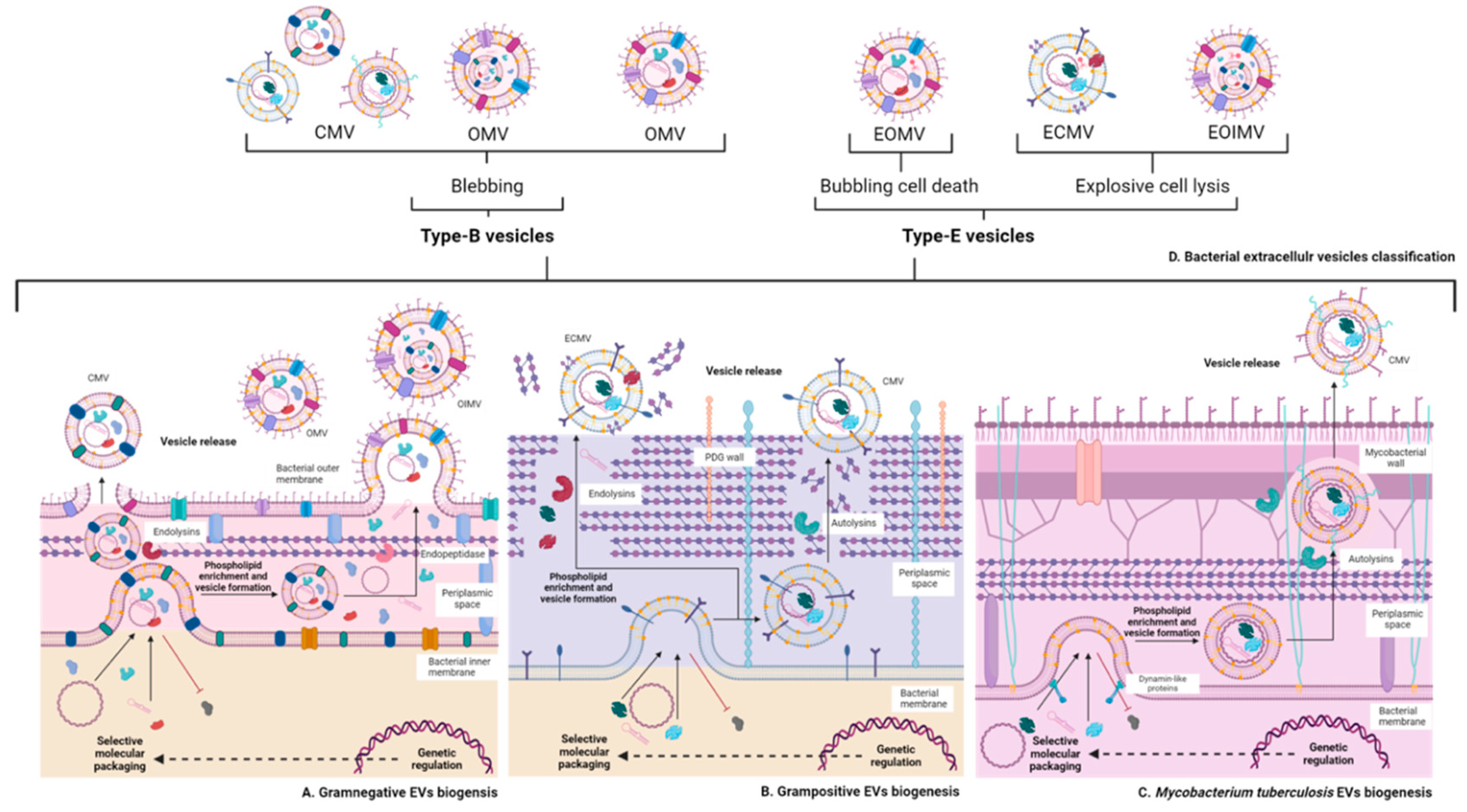
2.2. Extracellular Vesicles Produced by Bacteria
2.2.1. Vesicles of Gram-Negative Bacteria
Effect of Growth Conditions on Vesiculation of Gram-Negative Bacteria
2.2.2. Vesicles of Gram-Positive Bacteria
3. Role of Extracellular Vesicles in Host Interactions with Gram-Negative and Gram-Positive Bacteria
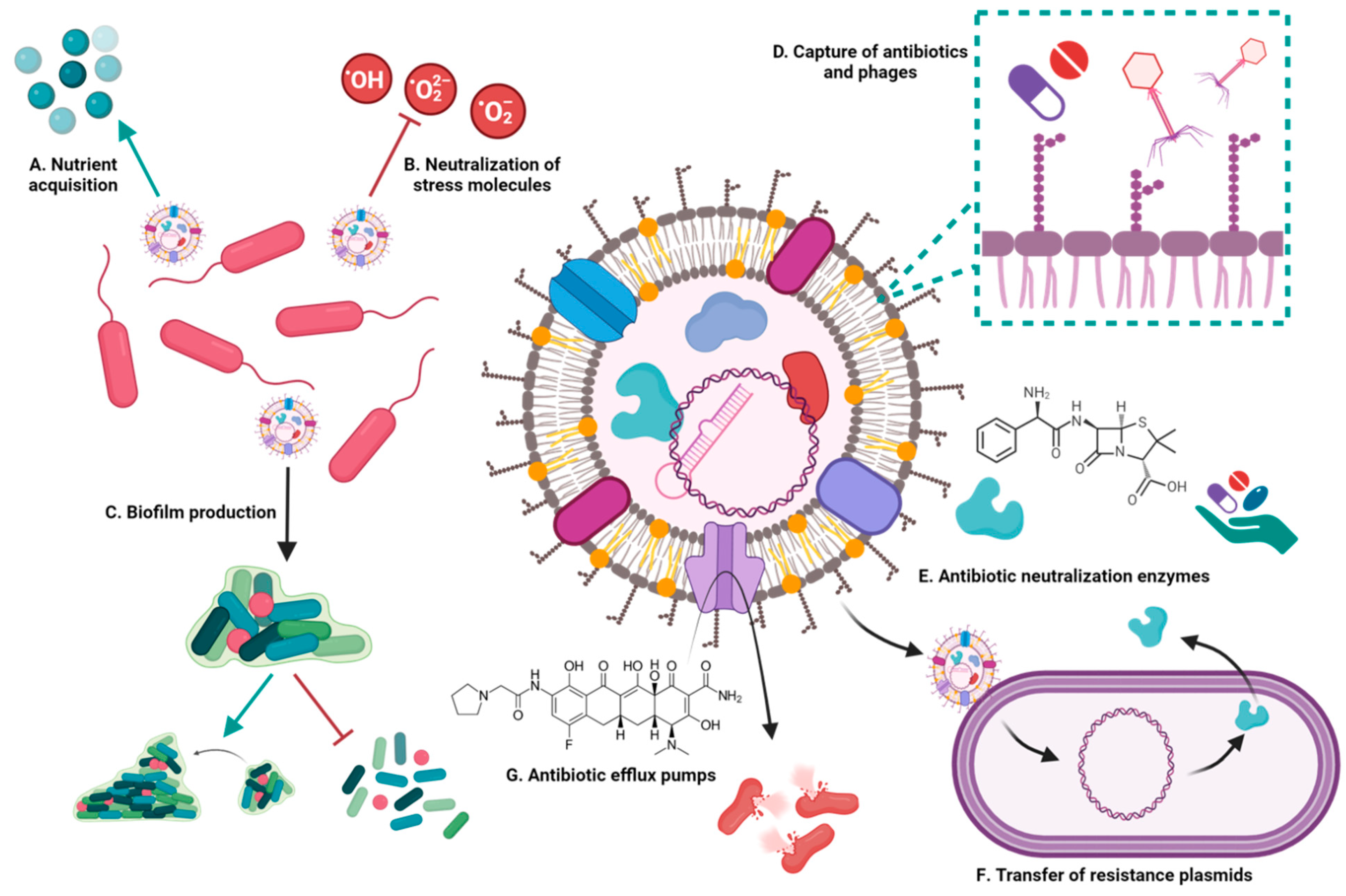
3.1. Involvement of Bacterial Extracellular Vesicles in Drug Resistance
3.1.1. Extracellular Vesicles Capture Drugs
3.1.2. Extracellular Vesicles Transfer Plasmids and Resistance Genes
3.1.3. Extracellular Vesicles Contain Molecules That Confer Resistance to Antibiotics
3.2. Extracellular Vesicles during Infection by Gram-Negative Bacteria
3.3. Extracellular Vesicles during Infection by Gram-Positive Bacteria
4. Extracellular Vesicles in Other Medically Important Bacteria
4.1. Extracellular Vesicles in Sporulating Bacteria: Bacillus and Clostridium
4.2. Extracellular Vesicles of Gram-Negative Bacteria Devoid of LPS: Treponema Pallidum
4.3. Extracellular Vesicles of Mycobacterium Tuberculosis
5. Immunological Effects of Bacterial Extracellular Vesicles
6. Therapeutic Proposals Based on Extracellular Vesicles
6.1. Treatments Based on the Use of Extracellular Vesicles
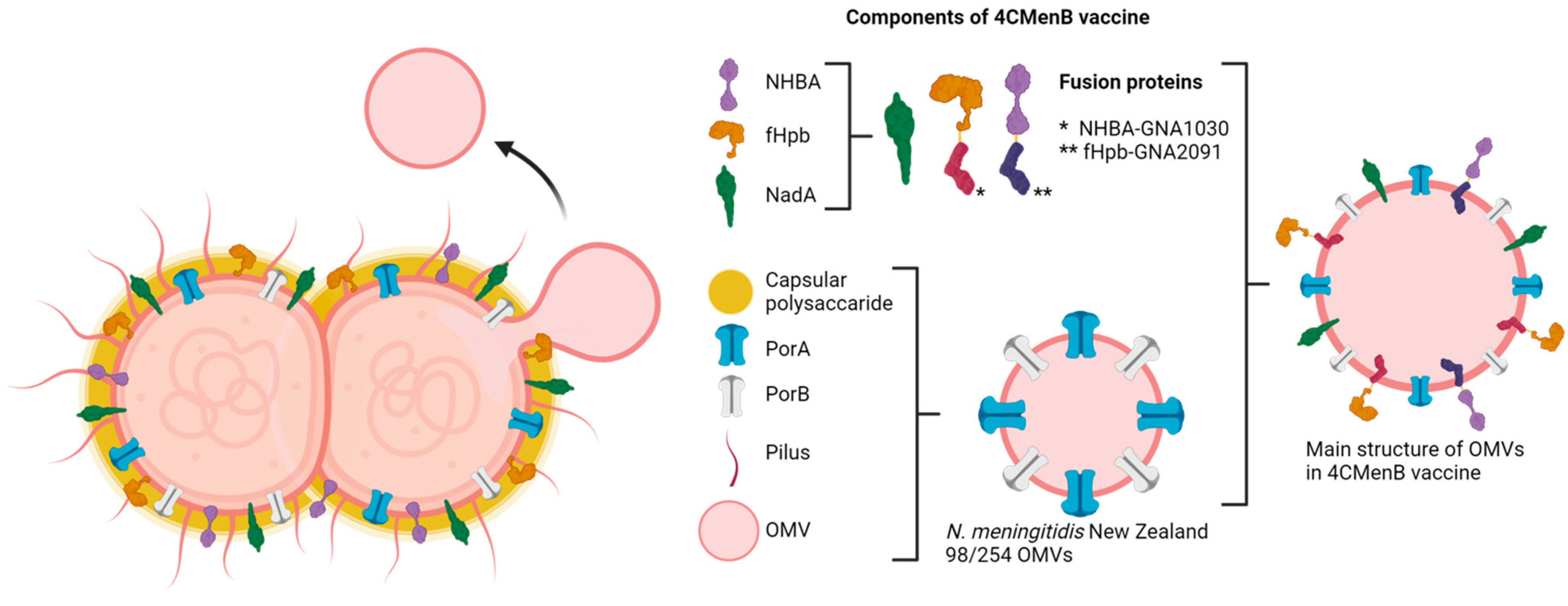
6.2. Vaccines Based on Extracellular Vesicles or Their Components
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chargaff, E. Cell Structure and the Problem of Blood Coagulation. J. Biol. Chem. 1945, 160, 351–359. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The Biological Significance of the Thromboplastic Protein of Blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M. Revisiting the Road to the Discovery of Exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Vizio, D.D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A Brief History of Nearly EV-Everything—The Rise and Rise of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.M.; Birss, A.J.; Smalley, J.W. Interaction of Extracellular Vesicles of Bacteroides gingivalis W50 with Human Polymorphonuclear Leucocytes. FEMS Microbiol. Lett. 1990, 72, 69–73. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Hardingfl Cornelis, C.; Melief, J.M.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stahl, P.D. Extracellular Vesicles: A New Communication Paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular Membrane Vesicles in the Three Domains of Life and Beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The Outer Membrane Vesicles: Secretion System Type Zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Thakur, M.; Spangler, J.R. Extracellular Vesicle Production in Gram-Positive Bacteria. Microb. Biotechnol. 2022, 15, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A.; Gupta, S.; Rodriguez, G.M.; Prados-Rosales, R. Extracellular Vesicles in the Context of Mycobacterium tuberculosis Infection. Mol. Immunol. 2021, 133, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, Z.; Liu, X.; Tyler, B.M. Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication with Microbes. Front. Microbiol. 2022, 13, 817844. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Smolarz, M.; Pietrowska, M.; Matysiak, N.; Mielańczyk, Ł.; Widłak, P. Proteome Profiling of Exosomes Purified from a Small Amount of Human Serum: The Problem of Co-Purified Serum Components. Proteomes 2019, 7, 18. [Google Scholar] [CrossRef]
- Panwar, D.; Shrivastava, D.; Bhawal, S.; Gupta, L.K.; Kumar, N.S.S.; Chintagunta, A.D. Detection of Exosomes in Various Biological Fluids Utilizing Specific Epitopes and Directed Multiple Antigenic Peptide Antibodies. Rev. Anal. Chem. 2023, 42, 20230056. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and Apoptotic Body: Disease Message and Therapeutic Target Potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Arteaga-Blanco, L.A.; Bou-Habib, D.C. The Role of Extracellular Vesicles from Human Macrophages on Host-Pathogen Interaction. Int. J. Mol. Sci. 2021, 22, 10262. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial Extracellular Vesicles and Their Novel Therapeutic Applications in Health and Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and Functions of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Charpentier, L.A.; Dolben, E.F.; Hendricks, M.R.; Hogan, D.A.; Bomberger, J.M.; Stanton, B.A. Bacterial Outer Membrane Vesicles and Immune Modulation of the Host. Membranes 2023, 13, 752. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bhagavathula, M.; Sharma, V.; Sharma, N.; Sharma, N.; Biswas, A.; Palacios, A.; Salgueiro, V.; Lavín, J.L.; Dogra, N.; et al. Dynamin-like Proteins Mediate Extracellular Vesicle Secretion in Mycobacterium tuberculosis. EMBO Rep. 2023, 24, e55593. [Google Scholar] [CrossRef] [PubMed]
- Zavan, L.; Fang, H.; Johnston, E.L.; Whitchurch, C.; Greening, D.W.; Hill, A.F.; Kaparakis-Liaskos, M. The Mechanism of Pseudomonas aeruginosa Outer Membrane Vesicle Biogenesis Determines Their Protein Composition. Proteomics 2023, 23, e2200464. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa Biofilm Membrane Vesicles Supports Multiple Mechanisms of Biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.L.; Zavan, L.; Bitto, N.J.; Petrovski, S.; Hill, A.F.; Kaparakis-Liaskos, M. Planktonic and Biofilm-Derived Pseudomonas aeruginosa Outer Membrane Vesicles Facilitate Horizontal Gene Transfer of Plasmid DNA. Microbiol. Spectr. 2023, 11, e0517922. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Lee, S.; Soper, S.A.; Mitchell, R.J. Staphylococcus aureus Extracellular Vesicles (EVs): Surface-Binding Antagonists of Biofilm Formation. Mol. Biosyst. 2017, 13, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef] [PubMed]
- Bordanaba-Florit, G.; Royo, F.; Kruglik, S.G.; Falcón-Pérez, J.M. Using Single-Vesicle Technologies to Unravel the Heterogeneity of Extracellular Vesicles. Nat. Protoc. 2021, 16, 3163–3185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1–9. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, E.J.; Lee, J.H.; Jun, S.H.; Choi, C.W.; Kim, S.I.; Kang, S.S.; Hyun, S. Klebsiella pneumoniae Secretes Outer Membrane Vesicles That Induce the Innate Immune Response. FEMS Microbiol. Lett. 2012, 331, 17–24. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Fu, H.; Wei, C.; Jin, Q. Comparative Proteomic Analysis of Outer Membrane Vesicles from Shigella flexneri under Different Culture Conditions. Biochem. Biophys. Res. Commun. 2014, 453, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Iida, K.I.; Takade, A.; Meno, Y.; Nair, G.B.; Yoshida, S.I. Release of Shiga Toxin by Membrane Vesicles in Shigella dysenteriae Serotype 1 Strains and in Vitro Effects of Antimicrobials on Toxin Production and Release. Microbiol. Immunol. 2004, 48, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Walczak, N.; Rudnicka, K. Helicobacter pylori Outer Membrane Vesicles Involvement in the Infection Development and Helicobacter pylori-Related Diseases. J. Biomed. Sci. 2018, 25, 78. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.K.; Liew, J.; Kwon, T.; Marcotte, E.M.; Whiteley, M. Role of Pseudomonas aeruginosa Peptidoglycan-Associated Outer Membrane Proteins in Vesicle Formation. J. Bacteriol. 2013, 195, 213–219. [Google Scholar] [CrossRef]
