Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System
Abstract
1. Introduction
2. Results and Discussion
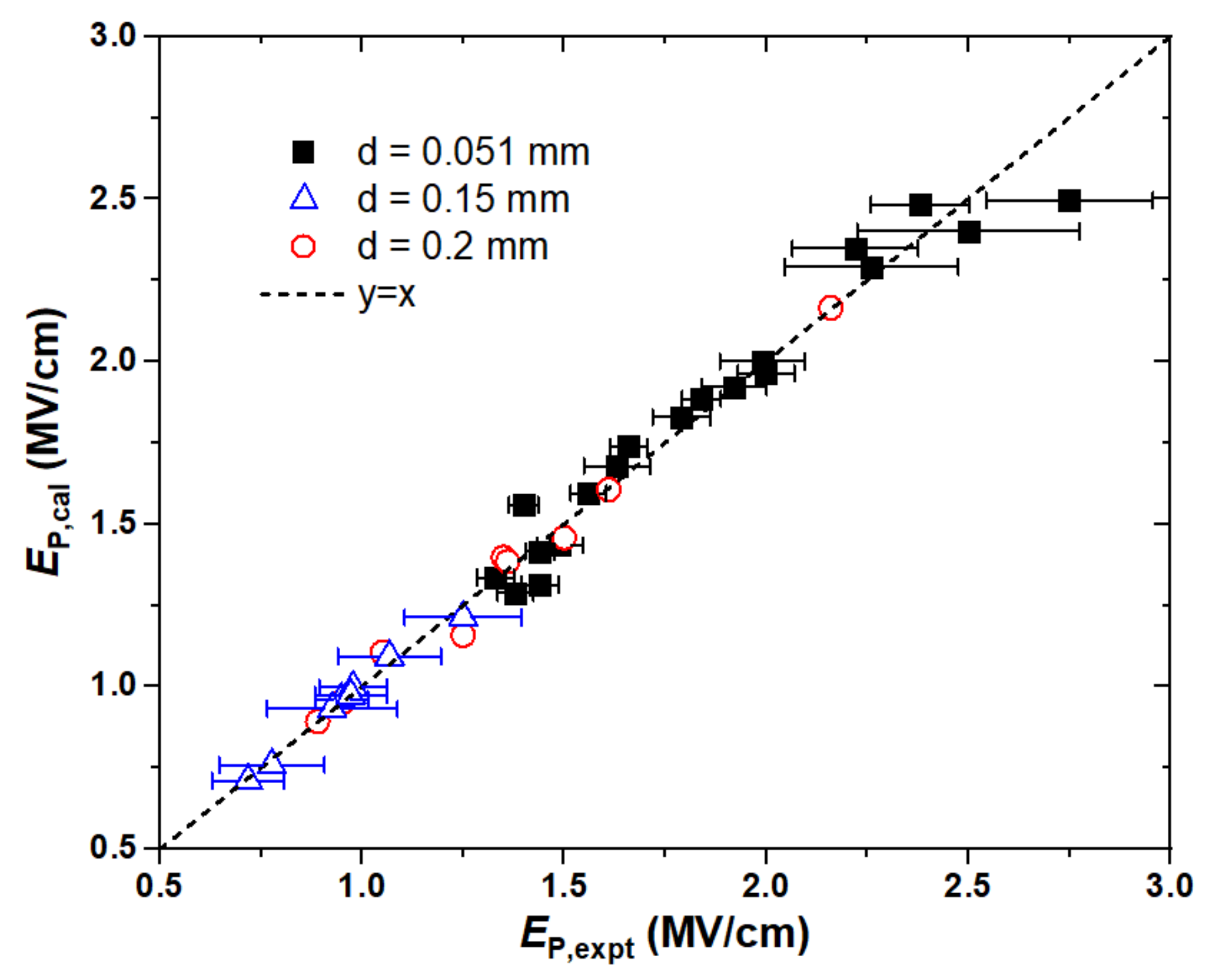
