TATA-Binding Protein-Based Virtual Screening of FDA Drugs Identified New Anti-Giardiasis Agents
Abstract
:1. Introduction
2. Results
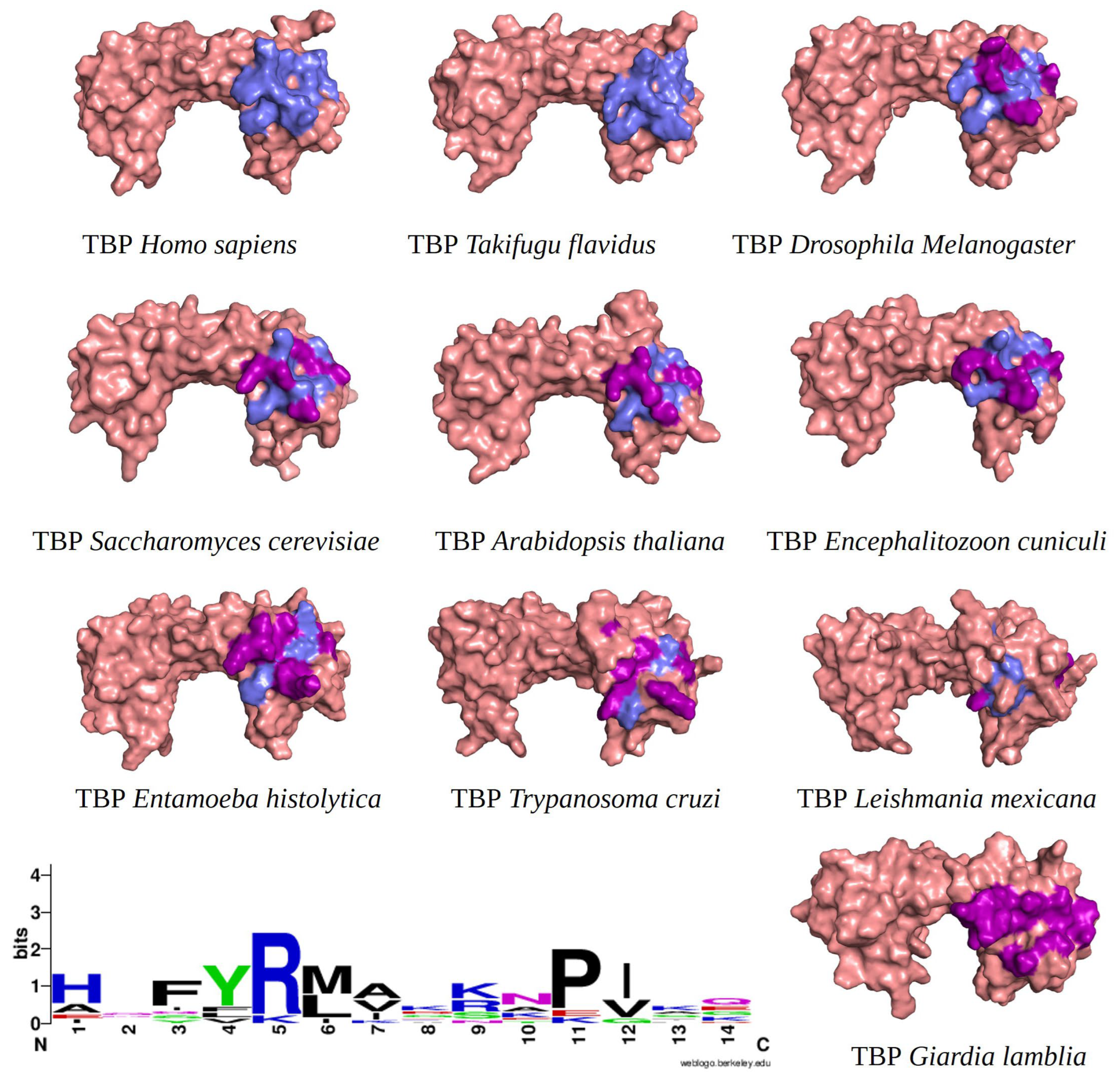
2.1. Multiple Sequence Alignments
2.2. Docking Molecular Analysis
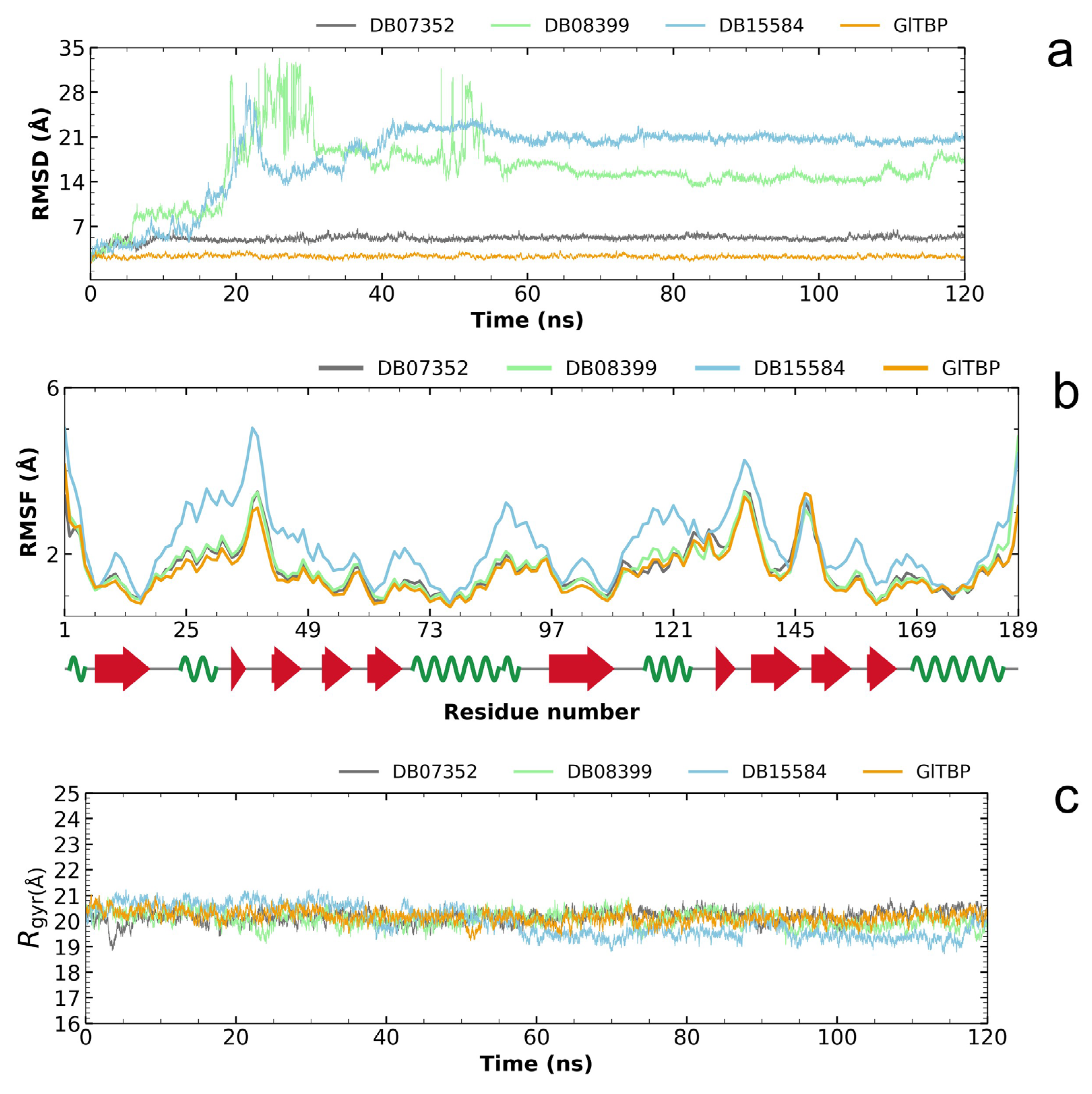
2.3. Molecular Dynamics Simulation
2.4. Molecular Dynamics Analysis of the Two Compounds with Potential for Repositioning in GlTBP and Their Evaluation on HsTBP
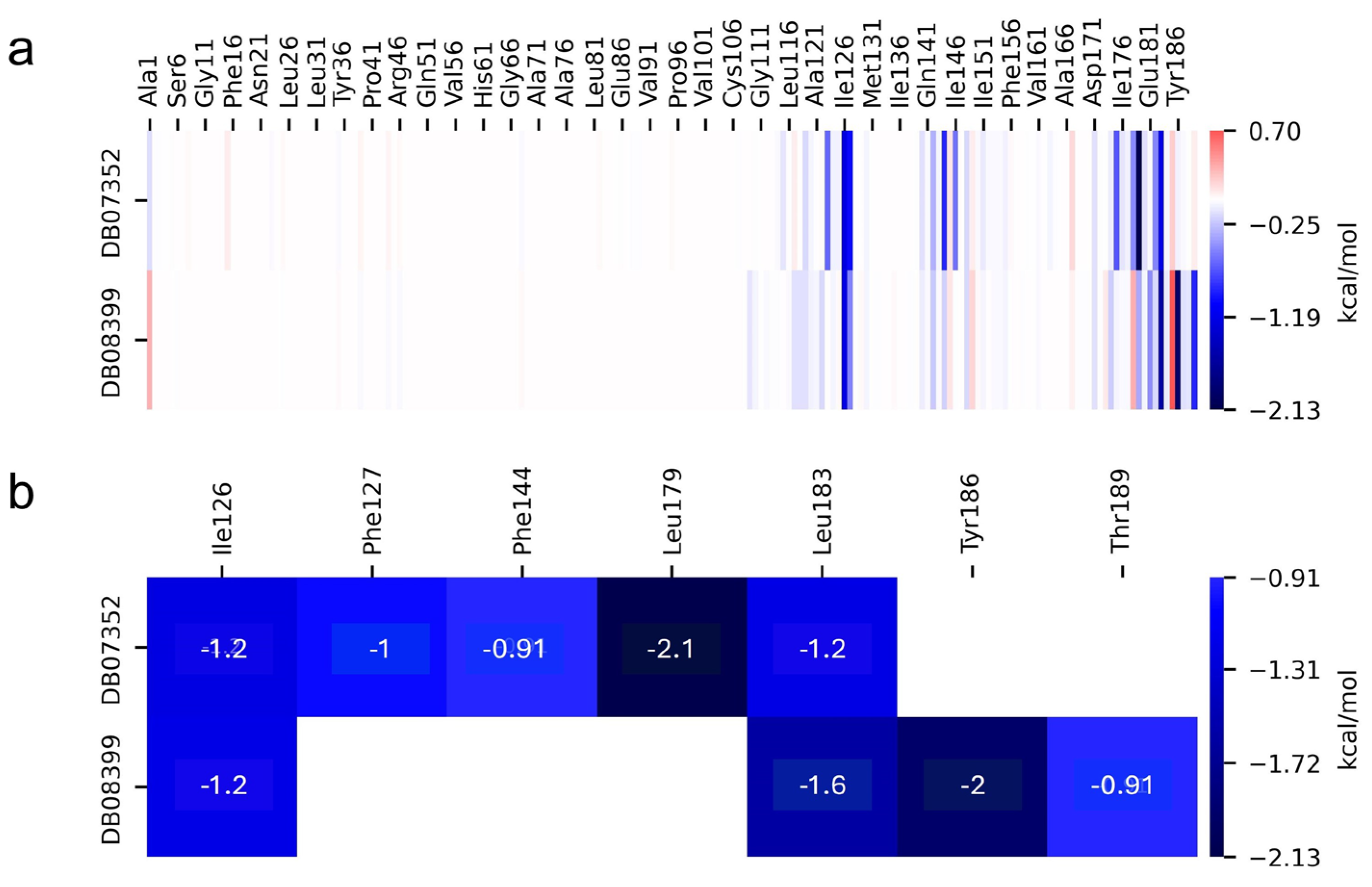
2.5. MM-PBSA Analysis
2.6. Giardia lamblia Viability and Adherence Assays
3. Discussion
4. Materials and Methods
4.1. Protein Sequence Retrieval
4.2. Multiple Sequence Alignments
4.3. Determination of Ligand Binding Site
4.4. Molecular Docking
4.5. Molecular Dynamics
4.6. MM-PBSA Analysis
4.7. Susceptibility Assays and Parasites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anisuzzaman; Hossain, M.S.; Hatta, T.; Labony, S.S.; Kwofie, K.D.; Kawada, H.; Tsuji, N.; Alim, M.A. Food- and Vector-Borne Parasitic Zoonoses: Global Burden and Impacts. Adv. Parasitol. 2023, 120, 87–136. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef]
- Steverding, D. The Spreading of Parasites by Human Migratory Activities. Virulence 2020, 11, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. World Neglected Tropical Diseases Day. PLoS Negl. Trop. Dis. 2020, 14, e0007999. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, M.-S.; Hayat, F.; Shin, D. Recent Advances in the Discovery of Novel Antiprotozoal Agents. Molecules 2019, 24, 3886. [Google Scholar] [CrossRef] [PubMed]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [PubMed]
- Cernikova, L.; Faso, C.; Hehl, A.B. Five Facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef] [PubMed]