- Henriquez, T.; Falciani, C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics 2023, 12, 703. [Google Scholar] [CrossRef]
- Martora, F.; Pinto, F.; Folliero, V.; Cammarota, M.; Dell’Annunziata, F.; Squillaci, G.; Galdiero, M.; Morana, A.; Schiraldi, C.; Giovane, A.; et al. Isolation, Characterization and Analysis of pro-Inflammatory Potential of Klebsiella pneumoniae Outer Membrane Vesicles. Microb. Pathog. 2019, 136, 103719. [Google Scholar] [CrossRef]
- Imamiya, R.; Shinohara, A.; Yakura, D.; Yamaguchi, T.; Ueda, K.; Oguro, A.; Minamiyama, Y.; Ichikawa, H.; Horiguchi, Y.; Osada-Oka, M. Escherichia coli-Derived Outer Membrane Vesicles Relay Inflammatory Responses to Macrophage-Derived Exosomes. MBio 2023, 14, e0305122. [Google Scholar] [CrossRef]
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia coli Extracellular Vesicle Proteome Identifies Markers of Purity and Culture Conditions. J. Extracell. Vesicles 2019, 8, 1632099. [Google Scholar] [CrossRef]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’Cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial Membrane Vesicles Transport Their DNA Cargo into Host Cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.; Kottwitz, H.; Benjamin, J.; Ryu, J.; Jarrar, A.; Garduno, R.; Rohde, J.R. A Shigella flexneri Virulence Plasmid Encoded Factor Controls Production of Outer Membrane Vesicles. G3 Genes Genomes, Genet. 2014, 4, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.O.; Dawson, R.A.; Alsharaf, L.M.; Winter, J.A. Protective Effects of Helicobacter pylori Membrane Vesicles against Stress and Antimicrobial Agents. Microbiology 2020, 166, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Malabirade, A.; Habier, J.; Heintz-Buschart, A.; May, P.; Godet, J.; Halder, R.; Etheridge, A.; Galas, D.; Wilmes, P.; Fritz, J.V. The RNA Complement of Outer Membrane Vesicles from Salmonella enterica Serovar Typhimurium under Distinct Culture Conditions. Front. Microbiol. 2018, 9, 2015. [Google Scholar] [CrossRef] [PubMed]
- Płaczkiewicz, J.; Gieczewska, K.; Musiałowski, M.; Adamczyk-Popławska, M.; Bącal, P.; Kwiatek, A. Availability of Iron Ions Impacts Physicochemical Properties and Proteome of Outer Membrane Vesicles Released by Neisseria gonorrhoeae. Sci. Rep. 2023, 13, 18733. [Google Scholar] [CrossRef] [PubMed]
- Dhurve, G.; Madikonda, A.K.; Jagannadham, M.V.; Siddavattam, D. Outer Membrane Vesicles of Acinetobacter baumannii DS002 Are Selectively Enriched with TonB-Dependent Transporters and Play a Key Role in Iron Acquisition. Microbiol. Spectr. 2022, 10, e0029322. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Subsomwong, P.; Narita, K.; Kawai, N.; Ishiai, T.; Teng, W.; Sukchawalit, R.; Nakane, A.; Tasaka, S.; Asano, K. Differential Proteomic Analysis and Pathogenic Effects of Outer Membrane Vesicles Derived from Acinetobacter baumannii under Normoxia and Hypoxia. PLoS ONE 2023, 18, e0283109. [Google Scholar] [CrossRef] [PubMed]
- Hadadi-Fishani, M.; Najar-Peerayeh, S.; Davar Siadat, S.; Sekhavati, M.; Mohabati Mobarez, A. Isolation and Immunogenicity of Extracted Outer Membrane Vesicles from Pseudomonas aeruginosa under Antibiotics Treatment Conditions. Iran. J. Microbiol. 2021, 13, 824. [Google Scholar] [CrossRef]
- McMahon, K.J.; Castelli, M.E.; Vescovi, E.G.; Feldman, M.F. Biogenesis of Outer Membrane Vesicles in Serratia marcescens Is Thermoregulated and Can Be Induced by Activation of the Rcs Phosphorelay System. J. Bacteriol. 2012, 194, 3241–3249. [Google Scholar] [CrossRef]
- Abdi, E.; Eleanor, W.; Pamela, S.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Brendan, W.W.; et al. Campylobacter jejuni Outer Membrane Vesicles Play an Important Role in Bacterial Interactions with Human Intestinal Epithelial Cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Taylor, A.J.; Elmi, A.; Winter, J.; Liaw, J.; Grabowska, A.D.; Gundogdu, O.; Wren, B.W.; Kelly, D.J.; Dorrell, N. Sodium Taurocholate Stimulates Campylobacter jejuni Outer Membrane Vesicle Production via Down-Regulation of the Maintenance of Lipid Asymmetry Pathway. Front. Cell. Infect. Microbiol. 2019, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Mahmud, A.K.M.F.; Sandblad, L.; Fällman, M.; Wai, S.N.; Fahlgren, A. Campylobacter jejuni Bile Exposure Influences Outer Membrane Vesicles Protein Content and Bacterial Interaction with Epithelial Cells. Sci. Rep. 2018, 8, 16996. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Kim, S.I.; Ryu, S.; Yoon, H. Identification and Characterization of Outer Membrane Vesicle-Associated Proteins in Salmonella enterica Serovar Typhimurium. Infect. Immun. 2014, 82, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Majcherczyk, P.A. Proinflammatory Activity of Cell-Wall Constituents from Gram-Positive Bacteria. Scand. J. Infect. Dis. 2003, 35, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, M.J.; Park, Y.; Chung, J.; Kweon, H.S.; Kang, N.G.; Hwang, S.J.; Youn, S.H.; Hwang, B.K.; Kim, D. Visualizing Extracellular Vesicle Biogenesis in Gram-Positive Bacteria Using Super-Resolution Microscopy. BMC Biol. 2022, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, C.; Liu, Y.; Qin, X.; Liu, J. An Update on Our Understanding of Gram-Positive Bacterial Membrane Vesicles: Discovery, Functions, and Applications. Front. Cell. Infect. Microbiol. 2023, 13, 1273813. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-Pubmed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Padfield, D.; Lear, L.; Bendall, R.; Vos, M. A Comprehensive List of Bacterial Pathogens Infecting Humans. Microbiol. 2022, 168, 001269. [Google Scholar] [CrossRef]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance from Salmonella enterica Sv. Typhi ΔtolR and ΔdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef]
- Schaar, V.; Nordström, T.; Mörgelin, M.; Riesbeck, K. Moraxella catarrhalis Outer Membrane Vesicles Carry β-Lactamase and Promote Survival of Streptococcus pneumoniae and Haemophilus influenzae by Inactivating Amoxicillin. Antimicrob. Agents Chemother. 2011, 55, 3845–3853. [Google Scholar] [CrossRef]