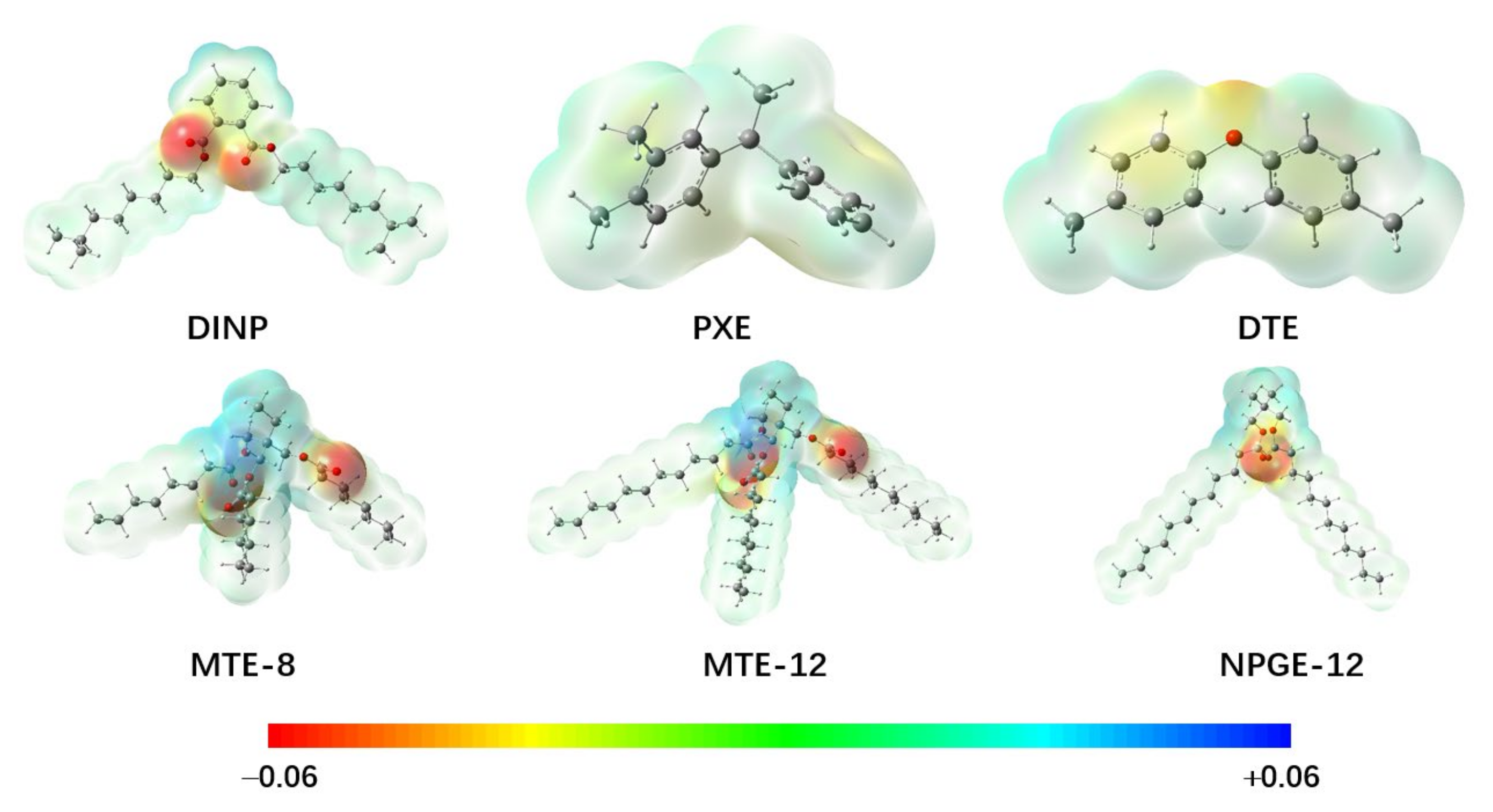
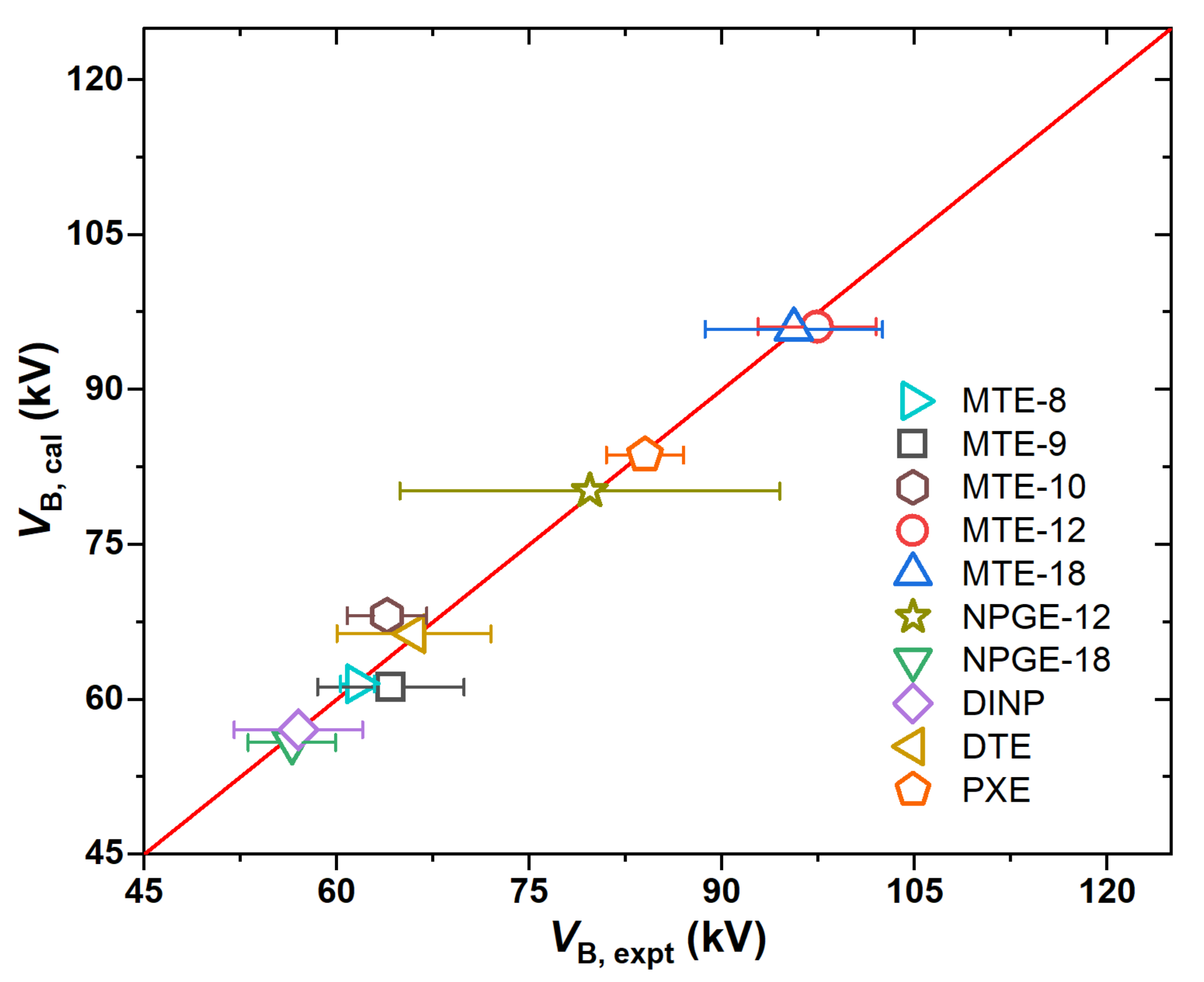
