Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis
Abstract
:1. Introduction
2. Results
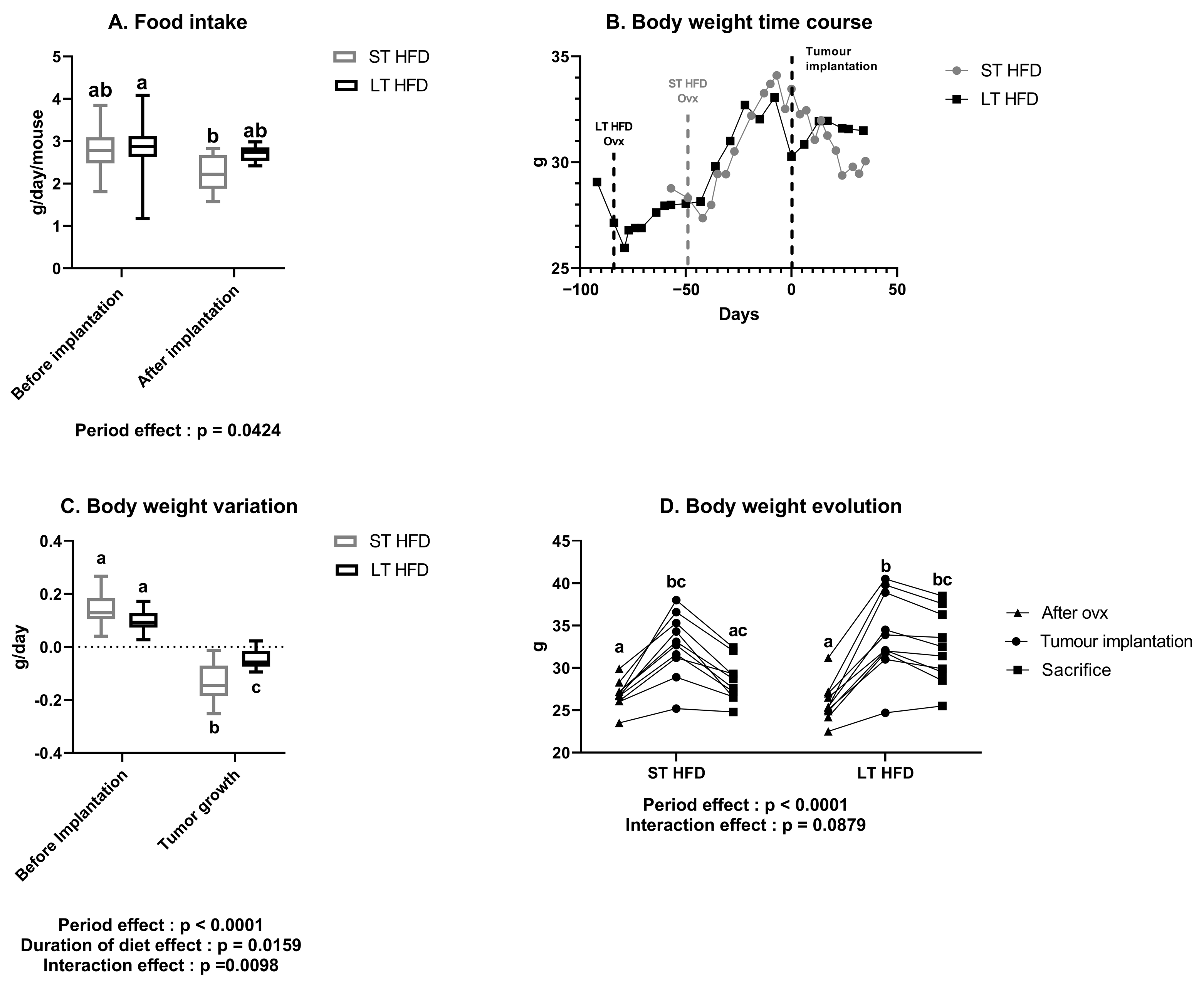
2.1. A Long-Term High-Fat Diet Tended to Influence Spontaneous Physical Activity but Not Food Intake
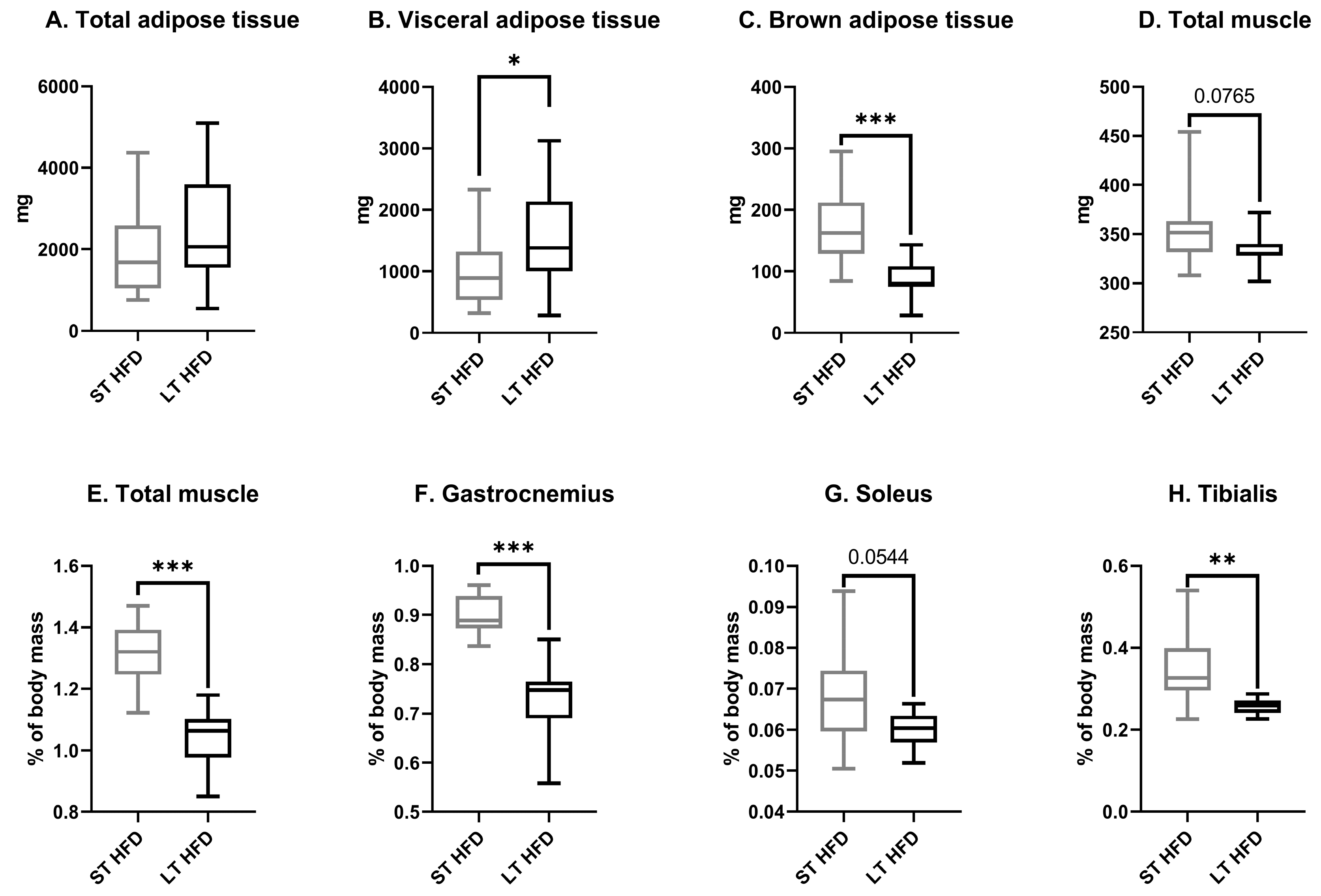
2.2. The Duration of Lipid Consumption Altered Anthropometric Parameters
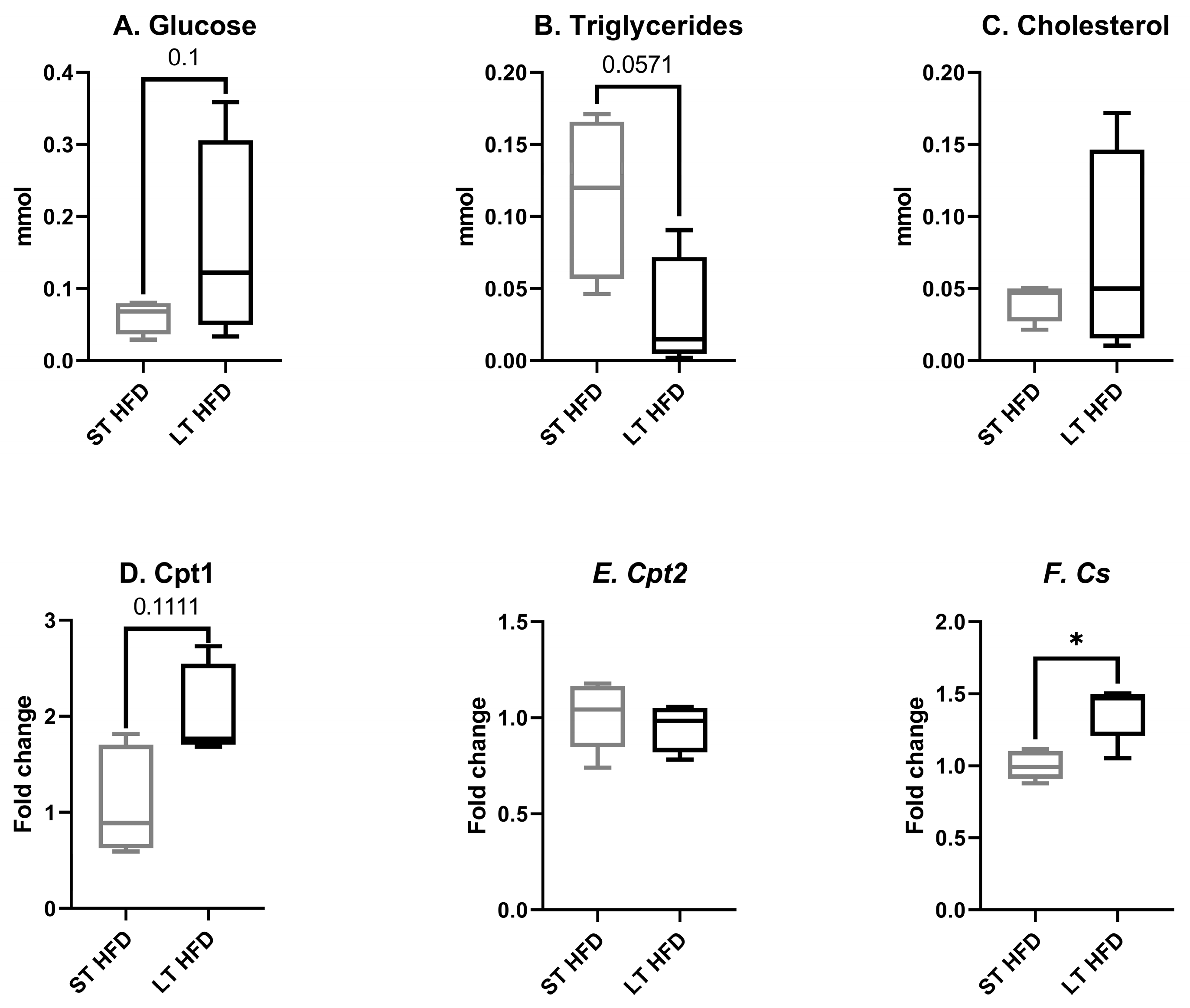
2.3. Biochemical Profiles in Liver and Plasma
2.4. Tumour Weight and Density
2.5. Tumour Growth
2.6. Tumour Characteristics
2.6.1. Tumour Metabolism
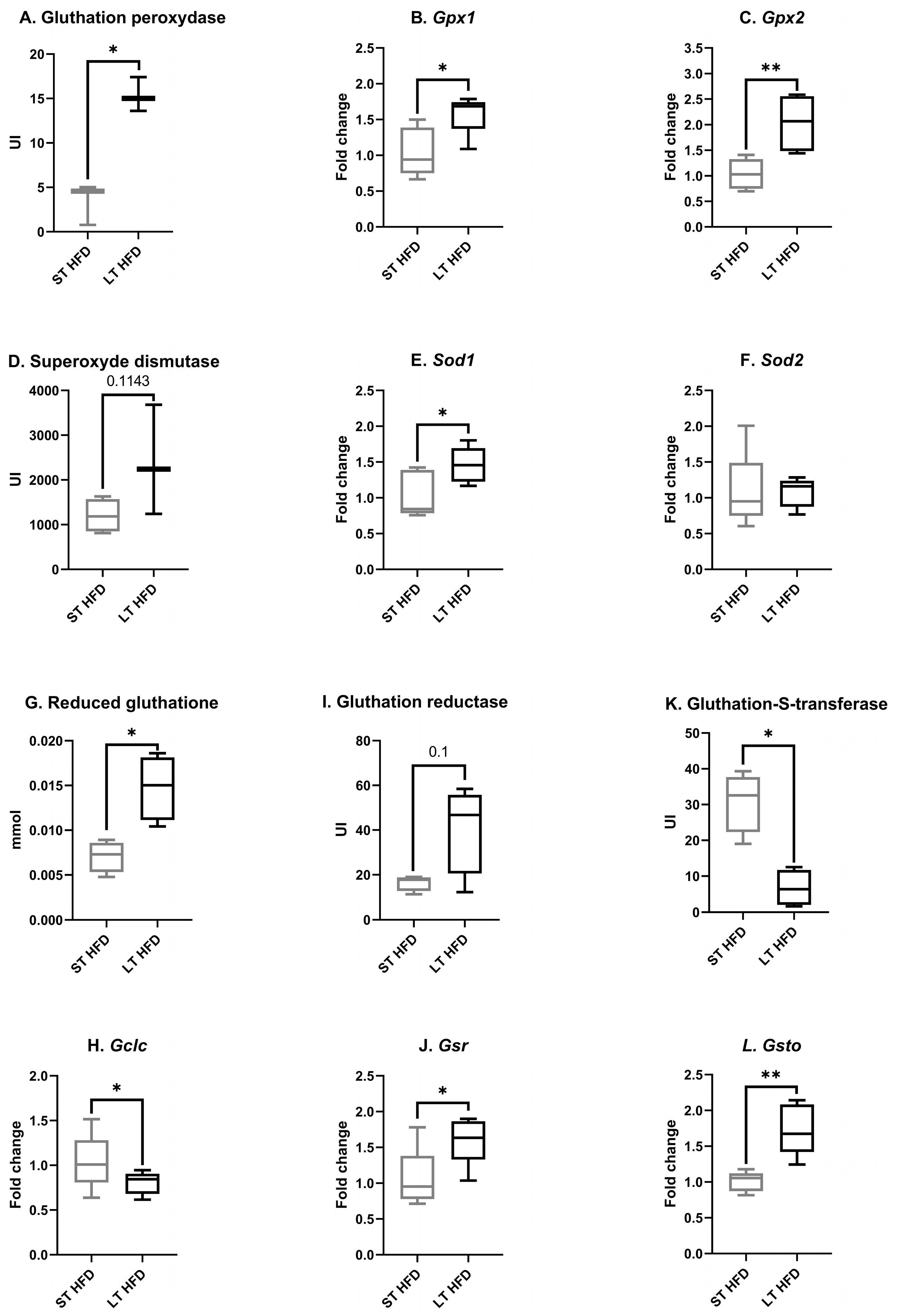
2.6.2. Tumour Oxidative Stress
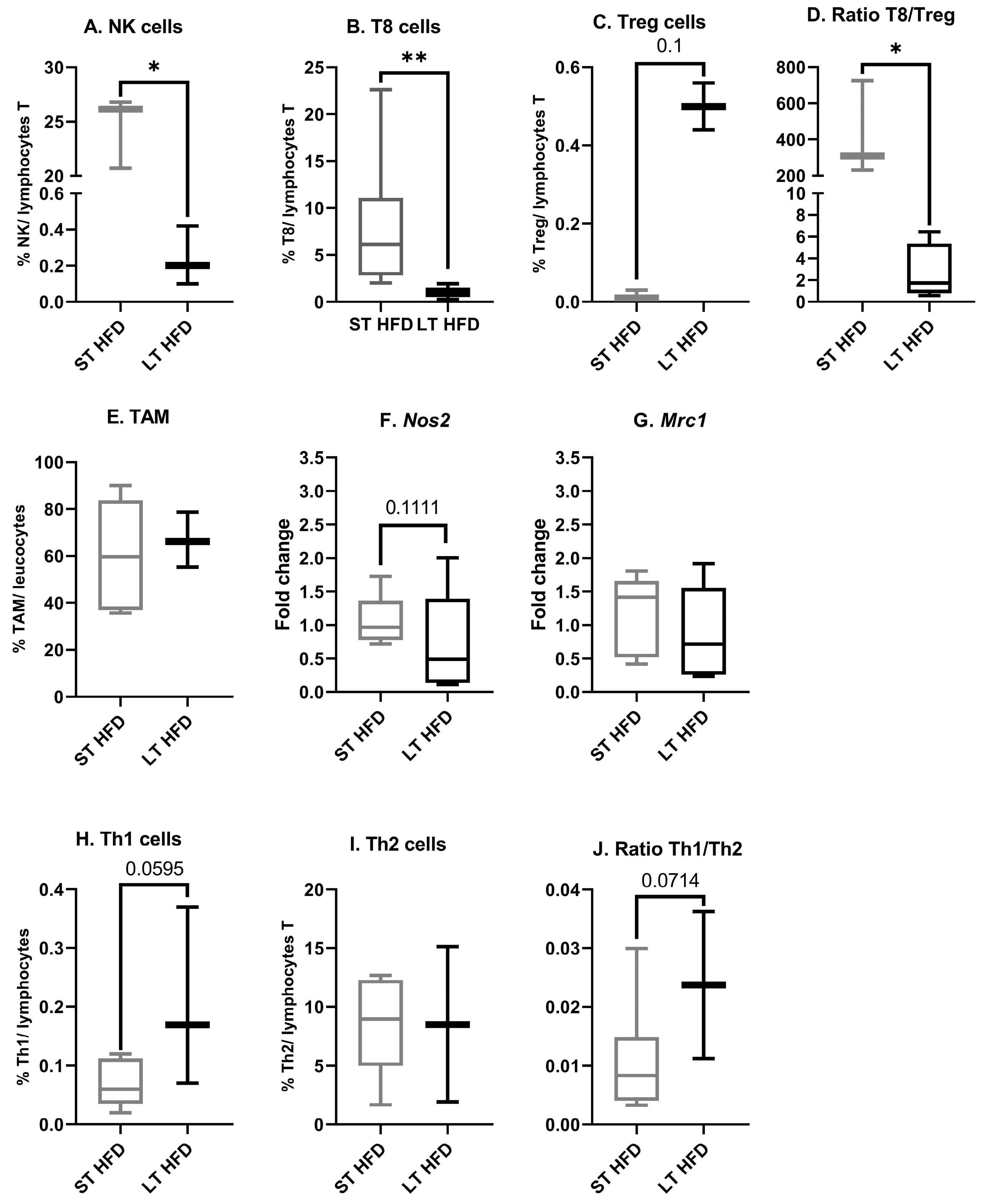
2.6.3. Tumour Immune Infiltrate
3. Discussion
4. Materials and Methods
4.1. Animal Model and Housing Conditions
4.2. Diet, Body Weight Follow Up and Physical Activity
4.3. Orthotopic Injection of EO771 Mammary Adenocarcinoma Cells and Tumour Monitoring
4.4. Sacrifice and Blood and Tissue Sampling
4.5. Tumour Immune Cell Infiltration
4.6. Tissue Exploration
4.6.1. Tissue Preparation
4.6.2. RNA Extraction
4.6.3. Quantitative Real-Time PCR
4.6.4. Protein Quantification
4.6.5. Enzymatic Activities
4.6.6. GSH Assay
4.7. Metabolism Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/today (accessed on 21 February 2024).
- Tung, N.; Lin, N.U.; Kidd, J.; Allen, B.A.; Singh, N.; Wenstrup, R.J.; Hartman, A.-R.; Winer, E.P.; Garber, J.E. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J. Clin. Oncol. 2024, 34, 1460. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association Analysis Identifies 65 New Breast Cancer Risk Loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Mucci, L.A.; Harris, J.R.; Scheike, T.; Holst, K.; Halekoh, U.; Adami, H.-O.; Czene, K.; Christensen, K.; Holm, N.V.; et al. The Heritability of Breast Cancer among Women in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of Breast Cancer: A Topic in Evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Nassiri, M.; Kooshyar, M.M.; Vakili-Azghandi, M.; Avan, A.; Sandry, R.; Pillai, S.; Lam, A.K.; Gopalan, V. Hereditary Breast Cancer; Genetic Penetrance and Current Status with BRCA. J. Cell Physiol. 2019, 234, 5741–5750. [Google Scholar] [CrossRef]