- Yun, S.H.; Park, E.C.; Lee, S.Y.; Lee, H.; Choi, C.W.; Yi, Y.S.; Ro, H.J.; Lee, J.C.; Jun, S.; Kim, H.Y.; et al. Antibiotic Treatment Modulates Protein Components of Cytotoxic Outer Membrane Vesicles of Multidrug-Resistant Clinical Strain, Acinetobacter baumannii DU202. Clin. Proteomics 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Jasim, R.; Han, M.L.; Zhu, Y.; Hu, X.; Hussein, M.H.; Lin, Y.W.; Tony Zhou, Q.; Da Dong, C.Y.; Li, J.; Velkov, T. Lipidomic Analysis of the Outer Membrane Vesicles from Paired Polymyxin-Susceptible and -Resistant Klebsiella pneumoniae Clinical Isolates. Int. J. Mol. Sci. 2018, 19, 2356. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, S.Y.; Son, J.H.; Kim, S.I.; Lee, H.; Kim, S.; Shin, M.; Lee, J.C. Production of Membrane Vesicles by Enterococcus Faecium Cultured with or without Subinhibitory Concentrations of Antibiotics and Their Pathological Effects on Epithelial Cells. Front. Cell. Infect. Microbiol. 2019, 9, 295. [Google Scholar] [CrossRef]
- da Luz, B.S.R.; de Rezende Rodovalho, V.; Nicolas, A.; Chabelskaya, S.; Jardin, J.; Briard-Bion, V.; Le Loir, Y.; de Carvalho Azevedo, V.A.; Guédon, É. Impact of Environmental Conditions on the Protein Content of Staphylococcus aureus and Its Derived Extracellular Vesicles. Microorganisms 2022, 10, 1808. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Hirose, S.; Narita, K.; Subsomwong, P.; Kawai, N.; Sukchawalit, R.; Nakane, A. Extracellular Vesicles from Methicillin Resistant Staphylococcus aureus Stimulate Proinflammatory Cytokine Production and Trigger IgE-Mediated Hypersensitivity. Emerg. Microbes Infect. 2021, 10, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Skerniškytė, J.; Karazijaitė, E.; Lučiūnaitė, A.; Sužiedėlienė, E. Ompa Protein-Deficient Acinetobacter baumannii Outer Membrane Vesicles Trigger Reduced Inflammatory Response. Pathogens 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.S.; Kinsella, R.L.; Harding, C.M.; Feldman, M.F. The Secrets of Acinetobacter Secretion. Trends Microbiol. 2017, 25, 532–545. [Google Scholar] [CrossRef]
- Dell’annunziata, F.; Dell’aversana, C.; Doti, N.; Donadio, G.; Dal Piaz, F.; Izzo, V.; De Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wang, J.; Huang, M.; Huang, Y.; Zhang, R.; Bu, F.; Yang, B.; Chen, J.; Lin, X.; Hu, X.; et al. Outer Membrane Vesicles-Transmitted Virulence Genes Mediate the Emergence of New Antimicrobial-Resistant Hypervirulent Klebsiella pneumoniae. Emerg. Microbes Infect. 2022, 11, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, A.; Liu, P.; Wang, Z.; Jian, Z.; Chen, X.; Yan, Q.; Liang, X.; Liu, W. Outer Membrane Vesicles Transmitting BlaNDM-1 Mediate the Emergence of Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2023, 67, e0144422. [Google Scholar] [CrossRef]
- Dhital, S.; Deo, P.; Bharathwaj, M.; Horan, K.; Nickson, J.; Azad, M.; Stuart, I.; Chow, S.H.; Gunasinghe, S.D.; Bamert, R.; et al. Neisseria gonorrhoeae-Derived Outer Membrane Vesicles Package β-Lactamases to Promote Antibiotic Resistance. MicroLife 2022, 3, uqac013. [Google Scholar] [CrossRef] [PubMed]
- Lucena, A.C.R.; Ferrarini, M.G.; de Oliveira, W.K.; Marcon, B.H.; Morello, L.G.; Alves, L.R.; Faoro, H. Modulation of Klebsiella pneumoniae Outer Membrane Vesicle Protein Cargo under Antibiotic Treatment. Biomedicines 2023, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Jasim, R.; Gocol, H.; Baker, M.; Thombare, V.J.; Ziogas, J.; Purohit, A.; Rao, G.G.; Li, J.; Velkov, T. Comparative Proteomics of Outer Membrane Vesicles from Polymyxin-Susceptible and Extremely Drug-Resistant Klebsiella pneumoniae. mSphere 2023, 8, e0053722. [Google Scholar] [CrossRef]
- Yao, L.; Wei, B.; Wang, Y.; Xu, B.; Yang, M.; Chen, X.; Chen, F. A Critical Role of Outer Membrane Vesicles in Antibiotic Resistance in Carbapenem-Resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, D.; Vasudevan, A.; Wu, L.; Chen, J.; Su, Z.; Wang, S.; Xu, H. Integrative Analysis of Outer Membrane Vesicles Proteomics and Whole-Cell Transcriptome Analysis of Eravacycline Induced Acinetobacter baumannii Strains. BMC Microbiol. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.S.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Clemons, W.M. Comprehensive Proteomic Profiling of Outer Membrane Vesicles from Campylobacter jejuni. J. Proteomics 2014, 98, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori Outer Membrane Vesicles by Gastric Epithelial Cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhao, G.; Wang, Z.; Zhang, X.; Wu, M.X.; Lu, M. Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications. Adv. Sci. 2023, 10, e2301357. [Google Scholar] [CrossRef]
- Sharpe, S.W.; Kuehn, M.J.; Mason, K.M. Elicitation of Epithelial Cell-Derived Immune Effectors by Outer Membrane Vesicles of Nontypeable Haemophilus influenzae. Infect. Immun. 2011, 79, 4361–4369. [Google Scholar] [CrossRef]
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter pylori Outer Membrane Vesicles Modulate Proliferation and Interleukin-8 Production by Gastric Epithelial Cells. Infect. Immun. 2003, 71, 5670–5675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Zhang, Y.; Zhao, P.; Li, Y. Aerosolization Inhalation of Non-Typeable Haemophilus influenzae Outer Membrane Vesicles Contributing to Neutrophilic Asthma. Front. Microbiol. 2023, 14, 1226633. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, K.; Park, K.S.; Wikström, J.; Lässer, C.; Crescitelli, R.; Shelke, G.V.; Jang, S.C.; Suzuki, S.; Bandeira, E.; Olofsson, C.S.; et al. Escherichia coli Outer Membrane Vesicles Can Contribute to Sepsis Induced Cardiac Dysfunction. Sci. Rep. 2017, 7, 17434. [Google Scholar] [CrossRef]
- Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Outer Membrane Vesicles Enhance the Carcinogenic Potential of Helicobacter pylori. Carcinogenesis 2008, 29, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Oh, M.H.; Jun, S.H.; Jeon, H.; Kim, S.I.; Kim, K.; Lee, Y.C.; Lee, J.C. Outer Membrane Protein A Plays a Role in Pathogenesis of Acinetobacter nosocomialis. Virulence 2016, 7, 413–426. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Hay, I.D.; Kleifeld, O.; Costin, A.; Elgass, K.D.; Jiang, J.H.; Ramm, G.; Gabriel, K.; Dougan, G.; et al. Outer Membrane vesicles from Neisseria gonorrhoeae Target PorB to Mitochondria and Induce Apoptosis. PLoS Pathog. 2018, 14, e1006945. [Google Scholar] [CrossRef]
- Moon, D.C.; Choi, C.H.; Lee, J.H.; Choi, C.W.; Kim, H.Y.; Park, J.S.; Kim, S.I.; Lee, J.C. Acinetobacter baumannii Outer Membrane Protein a Modulates the Biogenesis of Outer Membrane Vesicles. J. Microbiol. 2012, 50, 155–160. [Google Scholar] [CrossRef]
- Jin, J.S.; Kwon, S.O.; Moon, D.C.; Gurung, M.; Lee, J.H.; Kim, S.I.; Lee, J.C. Acinetobacter baumannii Secretes Cytotoxic Outer Membrane Protein a via Outer Membrane Vesicles. PLoS ONE 2011, 6, e17027. [Google Scholar] [CrossRef]