2.1. Electrical Strength
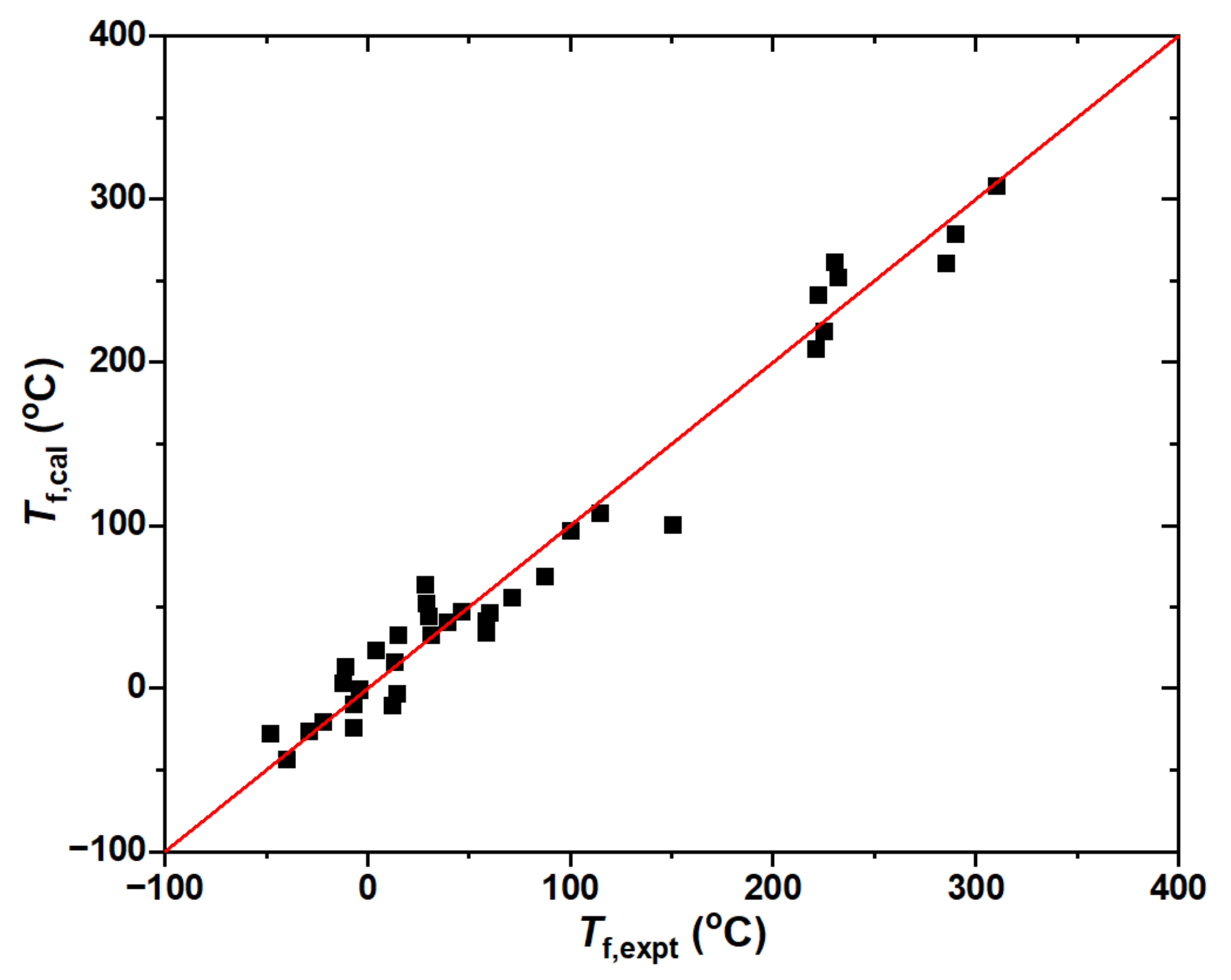