- Arthur, R.S.; Wang, T.; Xue, X.; Kamensky, V.; Rohan, T.E. Genetic Factors, Adherence to Healthy Lifestyle Behavior, and Risk of Invasive Breast Cancer Among Women in the UK Biobank. JNCI J. Natl. Cancer Inst. 2020, 112, 893–901. [Google Scholar] [CrossRef]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef]
- Arthur, R.; Wassertheil-Smoller, S.; Manson, J.E.; Luo, J.; Snetselaar, L.; Hastert, T.; Caan, B.; Qi, L.; Rohan, T. The Combined Association of Modifiable Risk Factors with Breast Cancer Risk in the Women’s Health Initiative. Cancer Prev. Res. 2018, 11, 317–326. [Google Scholar] [CrossRef]
- Breast Cancer. WCRF International. Available online: https://www.wcrf.org/diet-activity-and-cancer/cancer-types/breast-cancer/ (accessed on 18 May 2024).
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body Mass Index Had Different Effects on Premenopausal and Postmenopausal Breast Cancer Risks: A Dose-Response Meta-Analysis with 3,318,796 Subjects from 31 Cohort Studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef]
- Jesinger, R.A. Breast Anatomy for the Interventionalist. Tech. Vasc. Interv. Radiol. 2014, 17, 3–9. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5760. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose Tissue Macrophages as Potential Targets for Obesity and Metabolic Diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A Systematic Literature Review on Obesity: Understanding the Causes & Consequences of Obesity and Reviewing Various Machine Learning Approaches Used to Predict Obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 1–17. ISBN 978-3-319-48380-1. [Google Scholar]
- Grander, C.; Grabherr, F.; Tilg, H. Non-Alcoholic Fatty Liver Disease: Pathophysiological Concepts and Treatment Options. Cardiovasc. Res. 2023, 119, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Increased Risk of Incident Extrahepatic Cancers: A Meta-Analysis of Observational Cohort Studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Atoum, M.F.; Alzoughool, F.; Al-Hourani, H. Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer: Basic Clin. Res. 2020, 14, 117822341989845. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the Role of Obesity and Cholesterol in Breast Cancer. Breast Cancer Res. 2019, 21, 35. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and Breast Cancer: Mechanisms Involved in Obesity-Related Development, Growth and Progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.-M.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise Training in the Management of Overweight and Obesity in Adults: Synthesis of the Evidence and Recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22, e13273. [Google Scholar] [CrossRef] [PubMed]
- Olateju, I.V.; Opaleye-Enakhimion, T.; Udeogu, J.E.; Asuquo, J.; Olaleye, K.T.; Osa, E.; Oladunjoye, A.F. A Systematic Review on the Effectiveness of Diet and Exercise in the Management of Obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102759. [Google Scholar] [CrossRef]
- Dupuit, M.; Boscaro, A.; Bonnet, A.; Bouillon, P.; Bruno, P.; Morel, C.; Rance, M.; Boisseau, N. Acute Metabolic Responses after Continuous or Interval Exercise in Post-Menopausal Women with Overweight or Obesity. Scand. J. Med. Sci. Sports 2020, 30, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.T.V.; Drummond, A.E.; Boing, L.; Milne, R.L.; English, D.R.; Brown, K.A.; Van Roekel, E.H.; Dixon-Suen, S.C.; Lynch, M.J.; Moore, M.M.; et al. Linking Physical Activity to Breast Cancer via Sex Hormones, Part 1: The Effect of Physical Activity on Sex Steroid Hormones. Cancer Epidemiol. Biomark. Prev. 2022, 31, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, D.; Goepp, M.; Farges, M.-C.; Rougé, S.; Vasson, M.-P.; Caldefie-Chezet, F.; Rossary, A. Spontaneous Physical Activity in Obese Condition Favours Antitumour Immunity Leading to Decreased Tumour Growth in a Syngeneic Mouse Model of Carcinogenesis. Cancers 2021, 14, 59. [Google Scholar] [CrossRef]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-Cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Schneider, J.; Schlüter, K.; Steindorf, K.; Wiskemann, J.; Rosenberger, F.; Zimmer, P. Different Endurance Exercises Modulate NK Cell Cytotoxic and Inhibiting Receptors. Eur. J. Appl. Physiol. 