- Cooke, A.C.; Florez, C.; Dunshee, E.B.; Lieber, A.D.; Terry, M.L.; Light, C.J.; Schertzer, J.W. Pseudomonas quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas Aeruginosa. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, S.Q.; Zhang, J.; Sun, Y.; Xie, Y.L.; Liu, Y.B.; Ma, C.C.; Jiang, B.G.; Liao, X.Y.; Li, W.F.; et al. Proteomic Analysis of Vesicle-Producing Pseudomonas aeruginosa PAO1 Exposed to X-Ray Irradiation. Front. Microbiol. 2020, 11, 558233. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, Function and Regulation of Pseudomonas aeruginosa Porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Wispelwey, B.; Hansen, E.J.; Michael Scheldl, W. Haemophilus influenzae Outer Membrane Vesicle-Induced Blood-Brain Barrier Permeability during Experimental Meningitis. Infect. Immun. 1989, 57, 2559–2562. [Google Scholar] [CrossRef]
- Mustafa, M.M.; Ramilo, O.; Syrogiannopoulos, G.A.; Olsen, K.D.; Mccracken, G.H.; Hansen, E.J. Induction of Meningeal Inflammation by Outer Membrane Vesicles of Haemophilus influenzae Type B. J. Infect. Dis. 1989, 159, 917–922. [Google Scholar] [CrossRef]
- Xie, J.; Cools, L.; Van Imschoot, G.; Van Wonterghem, E.; Pauwels, M.J.; Vlaeminck, I.; De Witte, C.; EL Andaloussi, S.; Wierda, K.; De Groef, L.; et al. Helicobacter pylori-Derived Outer Membrane Vesicles Contribute to Alzheimer’s Disease Pathogenesis via C3-C3aR Signalling. J. Extracell. Vesicles 2023, 12, e12306. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori Outer Membrane Vesicles Induce Astrocyte Reactivity through Nuclear Factor-Κappa B Activation and Cause Neuronal Damage in Vivo in a Murine Model. J. Neuroinflammation 2023, 20, 66. [Google Scholar] [CrossRef]
- Chew, Y.; Chung, H.Y.; Lin, P.Y.; Wu, D.C.; Huang, S.K.; Kao, M.C. Outer Membrane Vesicle Production by Helicobacter pylori Represents an Approach for the Delivery of Virulence Factors Caga, Vaca and Urea into Human Gastric Adenocarcinoma (Ags) Cells. Int. J. Mol. Sci. 2021, 22, 3942. [Google Scholar] [CrossRef]
- Dehinwal, R.; Cooley, D.; Rakov, A.V.; Alugupalli, A.S.; Harmon, J.; Cunrath, O.; Vallabhajosyula, P.; Bumann, D.; Schifferli, D.M.; Dehinwal, C.R. Increased Production of Outer Membrane Vesicles by Salmonella Interferes with Complement-Mediated Innate Immune Attack. Mbio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Fernández, J.; Luque-García, J.L.; García-Lidón, C.; et al. Characterization of Protective Extracellular Membrane-Derived Vesicles Produced by Streptococcus pneumoniae. J. Proteomics 2014, 106, 46–60. [Google Scholar] [CrossRef]
- Macion, A.; Wyszyńska, A.; Godlewska, R. Delivery of Toxins and Effectors by Bacterial Membrane Vesicles. Toxins 2021, 13, 845. [Google Scholar] [CrossRef]
- Wagner, T.; Joshi, B.; Janice, J.; Askarian, F.; Škalko-Basnet, N.; Hagestad, O.C.; Mekhlif, A.; Wai, S.N.; Hegstad, K.; Johannessen, M. Enterococcus Faecium Produces Membrane Vesicles Containing Virulence Factors and Antimicrobial Resistance Related Proteins. J. Proteomics 2018, 187, 28–38. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Kumar Kopparapu, P.; Deshmukh, M.; Hu, Z.; Mohammad, M.; Maugeri, M.; Götz, F.; Valadi, H.; Jin, T. Lipoproteins Are Responsible for the Pro-Inflammatory Property of Staphylococcus aureus Extracellular Vesicles. Int. J. Mol. Sci. 2021, 13, 7099. [Google Scholar] [CrossRef]
- Briaud, P.; Frey, A.; Marino, E.C.; Bastock, R.A.; Zielinski, R.E.; Wiemels, R.E.; Keogh, R.A.; Murphy, E.R.; Shaw, L.N.; Carroll, R.K. Temperature Influences the Composition and Cytotoxicity of Extracellular Vesicles in Staphylococcus aureus. mSphere 2021, 6, e0067621. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Wang, X.; Lee, J.C. Contribution of Extracellular Membrane Vesicles To the Secretome of Staphylococcus aureus. MBio 2023, 14, e0357122. [Google Scholar] [CrossRef]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus Extracellular Vesicles and Their Application as a Vaccine Platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef]
- Luz, B.S.R.D.; Nicolas, A.; Chabelskaya, S.; Rodovalho, V.D.R.; Le Loir, Y.; Azevedo, V.A.d.C.; Felden, B.; Guédon, E. Environmental Plasticity of the RNA Content of Staphylococcus aureus Extracellular Vesicles. Front. Microbiol. 2021, 12, 634226. [Google Scholar] [CrossRef]
- Joshi, B.; Singh, B.; Nadeem, A.; Askarian, F.; Wai, S.N.; Johannessen, M.; Hegstad, K. Transcriptome Profiling of Staphylococcus aureus Associated Extracellular Vesicles Reveals Presence of Small RNA-Cargo. Front. Mol. Biosci. 2021, 7, 566207. [Google Scholar] [CrossRef]
- Han, F.; Wang, W.; Shi, M.; Zhou, H.; Yao, Y.; Li, C.; Shang, A. Outer Membrane Vesicles from Bacteria: Role and Potential Value in the Pathogenesis of Chronic Respiratory Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 1093327. [Google Scholar] [CrossRef]
- Jhelum, H.; Sori, H.; Sehgal, D. A Novel Extracellular Vesicle-Associated Endodeoxyribonuclease Helps Streptococcus pneumoniae Evade Neutrophil Extracellular Traps and Is Required for Full Virulence. Sci. Rep. 2018, 8, 7985. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.Y.; Kim, S.H.; Kim, D.K.; Park, K.S.; Kim, K.P.; Kim, Y.K.; Roh, T.Y.; Gho, Y.S. Staphylococcus aureus Extracellular Vesicles Carry Biologically Active β-Lactamase. Antimicrob. Agents Chemother. 2013, 57, 2589–2595. [Google Scholar] [CrossRef]
- Gurung, M.; Moon, D.C.; Choi, C.W.; Lee, J.H.; Bae, Y.C.; Kim, J.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, S., II; et al. Staphylococcus aureus Produces Membrane-Derived Vesicles That Induce Host Cell Death. PLoS ONE 2011, 6, e27958. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Yutin, N.; Wolf, Y.I.; Alvarez, R.V.; Koonin, E.V. Conservation and Evolution of the Sporulation Gene Set in Diverse Members of the Firmicutes. J. Bacteriol. 2022, 204, e0007922. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Orellana, C.A.; Moutafis, G.; Moonen, G.; Rincon, G.; Nielsen, L.K.; Marcellin, E.; Bapteste, E. Revisiting the Evolution and Taxonomy of Clostridia, a Phylogenomic Update. Genome Biol. Evol. 2019, 11, 2035–2044. [Google Scholar] [CrossRef]
- Brown, L.; Kessler, A.; Cabezas-Sanchez, P.; Luque-Garcia, J.L.; Casadevall, A. Extracellular Vesicles Produced by the Gram-Positive Bacterium bacillus Subtilis Are Disrupted by the Lipopeptide Surfactin. Mol. Microbiol. 2014, 93, 183–198. [Google Scholar] [CrossRef]
- Nicholas, A.; Jeon, H.; Selasi, G.N.; Na, S.H.; Kwon, H.I.; Kim, Y.J.; Choi, C.W.; Kim, S.I.; Lee, J.C. Clostridium Difficile-Derived Membrane Vesicles Induce the Expression of pro-Inflammatory Cytokine Genes and Cytotoxicity in Colonic Epithelial Cells in Vitro. Microb. Pathog. 2017, 107, 6–11. [Google Scholar] [CrossRef]