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF Global Causes of Diarrheal Disease Mortality in Children <5 Years of Age: A Systematic Review. PLoS ONE 2013, 8, e72788. [Google Scholar] [CrossRef]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium Join the “Neglected Diseases Initiative”. Trends Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef]
- Adam, R.D. Giardia Duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e0002419. [Google Scholar] [CrossRef]
- Escobedo, A.A.; Cimerman, S. Giardiasis: A Pharmacotherapy Review. Expert Opin. Pharmacother. 2007, 8, 1885–1902. [Google Scholar] [CrossRef] [PubMed]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure-Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Best, A.A.; Morrison, H.G.; McArthur, A.G.; Sogin, M.L.; Olsen, G.J. Evolution of Eukaryotic Transcription: Insights from the Genome of Giardia lamblia. Genome Res. 2004, 14, 1537–1547. [Google Scholar] [CrossRef]
- Parra-Marín, O.; López-Pacheco, K.; Hernández, R.; López-Villaseñor, I. The Highly Diverse TATA Box-Binding Proteins among Protists: A Review. Mol. Biochem. Parasitol. 2020, 239, 111312. [Google Scholar] [CrossRef]
- Santiago, Á.; Razo-Hernández, R.S.; Pastor, N. The TATA-binding Protein DNA-binding Domain of Eukaryotic Parasites Is a Potentially Druggable Target. Chem. Biol. Drug Des. 2020, 95, 130–149. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Ribosomal RNA Transcription Machineries in Intestinal Protozoan Parasites: A Bioinformatic Analysis. Acta Parasitol. 2022, 67, 1788–1799. [Google Scholar] [CrossRef]
- Drummond, D.A.; Bloom, J.D.; Adami, C.; Wilke, C.O.; Arnold, F.H. Why Highly Expressed Proteins Evolve Slowly. Proc. Natl. Acad. Sci. USA 2005, 102, 14338–14343. [Google Scholar] [CrossRef]