2021, 121, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Longobucco, Y.; Masini, A.; Marini, S.; Barone, G.; Fimognari, C.; Bragonzoni, L.; Dallolio, L.; Maffei, F. Exercise and Oxidative Stress Biomarkers among Adult with Cancer: A Systematic Review. Oxidative Med. Cell Longev. 2022, 2022, 2097318. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical Activity, Obesity and Sedentary Behavior in Cancer Etiology: Epidemiologic Evidence and Biologic Mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, D.; Hatte, V.; Farges, M.-C.; Rougé, S.; Goepp, M.; Caldefie-Chezet, F.; Vasson, M.-P.; Rossary, A. Modulation of Inter-Organ Signalling in Obese Mice by Spontaneous Physical Activity during Mammary Cancer Development. Sci. Rep. 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Aldekwer, S.; Desiderio, A.; Farges, M.-C.; Rougé, S.; Le Naour, A.; Le Guennec, D.; Goncalves-Mendès, N.; Mille-Hamard, L.; Momken, I.; Rossary, A.; et al. Vitamin D Supplementation Associated with Physical Exercise Promotes a Tolerogenic Immune Environment without Effect on Mammary Tumour Growth in C57BL/6 Mice. Eur J Nutr 2020, 60, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, A.; Koffi, Y.; Diab, M.; Le Guennec, D.; Rougé, S.; Aldekwer, S.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.-C.; Caldefie-Chezet, F.; et al. EO771, the First Luminal B Mammary Cancer Cell Line from C57BL/6 Mice. Cancer Cell Int. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, A.; Rossary, A.; Vasson, M. EO771, Is It a Well-characterized Cell Line for Mouse Mammary Cancer Model? Limit and Uncertainty. Cancer Med. 2020, 9, 8074–8085. [Google Scholar] [CrossRef] [PubMed]