- Rubio, A.P.D.; Martínez, J.; Palavecino, M.; Fuentes, F.; López, C.M.S.; Marcilla, A.; Pérez, O.E.; Piuri, M. Transcytosis of Bacillus Subtilis Extracellular Vesicles through an in Vitro Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 3120. [Google Scholar] [CrossRef]
- Buchacher, T.; Digruber, A.; Kanzler, M.; Del Favero, G.; Ehling-Schulz, M. Bacillus Cereus Extracellular Vesicles Act as Shuttles for Biologically Active Multicomponent Enterotoxins. Cell Commun. Signal. 2023, 21, 112. [Google Scholar] [CrossRef]
- Caballano-Infantes, E.; Ho-Plágaro, A.; López-Gómez, C.; Martín-Reyes, F.; Rodríguez-Pacheco, F.; Taminiau, B.; Daube, G.; Garrido-Sánchez, L.; Alcaín-Martínez, G.; Andrade, R.J.; et al. Membrane Vesicles of Toxigenic Clostridioides Difficile Affect the Metabolism of Liver HepG2 Cells. Antioxidants 2023, 12, 818. [Google Scholar] [CrossRef]
- Radolf, J.D.; Kumar, S. The Treponema pallidum Outer Membrane. In Spirochete Biology: The Post Genomic Era; Springer: Berlin/Heidelberg, Germany, 2018; Volume 415, pp. 1–38. [Google Scholar]
- Blanco, D.R.; Reimann, K.; Skare, J.; Champion, C.I.; Foley, D.; Exner, M.M.; Hancock, R.E.W.; Miller, J.N.; Lovewt1, M.A. Isolation of the Outer Membranes from Treponema pallidum and Treponema vincentii. J. Bacteriol. 1994, 176, 6088–6099. [Google Scholar] [CrossRef]
- Blanco, D.R.; Champion, C.I.; Lewinski, M.A.; Shang, E.S.; Simkins, S.G.; Miller, J.N.; Lovett, M.A. Immunization with Treponema pallidum Outer Membrane Vesicles Induces High-Titer Complement-Dependent Treponemicidal Activity and Aggregation of T. pallidum Rare Outer Membrane Proteins (TROMPs). J. Immunol. 1999, 163, 2741–2746. [Google Scholar] [CrossRef]
- Blanco, D.R.; Champion, C.I.; Dooley, A.; Cox, D.L.; Whitelegge, J.P.; Faull, K.; Lovett, M.A. A Monoclonal Antibody That Conveys in Vitro Killing and Partial Protection in Experimental Syphilis Binds a Phosphorylcholine Surface Epitope of Treponema pallidum. Infect. Immun. 2005, 73, 3083–3095. [Google Scholar] [CrossRef]
- Houston, S.; Taylor, J.S.; Denchev, Y.; Hof, R.; Zuerner, R.L.; Cameron, C.E. Conservation of the Host-Interacting Proteins Tp0750 and Pallilysin among Treponemes and Restriction of Proteolytic Capacity to Treponema pallidum. Infect. Immun. 2015, 83, 4204–4216. [Google Scholar] [CrossRef]
- Daniel, T.M. The History of Tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Zumla, A.; Raviglione, M.; Hafner, R.; Fordham von Reyn, C. Tuberculosis. N. Engl. J. Med. 2013, 368, 745–755. [Google Scholar] [CrossRef]
- Gupta, S.; Rodriguez, G.M. Mycobacterial Extracellular Vesicles and Host Pathogen Interactions. Pathog. Dis. 2018, 76, fty031. [Google Scholar] [CrossRef]
- Prados-Rosales, R.; Baena, A.; Martinez, L.R.; Luque-Garcia, J.; Kalscheuer, R.; Veeraraghavan, U.; Camara, C.; Nosanchuk, J.D.; Besra, G.S.; Chen, B.; et al. Mycobacteria Release Active Membrane Vesicles That Modulate Immune Responses in a TLR2-Dependent Manner in Mice. J. Clin. Investig. 2011, 121, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Rafael, P.-R.; Leandro, J.C.; Ana, B.-G.; Andres, B.; Manjunatha, M.V.; Jiayong, X.; Xiaobo, Y.; Garrick, W.; Mitchell, M.; Joshua, L.; et al. Mycobacterial Membrane Vesicles Administered Systemically in Mice a Protective Immune Response to Surface Compartments of Mycobacterium tuberculosis. MBio 2014, 5, 10–1128. [Google Scholar] [CrossRef]
- Rath, P.; Huang, C.; Wang, T.; Wang, T.; Li, H.; Prados-Rosales, R.; Elemento, O.; Casadevall, A.; Nathan, C.F. Genetic Regulation of Vesiculogenesis and Immunomodulation in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E4790–E4797. [Google Scholar] [CrossRef] [PubMed]
- White, D.W.; Elliott, S.R.; Odean, E.; Bemis, L.T.; Tischler, A.D. Mycobacterium tuberculosis Pst/SenX3-RegX3 Regulates Membrane Vesicle Production Independently of ESX-5 Activity. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Palacios, A.; Sampedro, L.; Sevilla, I.A.; Molina, E.; Gil, D.; Azkargorta, M.; Elortza, F.; Garrido, J.M.; Anguita, J.; Prados-Rosales, R. Mycobacterium tuberculosis Extracellular Vesicle-Associated Lipoprotein LpqH as a Potential Biomarker to Distinguish Paratuberculosis Infection or Vaccination from Tuberculosis Infection. BMC Vet. Res. 2019, 15, 188. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Choi, D.S.; Lee, J.S.; Kim, D.K.; Go, G.; Park, S.M.; Kim, S.H.; Shin, J.H.; Chang, C.L.; et al. Proteomic Analysis of Extracellular Vesicles Derived from Mycobacterium tuberculosis. Proteomics 2015, 15, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Flores, L.; Castañeda-Casimiro, J.; Vallejo-Castillo, L.; Álvarez-Jiménez, V.D.; Peregrino, E.S.; García-Martínez, M.; Barreda, D.; Rosales-García, V.H.; Segovia-García, C.D.; Santos-Mendoza, T.; et al. Extracellular Vesicles from Mycobacterium tuberculosis-Infected Neutrophils Induce Maturation of Monocyte-Derived Dendritic Cells and Activation of Antigen-Specific Th1 Cells. J. Leukoc. Biol. 2023, 113, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Athman, J.J.; Wang, Y.; McDonald, D.J.; Boom, W.H.; Harding, C.V.; Wearsch, P.A. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. J. Immunol. 2015, 195, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Athman, J.J.; Sande, O.J.; Groft, S.G.; Reba, S.M.; Nagy, N.; Wearsch, P.A.; Richardson, E.T.; Rojas, R.; Boom, W.H.; Shukla, S.; et al. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. 2017, 198, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Mehaffy, C.; Ryan, J.M.; Kruh-Garcia, N.A.; Dobos, K.M. Extracellular Vesicles in Mycobacteria and Tuberculosis. Front. Cell. Infect. Microbiol. 2022, 12, 912831. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Vaziri, F.; Riazi Rad, F.; Amanzadeh, A.; Fateh, A.; Moshiri, A.; Khatami, S.; Siadat, S.D. Comparative Study of Pathogenic and Non-Pathogenic Escherichia coli Outer Membrane Vesicles and Prediction of Host-Interactions with TLR Signaling Pathways. BMC Res. Notes 2018, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Cheng, L.; Johnston, E.L.; Pathirana, R.; Phan, T.K.; Poon, I.K.H.; O’Brien-Simpson, N.M.; Hill, A.F.; Stinear, T.P.; Kaparakis-Liaskos, M. Staphylococcus aureus Membrane Vesicles Contain Immunostimulatory DNA, RNA and Peptidoglycan That Activate Innate Immune Receptors and Induce Autophagy. J. Extracell. Vesicles 2021, 10, e12080. [Google Scholar] [CrossRef] [PubMed]