- Hoshiyama, D.; Kuma, K.; Miyata, T. Extremely Reduced Evolutionary Rate of TATA-Box Binding Protein in Higher Vertebrates and Its Evolutionary Implications. Gene 2001, 280, 169–173. [Google Scholar] [CrossRef]
- Walters, H.A.; Temesvari, L.A. Target Acquired: Transcriptional Regulators as Drug Targets for Protozoan Parasites. Int. J. Parasitol. 2021, 51, 599–611. [Google Scholar] [CrossRef]
- Kramm, K.; Engel, C.; Grohmann, D. Transcription Initiation Factor TBP: Old Friend New Questions. Biochem. Soc. Trans. 2019, 47, 411–423. [Google Scholar] [CrossRef]
- Ferreira, R.; Schneekloth, J.S.; Panov, K.I.; Hannan, K.M.; Hannan, R.D. Targeting the RNA Polymerase I Transcription for Cancer Therapy Comes of Age. Cells 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.-H. Targeting Transcription Factors for Cancer Treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Holm, L. Advances and Pitfalls of Protein Structural Alignment. Curr. Opin. Struct. Biol. 2009, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.L. Enhancing Statistical Multiple Sequence Alignment and Tree Inference Using Structural Information. Methods Mol. Biol. 2019, 1851, 183–214. [Google Scholar] [CrossRef]
- Hurt, D.E.; Sutton, A.E.; Clardy, J. Brequinar Derivatives and Species-Specific Drug Design for Dihydroorotate Dehydrogenase. Bioorg. Med. Chem. Lett. 2006, 16, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Kulakova, L.; Southall, N.; Marugan, J.J.; Galkin, A.; Austin, C.P.; Herzberg, O.; Zheng, W. High-Throughput Giardia lamblia Viability Assay Using Bioluminescent ATP Content Measurements. Antimicrob. Agents Chemother. 2011, 55, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.W.; Walker, J.E. Mechanism of Inhibition of Bovine F1-ATPase by Resveratrol and Related Polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef]