- Mahbouli, S.; Rossary, A.; Nachat-Kappes, R.; Combe, K.; Basu, S.; Vasson, M.-P. Leptin Induces in Vitro and in Vivo a Lipid Peroxidation and an Inflammatory Response, in Neoplasic Mammary Epithelial Cells. In Proceedings of the 11th Biennial ISSFAL Congress, Stockholm, Sweden, 1 July 2014. [Google Scholar]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of Exercise Interventions on Different Diseases and Organ Functions in Mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Nachat-Kappes, R.; Pinel, A.; Combe, K.; Lamas, B.; Farges, M.-C.; Rossary, A.; Goncalves-Mendes, N.; Caldefie-Chezet, F.; Vasson, M.-P.; Basu, S. Effects of Enriched Environment on COX-2, Leptin and Eicosanoids in a Mouse Model of Breast Cancer. PLoS ONE 2012, 7, e51525. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Basen-Engquist, K.; Bea, J.W. Weight Management and Physical Activity for Breast Cancer Prevention and Control. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, L.M.; Wadden, T.A.; Goodwin, P.J.; Alfano, C.M.; Thomson, C.A.; Irwin, M.L.; Neuhouser, M.L.; Crane, T.E.; Frank, E.; Spears, P.A.; et al. The Breast Cancer Weight Loss (BWEL) Trial (Alliance A011401): A Description and Evidence for the Lifestyle Intervention. Obesity 2022, 30, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Barchiesi, G.; Resuli, B.; Verrico, M.; Speranza, I.; Cristofani, L.; Pediconi, F.; Tomao, F.; Botticelli, A.; Santini, D. Sarcopenia in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 596. [Google Scholar] [CrossRef] [PubMed]
- Morlino, D.; Marra, M.; Cioffi, I.; Santarpia, L.; De Placido, P.; Giuliano, M.; De Angelis, C.; Carrano, S.; Verrazzo, A.; Buono, G.; et al. Prevalence of Sarcopenia in Women with Breast Cancer. Nutrients 2022, 14, 1839. [Google Scholar] [CrossRef]
- D’Souza, S.S.; Asha, A. A Systematic Study of Biochemical Profile during the Induction and Development of an Animal Model for Metabolic Syndrome. IOSR J. Pharm. Biol. Sci. 2014, 9, 109–113. [Google Scholar] [CrossRef]
- Kalezic, A.; Udicki, M.; Srdic Galic, B.; Aleksic, M.; Korac, A.; Jankovic, A.; Korac, B. Tissue-Specific Warburg Effect in Breast Cancer and Cancer-Associated Adipose Tissue—Relationship between AMPK and Glycolysis. Cancers 2021, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; Van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte Lipolysis Links Obesity to Breast Cancer Growth: Adipocyte-Derived Fatty Acids Drive Breast Cancer Cell Proliferation and Migration. Cancer Metab 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxidative Med. Cell Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef] [PubMed]