- Laakmann, K.; Eckersberg, J.M.; Hapke, M.; Wiegand, M.; Bierwagen, J.; Beinborn, I.; Preußer, C.; Pogge von Strandmann, E.; Heimerl, T.; Schmeck, B.; et al. Bacterial Extracellular Vesicles Repress the Vascular Protective Factor RNase1 in Human Lung Endothelial Cells. Cell Commun. Signal. 2023, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, X.; Wang, J.; Wang, Y.; Zhang, C.; Dai, S.; Wang, X.; Deng, X.; Zhao, L.; Shan, B. Outer Membrane Vesicles Secreted by Helicobacter pylori Transmitting Gastric Pathogenic Virulence Factors. ACS Omega 2022, 7, 240–258. [Google Scholar] [CrossRef]
- Choi, M.S.; Ze, E.Y.; Park, J.Y.; Shin, T.S.; Kim, J.G. Helicobacter pylori-Derived Outer Membrane Vesicles Stimulate Interleukin 8 Secretion through Nuclear Factor Kappa B Activation. Korean J. Intern. Med. 2021, 36, 857–867. [Google Scholar] [CrossRef]
- Hock, B.D.; McKenzie, J.L.; Keenan, J.I. Helicobacter pylori Outer Membrane Vesicles Inhibit Human T Cell Responses via Induction of Monocyte COX-2 Expression. Pathog. Dis. 2017, 75, ftx034. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Nasher, F.; Jagatia, H.; Gundogdu, O.; Bajaj-Elliott, M.; Wren, B.; Dorrell, N. Campylobacter jejuni Outer Membrane Vesicle-Associated Proteolytic Activity Promotes Bacterial Invasion by Mediating Cleavage of Intestinal Epithelial Cell E-Cadherin and Occludin. Cell. Microbiol. 2016, 18, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.A.; Lee, M.K.; Hazlett, H.F.; Dessaint, J.A.; Mellinger, D.L.; Aridgides, D.S.; Hendricks, G.M.; Abdalla, M.A.K.; Christensen, B.C.; Ashare, A. Extracellular Vesicles from Pseudomonas aeruginosa Suppress MHC-Related Molecules in Human Lung Macrophages. ImmunoHorizons 2020, 4, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hwang, I.; Lee, E.; Shin, S.J.; Lee, E.J.; Rhee, J.H.; Yu, J.W. Bacterial Outer Membrane Vesicle-Mediated Cytosolic Delivery of Flagellin Triggers Host NLRC4 Canonical Inflammasome Signaling. Front. Immunol. 2020, 11, 581165. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.H.; Lee, J.H.; Kim, B.R.; Kim, S.I.; Park, T.I.; Lee, J.C.; Lee, Y.C. Acinetobacter baumannii Outer Membrane Vesicles Elicit a Potent Innate Immune Response via Membrane Proteins. PLoS ONE 2013, 8, e71751. [Google Scholar] [CrossRef] [PubMed]
- Lekmeechai, S.; Su, Y.C.; Brant, M.; Alvarado-Kristensson, M.; Vallström, A.; Obi, I.; Arnqvist, A.; Riesbeck, K. Helicobacter pylori Outer Membrane Vesicles Protect the Pathogen from Reactive Oxygen Species of the Respiratory Burst. Front. Microbiol. 2018, 9, 1837. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Koch, M.; Lehr, C.M.; Fuhrmann, G. Streptococcal Extracellular Membrane Vesicles Are Rapidly Internalized by Immune Cells and Alter Their Cytokine Release. Front. Immunol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, S.S.; Werner, S.; Azambuja, J.H.; Ludwig, N.; Eutsey, R.; Lucas, P.C.; Bailey, N.; Whiteside, T.L.; Campbell, P.G.; Hiller, N.L.; et al. Pneumococcal Extracellular Vesicles Modulate Host Immunity. MBio 2021, 12, e0165721. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef]
- Allen, E.R.; Lempke, S.L.; Miller, M.M.; Bush, D.M.; Braswell, B.G.; Estes, C.L.; Benedict, E.L.; Mahon, A.R.; Sabo, S.L.; Greenlee-Wacker, M.C. Effect of Extracellular Vesicles from S. aureus-Challenged Human Neutrophils on Macrophages. J. Leukoc. Biol. 2020, 108, 1841–1850. [Google Scholar] [CrossRef]
- Hong, S.W.; Kim, M.R.; Lee, E.Y.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Yang, J.M.; Lee, B.J.; Pyun, B.Y.; Gho, Y.S.; et al. Extracellular Vesicles Derived from Staphylococcus aureus Induce Atopic Dermatitis-like Skin Inflammation. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 351–359. [Google Scholar] [CrossRef]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar]
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mihret, A. The Role of Dendritic Cells in Mycobacterium tuberculosis Infection. Virulence 2012, 3, 654–659. [Google Scholar] [CrossRef]
- Mihret, A.; Mamo, G.; Tafesse, M.; Hailu, A.; Parida, S. Dendritic Cells Activate and Mature after Infection with Mycobacterium tuberculosis. BMC Res. Notes 2011, 4, 247. [Google Scholar] [CrossRef]
- Behrouzi, A.; Mianroodi, R.A.; Afrough, P.; Ayadi, A.; Serajian, A. Evaluation of Immunological Responses against Outer Membrane Vesicles (OMV) of Nontypeable Haemophilus influenzae Using MPLA-CpG Adjuvant as a Vaccine Candidate. Iran. J. Microbiol. 2020, 12, 417. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High Yield Production Process for Shigella Outer Membrane Particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Wrage, M.; Nüse, B.; Mattner, J. Shigella Outer Membrane Vesicles as Promising Targets for Vaccination. Int. J. Mol. Sci. 2022, 23, 994. [Google Scholar] [CrossRef]
- Mitra, S.; Chakrabarti, M.K.; Koley, H. Multi-Serotype Outer Membrane Vesicles of Shigellae Confer Passive Protection to the Neonatal Mice against Shigellosis. Vaccine 2013, 31, 3163–3173. [Google Scholar] [CrossRef]
- Mitra, S.; Barman, S.; Nag, D.; Sinha, R.; Saha, D.R.; Koley, H. Outer Membrane Vesicles of Shigella Boydii Type 4 Induce Passive Immunity in Neonatal Mice. FEMS Immunol. Med. Microbiol. 2012, 66, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.I.; De Souza, J.; Sánchez-Gómez, S.; Pardo-Ros, M.; Irache, J.M.; Gamazo, C. Mucosal Immunization with Shigella flexneri Outer Membrane Vesicles Induced Protection in Mice. Vaccine 2011, 29, 8222–8229. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, B.; Zhang, Y.; Li, R.; Ruan, H.; Wu, J.; Liu, Q. Outer Membrane Vesicles of Helicobacter pylori 7.13 as Adjuvants Promote Protective Efficacy Against Helicobacter pylori Infection. Front. Microbiol. 2020, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Hamilton, D.J.; Munson, G.P.; Fleckenstein, J.M. Outer Membrane Vesicles Induce Immune Responses to Virulence Proteins and Protect against Colonization by Enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 2011, 18, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Choi, K.H.; Kim, Y.S.; Hong, B.S.; Kim, O.Y.; Kim, J.H.; Yoon, C.M.; Koh, G.Y.; Kim, Y.K.; Gho, Y.S. Outer Membrane Vesicles Derived from Escherichia coli Induce Systemic Inflammatory Response Syndrome. PLoS ONE 2010, 5, e11334. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Hong, B.S.; Park, K.-S.; Yoon, Y.J.; Choi, S.J.; Lee, W.H.; Roh, T.-Y.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Immunization with Escherichia coli Outer Membrane Vesicles Protects Bacteria—Induced Lethality via Th1 and Th17 Cell Responses. J. Immunol. 2013, 190, 4092–4102. [Google Scholar] [CrossRef]