- Palomo-Ligas, L.; Estrada-Camacho, J.; Garza-Ontiveros, M.; Vargas-Villanueva, J.R.; Gutiérrez-Gutiérrez, F.; Nery-Flores, S.D.; Cañas Montoya, J.A.; Ascacio-Valdés, J.; Campos-Muzquiz, L.G.; Rodriguez-Herrera, R. Polyphenolic Extract from Punica Granatum Peel Causes Cytoskeleton-Related Damage on Giardia lamblia Trophozoites in Vitro. PeerJ 2022, 10, e13350. [Google Scholar] [CrossRef]
- Puhl, A.C.; Bernardes, A.; Silveira, R.L.; Yuan, J.; Campos, J.L.O.; Saidemberg, D.M.; Palma, M.S.; Cvoro, A.; Ayers, S.D.; Webb, P.; et al. Mode of Peroxisome Proliferator-Activated Receptor γ Activation by Luteolin. Mol. Pharmacol. 2012, 81, 788–799. [Google Scholar] [CrossRef]
- Mewshaw, R.E.; Edsall, R.J.; Yang, C.; Manas, E.S.; Xu, Z.B.; Henderson, R.A.; Keith, J.C.; Harris, H.A. ERbeta Ligands. 3. Exploiting Two Binding Orientations of the 2-Phenylnaphthalene Scaffold to Achieve ERbeta Selectivity. J. Med. Chem. 2005, 48, 3953–3979. [Google Scholar] [CrossRef]
- Guo, R.-T.; Cao, R.; Liang, P.-H.; Ko, T.-P.; Chang, T.-H.; Hudock, M.P.; Jeng, W.-Y.; Chen, C.K.-M.; Zhang, Y.; Song, Y.; et al. Bisphosphonates Target Multiple Sites in Both Cis- and Trans-Prenyltransferases. Proc. Natl. Acad. Sci. USA 2007, 104, 10022–10027. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Ye, Q.; Dinaut, A.N.; Wang, Q.; Waddleton, D.; Payette, P.; Ramachandran, C.; Kennedy, B.; Hum, G.; Taylor, S.D. Structure of Protein Tyrosine Phosphatase 1B in Complex with Inhibitors Bearing Two Phosphotyrosine Mimetics. J. Med. Chem. 2001, 44, 4584–4594. [Google Scholar] [CrossRef]