- Mahbouli, S.; Talvas, J.; der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.-P.; Rossary, A. Activation of Antioxidant Defences of Human Mammary Epithelial Cells under Leptin Depend on Neoplastic State. BMC Cancer 2018, 18, 1264. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative Stress and Its Role in Cancer. J. Can. Res. Ther. 2021, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Fahrmann, J.F.; Aftabizadeh, M.; Zhao, Q.; Tripathi, S.C.; Zhang, C.; Yuan, Y.; Ann, D.; Hanash, S.; Yu, H. Fatty Acid Oxidation Protects Cancer Cells from Apoptosis by Increasing Mitochondrial Membrane Lipids. Cell Rep. 2022, 39, 110870. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, L.; Dalle, C.; Lyan, B.; Demidem, A.; Rossary, A.; Vasson, M.-P.; Petera, M.; Lagree, M.; Ferreira, T.; Centeno, D.; et al. Plasma Metabolomic Signatures Associated with Long-Term Breast Cancer Risk in the SU.VI.MAX Prospective Cohort. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, L.; Victor Bala, A.; Deschasaux, M.; Bouchemal, N.; Nawfal Triba, M.; Vasson, M.-P.; Rossary, A.; Demidem, A.; Galan, P.; Hercberg, S.; et al. NMR Metabolomic Signatures Reveal Predictive Plasma Metabolites Associated with Long-Term Risk of Developing Breast Cancer. Int. J. Epidemiol. 2018, 47, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing Cancer Complexity: Hallmarks of Systemic Disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of Reactive Oxygen Species in Inflammation and Cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Gil Del Alcazar, C.R.; Alečković, M.; Polyak, K. Immune Escape during Breast Tumor Progression. Cancer Immunol. Res. 2020, 8, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Mortimer, J.E.; Natarajan, R.; Dietze, E.C.; Seewaldt, V.L. Metabolic Health, Insulin, and Breast Cancer: Why Oncologists Should Care About Insulin. Front. Endocrinol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Crudele, L.; Piccinin, E.; Moschetta, A. Visceral Adiposity and Cancer: Role in Pathogenesis and Prognosis. Nutrients 2021, 13, 2101. [Google Scholar] [CrossRef]