- Lee, W.H.; Choi, H.I.; Hong, S.W.; Kim, K.S.; Gho, Y.S.; Jeon, S.G. Vaccination with Klebsiella pneumoniae-Derived Extracellular Vesicles Protects against Bacteria-Induced Lethality via Both Humoral and Cellular Immunity. Exp. Mol. Med. 2015, 47, e183. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.M.; Romero-Saavedra, F.; Laverde, D.; Johannessen, M.; Hübner, J.; Hegstad, K. Enterococcal Membrane Vesicles as Vaccine Candidates. Int. J. Mol. Sci. 2023, 24, 16051. [Google Scholar] [CrossRef] [PubMed]
- Emerson, L.E.; Barker, H.; Tran, T.; Barker, S.; Enslow, S.; Ou, M.; Hoffman, C.; Jones, M.; Pascual, D.W.; Edelmann, M.J. Extracellular Vesicles Elicit Protective Immune Responses against Salmonella Infection. J. Extracell. Vesicles 2022, 11, e12267. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Hu, B.; Zhang, X.; Curtiss, R.; Kong, Q. Outer Membrane Vesicles from Flagellin-Deficient Salmonella enterica Serovar Typhimurium Induce Cross-Reactive Immunity and Provide Cross-Protection against Heterologous Salmonella Challenge. Sci. Rep. 2016, 6, 34776. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, M.H.; Jeon, J.; Kim, O.Y.; Choi, Y.; Seo, J.; Hong, S.W.; Lee, W.H.; Jeon, S.G.; Gho, Y.S.; et al. Active Immunization with Extracellular Vesicles Derived from Staphylococcus aureus Effectively Protects against Staphylococcal Lung Infections, Mainly via Th1 Cell-Mediated Immunity. PLoS ONE 2015, 10, e0136021. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Beveridge, T.J. Delivery of the Non-Membrane-Permeative Antibiotic Gentamicin into Mammalian Cells by Using Shigella flexneri Membrane Vesicles. Antimicrob. Agents Chemother. 1998; 42, 1476–1483. [Google Scholar]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Guescini, M.; Gundogdu, O.; Luchetti, F.; Lanuti, P.; Ciacci, C.; Burattini, S.; Campana, R.; Ortolani, C.; Papa, S.; et al. Extracellular Vesicles from Campylobacter jejuni CDT-Treated Caco-2 Cells Inhibit Proliferation of Tumour Intestinal Caco-2 Cells and Myeloid U937 Cells: Detailing the Global Cell Response for Potential Application in Anti-Tumour Strategies. Int. J. Mol. Sci. 2023, 24, 487. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, Z.; Tan, X.; Wang, Z.; Tang, B.; Wang, Z.; Li, M.; Mi, T.; Shen, L.; Long, C.; et al. Antitumor Effect of Escherichia coli-Derived Outer Membrane Vesicles on Neuroblastoma in Vitro and in Vivo. Acta Biochim. Biophys. Sin. 2022, 54, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Zare Banadkoki, E.; Rasooli, I.; Ghazanfari, T.; Siadat, S.D.; Shafiee Ardestani, M.; Owlia, P. Pseudomonas aeruginosa PAO1 Outer Membrane Vesicles-Diphtheria Toxoid Conjugate as a Vaccine Candidate in a Murine Burn Model. Sci. Rep. 2022, 12, 22324. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Sun, X.; Cimino, J.; Guan, Z.; Sun, W. Recombinant Pseudomonas Bionanoparticles Induce Protection against Pneumonic Pseudomonas aeruginosa Infection. Infect. Immun. 2021, 89. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, B.; Xu, T.; Yu, H.; Chen, J.; Yu, H.; Li, S.; Zeng, L.; Huang, X.; Liua, Q. Outer Membrane Vesicles Derived From Salmonella enterica Serotype Typhimurium Can Deliver Shigella flexneri 2a O-Polysaccharide Antigen To Prevent Shigella flexneri 2a Infection In Mice. Appl. Environ. Microbiol. 2021, 87, e00968-21. [Google Scholar] [CrossRef]
- Harrell, J.E.; Kurtz, J.R.; Bauer, D.L.; Timothy Prior, J.; Gellings, P.S.; Morici, L.A.; McLachlan, J.B. An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects against Lethal, Oral Salmonella Infection. Pathogens 2021, 10, 616. [Google Scholar] [CrossRef]
- Badmasti, F.; Ajdary, S.; Bouzari, S.; Fooladi, A.A.I.; Shahcheraghi, F.; Siadat, S.D. Immunological Evaluation of OMV(PagL)+Bap(1-487aa) and AbOmpA(8-346aa)+Bap(1-487aa) as Vaccine Candidates against Acinetobacter baumannii Sepsis Infection. Mol. Immunol. 2015, 67, 552–558. [Google Scholar] [CrossRef]
- Matthias, K.A.; Connolly, K.L.; Begum, A.A.; Jerse, A.E.; MacIntyre, A.N.; Sempowski, G.D.; Bash, M.C. Meningococcal Detoxified Outer Membrane Vesicle Vaccines Enhance Gonococcal Clearance in a Murine Infection Model. J. Infect. Dis. 2022, 225, 650–660. [Google Scholar]
- Li, W.; Hu, Y.; Zhang, Q.; Hua, L.; Yang, Z.; Ren, Z.; Zheng, X.; Huang, W.; Ma, Y. Development of Drug-Resistant Klebsiella pneumoniae Vaccine via Novel Vesicle Production Technology. ACS Appl. Mater. Interfaces 2021, 13, 32703–32715. [Google Scholar] [CrossRef]
- Parveen, S.; Subramanian, K. Emerging Roles of Extracellular Vesicles in Pneumococcal Infections: Immunomodulators to Potential Novel Vaccine Candidates. Front. Cell. Infect. Microbiol. 2022, 12, 836070. [Google Scholar] [CrossRef] [PubMed]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R. Immunization with Salmonella enterica Serovar Typhimurium-Derived Outer Membrane Vesicles Delivering the Pneumococcal Protein PspA Confers Protection against Challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Irene, C.; Fantappiè, L.; Caproni, E.; Zerbini, F.; Anesi, A.; Tomasi, M.; Zanella, I.; Stupia, S.; Prete, S.; Valensin, S.; et al. Bacterial Outer Membrane Vesicles Engineered with Lipidated Antigens as a Platform for Staphylococcus aureus Vaccine. Proc. Natl. Acad. Sci. USA 2019, 116, 21780–21788. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Gagliardi, A.; Riedmiller, I.; Andretta, C.; Tomasi, M.; Irene, C.; Frattini, L.; Zanella, I.; Berti, F.; Grandi, A.; et al. Multi-Antigen Outer Membrane Vesicle Engineering to Develop Polyvalent Vaccines: The Staphylococcus aureus Case. Front. Immunol. 2021, 12, 752168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, X.; He, Y.; Zhang, B.; Zhou, N.; Huang, J.D. A Bacterial Outer Membrane Vesicle-Based Click Vaccine Elicits Potent Immune Response against Staphylococcus aureus in Mice. Front. Immunol. 2023, 14, 1088501. [Google Scholar] [CrossRef] [PubMed]
- Zanella, I.; König, E.; Tomasi, M.; Gagliardi, A.; Frattini, L.; Fantappiè, L.; Irene, C.; Zerbini, F.; Caproni, E.; Isaac, S.J.; et al. Proteome-Minimized Outer Membrane Vesicles from Escherichia coli as a Generalized Vaccine Platform. J. Extracell. Vesicles 2021, 10, e12066. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1852. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, P.D.; Canals, R.; Ramasamy, M.N. The INTS-GMMA Vaccine: A Promising Step in Non-Typhoidal Salmonella Vaccine Development. Expert Rev. Vaccines 2023, 22, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Izeli Portilho, A.; De Gaspari, E. Outer Membrane Vesicles: A Challenging Yet Promising Platform for COVID-19 Vaccines. In COVID-19 Vaccines—Current State and Perspectives; IntechOpen: London, UK, 2023. [Google Scholar]