- D’Alessio, R.; Bargiotti, A.; Metz, S.; Brasca, M.G.; Cameron, A.; Ermoli, A.; Marsiglio, A.; Polucci, P.; Roletto, F.; Tibolla, M.; et al. Benzodipyrazoles: A New Class of Potent CDK2 Inhibitors. Bioorg Med. Chem. Lett. 2005, 15, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, J.; Abbasi-Maleki, S. Effect of Origanum Vulgare Hydroalcoholic Extract on Giardia lamblia Cysts Compared with Metronidazole in Vitro. Iran. J. Parasitol. 2018, 13, 486–492. [Google Scholar] [PubMed]
- Narwal, M.; Haikarainen, T.; Fallarero, A.; Vuorela, P.M.; Lehtiö, L. Screening and Structural Analysis of Flavones Inhibiting Tankyrases. J. Med. Chem. 2013, 56, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Cianci, M.; Folli, C.; Zonta, F.; Florio, P.; Berni, R.; Zanotti, G. Structural Evidence for Asymmetric Ligand Binding to Transthyretin. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Structural Insight into the Interactions between Death-Associated Protein Kinase 1 and Natural Flavonoids. J. Med. Chem. 2015, 58, 7400–7408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three Flavonoids Targeting the Beta-Hydroxyacyl-Acyl Carrier Protein Dehydratase from Helicobacter Pylori: Crystal Structure Characterization with Enzymatic Inhibition Assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Trivella, D.B.B.; dos Reis, C.V.; Lima, L.M.T.R.; Foguel, D.; Polikarpov, I. Flavonoid Interactions with Human Transthyretin: Combined Structural and Thermodynamic Analysis. J. Struct. Biol. 2012, 180, 143–153. [Google Scholar] [CrossRef]
- DRUGBANK. Available online: https://go.drugbank.com/ (accessed on 13 August 2023).