- Perry, R.J.; Shulman, G.I. Mechanistic Links between Obesity, Insulin, and Cancer. Trends Cancer 2020, 6, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-Tumor Crosstalk: The Effect of Exercise-Induced Myokine on Cancer Progression. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef] [PubMed]


| Mmol/Tissue | ST HFD | LT HFD |
|---|---|---|
| Glucose | 0.045 ± 0.011 | 0.033 ± 0.006 |
| Triglycerides | 0.031 ± 0.011 | 0.024 ± 0.003 |
| Cholesterol | 0.007 ± 0.0003 | 0.007 ± 0.001 |
| Total bilirubin | 0.062 ± 0.018 | 0.099 ± 0.006 * |
| Urea | 1.61 ± 0.524 | 1.82 ± 0.099 |
| Uric acid | 2.72 ± 0.498 | 2.50 ± 1.422 |
| Creatinine | 0.129 ± 0.043 | 0.324 ± 0.068 |
| Biological Marker | ST HFD | LT HFD |
|---|---|---|
| Glucose (mmol/L) | 15.1 ± 5.2 | 10.8 ± 3.0 |
| Triglycerides (mmol/L) | 1.05 ± 0.60 | 1.11 ± 0.08 |
| Cholesterol (mmol/L) | 2.95 ± 0.18 | 5.65 ± 0.23 |
| Total bilirubin (µmol/L) | 8.94 ± 0.39 | 7.10 ± 1.06 |
| Urea (mmol/L) | 12.1 ± 3.9 | 7.6 ± 0.6 |
| Uric acid (µmol/L) | 208 ± 48 | 142 ± 33 |
| Creatinine (µmol/L) | 0.104 ± 0.042 | 0.042 ± 0.018 |
| Cell Type | Antibody | Fluorochrome | Clone | Provider |
|---|---|---|---|---|
| TAM | Cd11b | PerCP Cy5.5 | M1/70.15 | eBioscience |
| F4/80 | PE | REA126 | MACS Miltenyi | |
| Natural Killer | NK1.1 | PerCP Vio®700 | PK136 | MACS Miltenyi |
| CD49b (DX5) | PE | DX5 | MACS Miltenyi | |
| T4 | CD4 | PE | GK1.5 | MACS Miltenyi |
| T8 | CD8α | FITC | 53-6.7 | MACS Miltenyi |
| Th1 | CD4 | AF700 | GK1.5 | eBioscience |
| CD119 | PE | REA189 | MACS Miltenyi | |
| Th2 | CD4 | AF700 | GK1.5 | eBioscience |
| CD124 | PE | REA235 | MACS Miltenyi | |
| Treg | CD25 | PE | PC61.5 | eBioscience |
| FoxP3 | Biotin | FJK-16s | eBioscience |
| Gene | Species | Sequence Reference | Amplicon Size | Forward Primer | Reverse Primer |
|---|---|---|---|---|---|