- Isitt, C.; Cosgrove, C.A.; Ramsay, M.E.; Ladhani, S.N. Success of 4CMenB in Preventing Meningococcal Disease: Evidence from Real-World Experience. Arch. Dis. Child. 2020, 105, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The New Multicomponent Vaccine against Meningococcal Serogroup B, 4CMenB: Immunological, Functional and Structural Characterization of the Antigens. Vaccine 2012, 30, B87–B97. [Google Scholar] [CrossRef] [PubMed]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Viviani, V.; Biolchi, A.; Pizza, M. Synergistic Activity of Antibodies in the Multicomponent 4CMenB Vaccine. Expert Rev. Vaccines 2022, 21, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Semchenko, E.A.; Seib, K.A. Outer Membrane Vesicle Vaccines for Neisseria gonorrhoeae. Nat. Rev. Urol. 2022, 225, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hafez, I.M.; Maurer, N.; Cullis, P.R. On the Mechanism Whereby Cationic Lipids Promote Intracellular Delivery of Polynucleic Acids. Gene Ther. 2001, 8, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of MRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Rosenecker, J. Nanotechnologies in Delivery of MRNA Therapeutics Using Nonviral Vector-Based Delivery Systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]


| Bacteria Producing the EVs | Target Cell | Effects on the Immune System | References |
|---|---|---|---|
| EVs contain PAMPs recognized by PRRs | |||
| S. aureus | Mouse macrophages | Lipoproteins recognized by TLR2 and TLR4 induce IL-6, MIP-2, and TNFα production | [113,149] |
| HEK-Blue reporter cells | RNA and DNA detected by TLR7, TLR8 and TLR9 | [164] | |
| M. tuberculosis | Mouse macrophages | LpqH, LprG, PhoS1 and LAM lipoproteins recognized by TLR2 | [138,145] |
| Mouse macrophages | RNA activates RIG-1 receptors, inducing IRF3 expression, and an increase in macrophage bactericidal activity | [90] | |
| EVs have a proinflammatory effect | |||
| H. pylori | Human gastric epithelial cells | Induction of NF-κB results in the production of TNFα, IFNγ, IL-5, IL-6, IL-12 and IL-8 | [51,151,152] |
| C. jejuni | Human intestinal epithelial cells | Secretion of TNFα, IL-8, IL-6 and hBD-3 | [66,154] |
| P. aeruginosa | Human macrophages | Production of IL-1β, IL-6 and IL-8 | [155] |
| K. pneumoniae | Human bronchial epithelial cells | Increased TNFα, IL-8, IL-6 and IL1β production | [54] |
| C. difficile | Human intestinal and hepatic cells | Induces the production of MCP-1, IL-1β, IL-6 and IL-8 | [126,129] |
| EVs have an anti-inflammatory effect | |||
| H. pylori | Human monocytes | Secretion of IL-10 induced by MyD88-dependent signaling pathway | [153] |
| P. aeruginosa | Human lung macrophages | Upregulation of IL-10 expression | [155] |
| S. pneumoniae | Murine dendritic cells | Increased IL-10 production | [159] |
| EVs contain virulence factors | |||
| S. Typhimurium | Mouse macrophages | Flagellin increases IL-1β production, via NLRC4 | [156] |
| A. baumannii | Murine model | OmpA porin stimulates IL-1α/IL-1β, IL-6, MIP-1α, and neutrophil infiltration | [78,157] |
| EVs induce adaptative immune responses | |||
| Non-typeable H. influenzae | Murine model | Increased levels of IgG1, IgG2, IL-10, IL-4 and IFNγ | [169] |
| H. pylori | Murine model | Increased levels of IgG1 and IgG2, related to a change toward a Th2 profile | [175] |
| E. coli | Murine model | Induction of Th1 and Th17 responses | [176,177,178] |
| S. pneumoniae | Murine model | Induction of IgG production, protection and survival | [109] |
| K. pneumoniae | Murine model | Increased IgG production and decrease of bacterial load | [179] |
| Mouse macrophages | Increased IFNγ production by T cells | [179] | |
| EV from S. Typhimurium-infected macrophages | Murine model | Induction of IgA and IgG in mucous membranes | [181,182] |
| EV from M. tuberculosis-infected neutrophils | Human dendritic cells | Induction of DC maturation and increased IFNγ production by T cells | [144] |
| Vaccine | Components | Targeted Disease | Phase |
|---|---|---|---|
| VA-MENGO-BC | EVs derived from N. meningitidis group B and capsular polysaccharide from N. meningitidis group C | Meningococcal infections | Licensed |
| MenBvac | EVs derived from N. meningitidis P1.7,16 strains | Meningococcal infections | Licensed |
| MeNZB | EVs derived from N. meningitidis New Zealand 98/254 strain | Meningococcal infections | Licensed |
| GSK 4CMenB | EVs derived from N. meningitidis New Zealand 98/254 strain with additional antigens | Meningococcal infections | Licensed |
| GSK meningococcal group B vaccine administered concomitantly with GSK meningococcal MenACWY conjugate vaccine | Recombinant membrane proteins (rMenB) with EVs from the New Zealand B strain, administered concomitantly with a quadrivalent meningococcal tetanus toxoid conjugate vaccine (MenACWY) | Meningococcal infections | 3 |
| GSK meningococcal group B vaccine and 13-valent pneumococcal vaccine administered concomitantly with routine infant vaccines | Recombinant membrane proteins (rMenB) with EVs from the New Zealand B strain, administered concomitantly with pneumococcal conjugate vaccine (PCV 13) and other routine infant vaccines | Meningococcal and other infections | 3 |
| GSK Vaccines Institute for Global Health (GVGH) invasive non-typhoidal salmonellosis (iNTS)-GMMA | EVs derived from modified S. Typhimurium and S. Enteritidis | Invasive non-typhoidal salmonellosis | 2 |
| GVGH iNTS-GMMA-typhoid Vi polysaccharide-conjugate vaccines (TCV) | EVs derived from modified S. Typhimurium and S. Enteritidis, with the addition of TCV | Invasive non-typhoidal salmonellosis and typhoid fever | 1/2 |
| GSK N. gonorrhoeae GMMA (NgG) | N. gonorrhoeae GMMA | Gonorrhea | 1/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peregrino, E.S.; Castañeda-Casimiro, J.; Vázquez-Flores, L.; Estrada-Parra, S.; Wong-Baeza, C.; Serafín-López, J.; Wong-Baeza, I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 6210. https://doi.org/10.3390/ijms25116210
Peregrino ES, Castañeda-Casimiro J, Vázquez-Flores L, Estrada-Parra S, Wong-Baeza C, Serafín-López J, Wong-Baeza I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. International Journal of Molecular Sciences. 2024; 25(11):6210. https://doi.org/10.3390/ijms25116210
Chicago/Turabian StylePeregrino, Eliud S., Jessica Castañeda-Casimiro, Luis Vázquez-Flores, Sergio Estrada-Parra, Carlos Wong-Baeza, Jeanet Serafín-López, and Isabel Wong-Baeza. 2024. "The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities" International Journal of Molecular Sciences 25, no. 11: 6210. https://doi.org/10.3390/ijms25116210
APA StylePeregrino, E. S., Castañeda-Casimiro, J., Vázquez-Flores, L., Estrada-Parra, S., Wong-Baeza, C., Serafín-López, J., & Wong-Baeza, I. (2024). The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. International Journal of Molecular Sciences, 25(11), 6210. https://doi.org/10.3390/ijms25116210