- Morachis, J.M.; Huang, R.; Emerson, B.M. Identification of Kinase Inhibitors That Target Transcription Initiation by RNA Polymerase II. Oncotarget 2011, 2, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T. Targeting Transcription Through Inhibition of TBP. Oncotarget 2011, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Parra-Marín, O.; Rosas-Hernández, L.; López-Pacheco, K.; Franco, B.; Ibáñez-Escribano, A.; Hernández, R.; López-Villaseñor, I. An in Vitro Characterisation of the Trichomonas vaginalis TATA Box-Binding Proteins (TBPs). Parasitol. Res. 2019, 118, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Henley, M.J.; Koehler, A.N. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat. Rev. Drug Discov. 2021, 20, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Volkamer, A.; Kuhn, D.; Rippmann, F.; Rarey, M. DoGSiteScorer: A Web Server for Automatic Binding Site Prediction, Analysis and Druggability Assessment. Bioinformatics 2012, 28, 2074–2075. [Google Scholar] [CrossRef] [PubMed]
- Ghersi, D.; Sanchez, R. Improving Accuracy and Efficiency of Blind Protein-Ligand Docking by Focusing on Predicted Binding Sites. Proteins 2009, 74, 417–424. [Google Scholar] [CrossRef]
- Hetényi, C.; van der Spoel, D. Efficient Docking of Peptides to Proteins without Prior Knowledge of the Binding Site. Protein Sci. 2009, 11, 1729–1737. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein-Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser InterfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Reyes-Vivas, H.; de la Mora-de la Mora, I.; Castillo-Villanueva, A.; Yépez-Mulia, L.; Hernández-Alcántara, G.; Figueroa-Salazar, R.; García-Torres, I.; Gómez-Manzo, S.; Méndez, S.T.; Vanoye-Carlo, A.; et al. Giardial triosephosphate isomerase as possible target of the cytotoxic effect of omeprazole in Giardia lamblia. Antimicrob. Agents Chemother. 2014, 58, 7072–7082. [Google Scholar] [CrossRef]





| Compound | Target Reported | Giardicidal Effect | BFE and IP | Other Functions |
|---|---|---|---|---|
 DB04583 | Dihydroorotate dehydrogenase (quinone), mitochondrial | Negative | −8.365 kcal/mol. HI. I126, F127, F144, K178, E181; HB. S128, S129, K145; SB. K178; π-stacking. F127. | New inhibitor of Human dihydroorotate dehydrogenase [25]. |
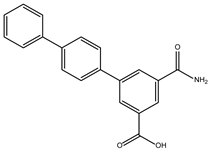
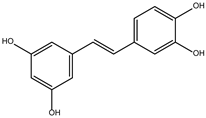 DB08399 (Piceatannol) | ATP synthase subunit (alpha, beta, gamma) mitochondrial. | Positive | −7.318 kcal/mol. HI. F127, F144, K178; HB. S128, S129, K145, E181. | Effect on the assembly of microtubules [26]. Piceatannol blocks both the hydrolysis and synthesis of ATP through the ATP synthase mitochondrial [27]. |
 DB06927 | Estrogen receptor beta. Nuclear receptor coactivator 1. Estrogen receptor alpha. | Negative | −7.749 kcal/mol. HI. F127, F144, K178; HB. S128, S129, K145, E181. | Not available. |
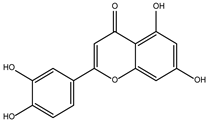 DB15584 (Luteoline) | Not Available. | Positive | −7.720 kcal/mol. HI. F127, K178; HB. S128, S129, K145, K178, G182. | Effect on the growth and decrease in the adhesion of trophozoites treated with 200 μg/mL. IC50 of 179 μg/mL [28]. Luteolin is a flavonoid with anti-inflammatory actions that bind PPARγ [29]. |