| Hprt | mouse | NM_013556.2 | 176 | GTAATGATCAGTCAACGGGGGAC | CCAGCAAGCTTGCAACCTTAACCA |
| Cd45 | mouse | NM_001111316.2 | 158 | GCTGATGGATGTGGAGCCAA | TCTGATTGTGGGGCTTTCGG |
| Cpt1 | mouse | NM_013495.2 | 336 | CTGGCTCTACCATGACGGGA | ATGGACTTGTCAAACCACCTGTC |
| Cpt2 | mouse | NM_009949 | 241 | CATCGTACCCACCATGCACT | ATCAAACCAGGGGCCTGAGA |
| Cs | mouse | NM_026444.4 | 133 | CAGCAGTATCGGAGCCATTGAC | ACCACCCTCATGGTCACTATGGAT |
| Gclc | mouse | NM_010295.2 | 375 | TCGACCTGACCATCGATAAGGA | TCATGTTCTCGTCAACCTTGG |
| Gpx 1 | mouse | NM_008160.6 | 150 | GCTCATTGAGAATGTCGCGT | TCATTCTTGCCATTCTCCTGGT |
| Gpx 2 | mouse | NM_030677.2 | 108 | CGGGACTACAACCAGCTCAA | CTCGTTCTGACAGTTCTCCTGA |
| Gsr | mouse | NM_010344.4 | 177 | GGCACTTGCGTGAATGTTGG | ATAGATGGTGTTCAGGCGGC |
| Gsto1 | mouse | NM_010362.3 | 168 | CTCCGAACCTAAGGGAAGCG | TGTGGGCTAGACACTCCTTG |
| Sod1 | mouse | NM_011434.1 | 136 | GGAACCATCCACTTCGAGCA | CTGCACTGGTACAGCCTTGT |
| Sod2 | mouse | NM_013671.3 | 179 | GAACAATCTCAACGCCACCG | CCAGCAACTCTCCTTTGGGTT |
| Nos2 | mouse | NM_010927.4 | 131 | AGGGTCACAACTTTACAGGGAG | GTGAGGAGCCTCAGAAGTGTC |
| Mrc1 | mouse | NM_008625.2 | 102 | TTGCACTTTGAGGGAAGCGA | CCTTGCCTGATGCCAGGTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marlin, S.; Goepp, M.; Desiderio, A.; Rougé, S.; Aldekwer, S.; Le Guennec, D.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.-C.; Rossary, A. Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 6221. https://doi.org/10.3390/ijms25116221
Marlin S, Goepp M, Desiderio A, Rougé S, Aldekwer S, Le Guennec D, Goncalves-Mendes N, Talvas J, Farges M-C, Rossary A. Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis. International Journal of Molecular Sciences. 2024; 25(11):6221. https://doi.org/10.3390/ijms25116221
Chicago/Turabian StyleMarlin, Sébastien, Marie Goepp, Adrien Desiderio, Stéphanie Rougé, Sahar Aldekwer, Delphine Le Guennec, Nicolas Goncalves-Mendes, Jérémie Talvas, Marie-Chantal Farges, and Adrien Rossary. 2024. "Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis" International Journal of Molecular Sciences 25, no. 11: 6221. https://doi.org/10.3390/ijms25116221