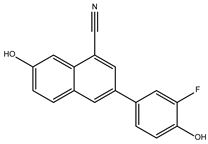 DB06875 (ERB-196) | Estrogen receptor beta. Nuclear receptor coactivator 1. | Negative | −8.090 kcal/mol. HI. F127, F144, K178; HB. V143, K145, K178, E181. | WAY-202196 (DB06875) was effective in two inflammation models, suggesting that targeting estrogen receptors may be medically helpful in treating certain chronic inflammatory diseases [30]. |
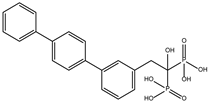 DB07409 | Ditrans-polycis-undecaprenyl-diphosphate synthase ((2E,6E)-farnesyl-diphosphate specific). | Negative | −7.888 kcal/mol. HI. I126, F127, F144, K178, E181; HB. I126, S128, S129, V143, K145. | Inhibitor of undecaprenyl diphosphate synthase [31]. |
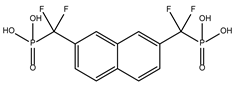 DB03154 | Tyrosine-protein phosphatase non-receptor type 1. | Negative | −7.712 kcal/mol. HI. F127, F144, K178; HB. S128, S129; HalB. E181. | Inhibitor of Human Tyrosine Phosphatase 1B [32]. This enzyme is an attractive therapeutic target because it regulates insulin sensitivity [32]. |
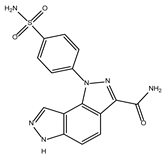 DB08527 | Cyclin-A2. Cyclin-dependent kinase 2. | Negative | −7.743 kcal/mol. HI. I126, F127, F144, K178; HB. S128, S129, K145, K178. | A new class of CDK2 inhibitors [33]. Anti-proliferative activity [33]. |
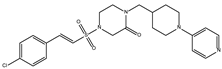 DB08745 | Coagulation factor X. | Negative | −7.355 kcal/mol. HI. I126, F127, V143, K145; HB. S129, N150. | Not available. |
 DB07352 (Apigenin) | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ. | Positive | −7.459 kcal/mol. HI. F127, F144, K178; HB. S128, S129, K145, K178. | Anti-giardia activity is due to the presence of phenolic compounds and flavonoids, among which is Apigenin [34]. tankyrase inhibitor [35]. This compound has been proposed as effective drugs in the therapy of TTR amyloidosis [36]. DAPK1 (inhibitor) is a possible target for treating acute ischemic stroke and endometrial adenocarcinomas [37]. Competitive inhibitor against protein dehydratase from Helicobacter pylori (HpFabZ) [38]. This compound may act as Transthyretin (TTR) amyloid inhibitor [39]. |
| TBP/Ligand | ∆Evdw | ∆Eele | ∆Gpolar | ∆GSA | ∆Gb |
|---|---|---|---|---|---|
| GlTBP/DB07352 | −29.58 ± 0.17 | −1.64 ± 0.12 | 15.25 ± 0.16 | −3.31 ± 0.01 | −19.28 ± 0.15 |
| GlTBP/DB08399 | −23.53 ± 0.19 | −9.32 ± 0.17 | 21.89 ± 0.18 | −3.21 ± 0.01 | −14.16 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaona-López, C.; Méndez-Álvarez, D.; Moreno-Rodríguez, A.; Bautista-Martínez, J.L.; De Fuentes-Vicente, J.A.; Nogueda-Torres, B.; García-Torres, I.; López-Velázquez, G.; Rivera, G. TATA-Binding Protein-Based Virtual Screening of FDA Drugs Identified New Anti-Giardiasis Agents. Int. J. Mol. Sci. 2024, 25, 6238. https://doi.org/10.3390/ijms25116238
Gaona-López C, Méndez-Álvarez D, Moreno-Rodríguez A, Bautista-Martínez JL, De Fuentes-Vicente JA, Nogueda-Torres B, García-Torres I, López-Velázquez G, Rivera G. TATA-Binding Protein-Based Virtual Screening of FDA Drugs Identified New Anti-Giardiasis Agents. International Journal of Molecular Sciences. 2024; 25(11):6238. https://doi.org/10.3390/ijms25116238
Chicago/Turabian StyleGaona-López, Carlos, Domingo Méndez-Álvarez, Adriana Moreno-Rodríguez, Juan Luis Bautista-Martínez, José Antonio De Fuentes-Vicente, Benjamín Nogueda-Torres, Itzhel García-Torres, Gabriel López-Velázquez, and Gildardo Rivera. 2024. "TATA-Binding Protein-Based Virtual Screening of FDA Drugs Identified New Anti-Giardiasis Agents" International Journal of Molecular Sciences 25, no. 11: 6238. https://doi.org/10.3390/ijms25116238