2.2. Flash Point
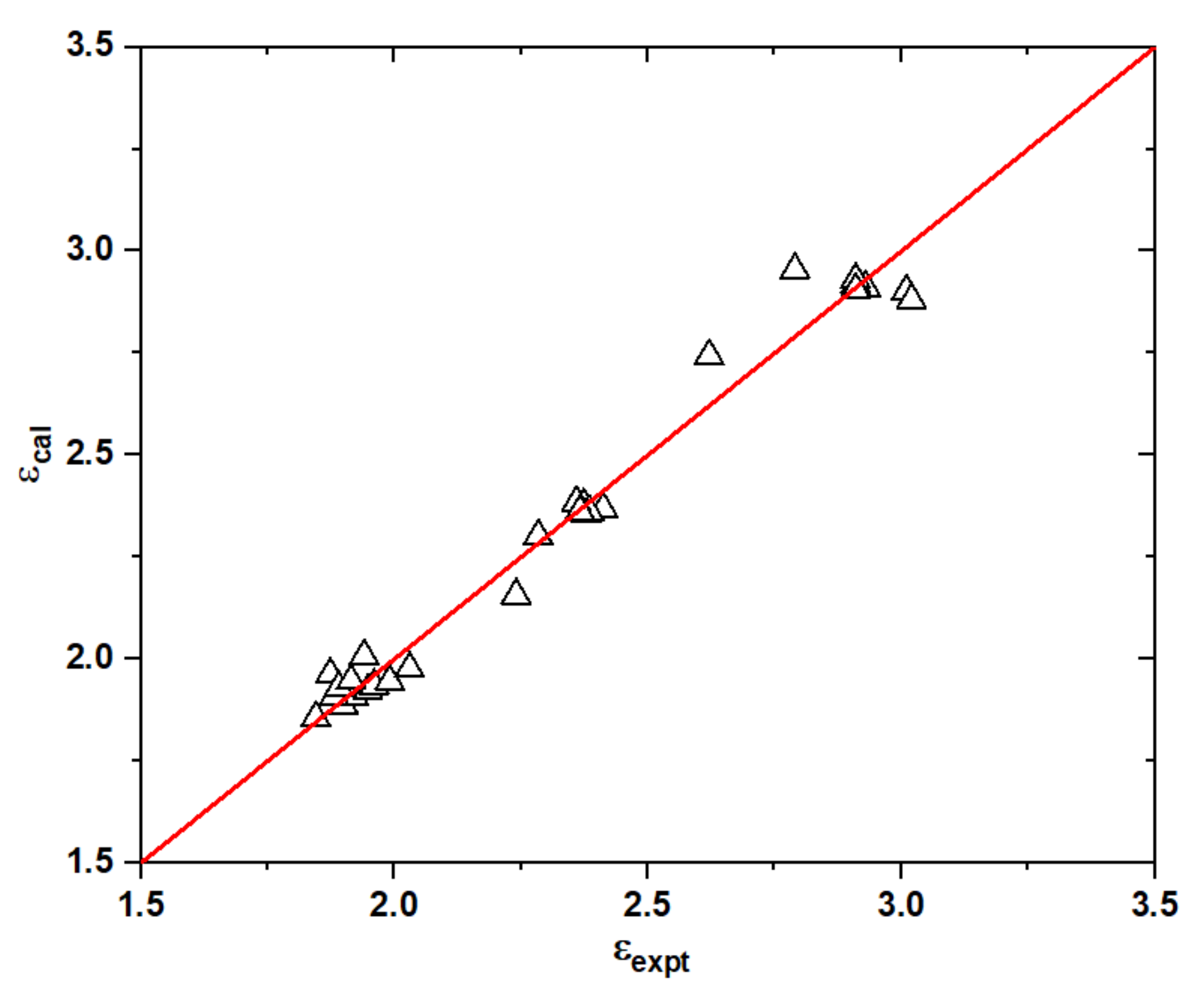
2.3. Dielectric Constant
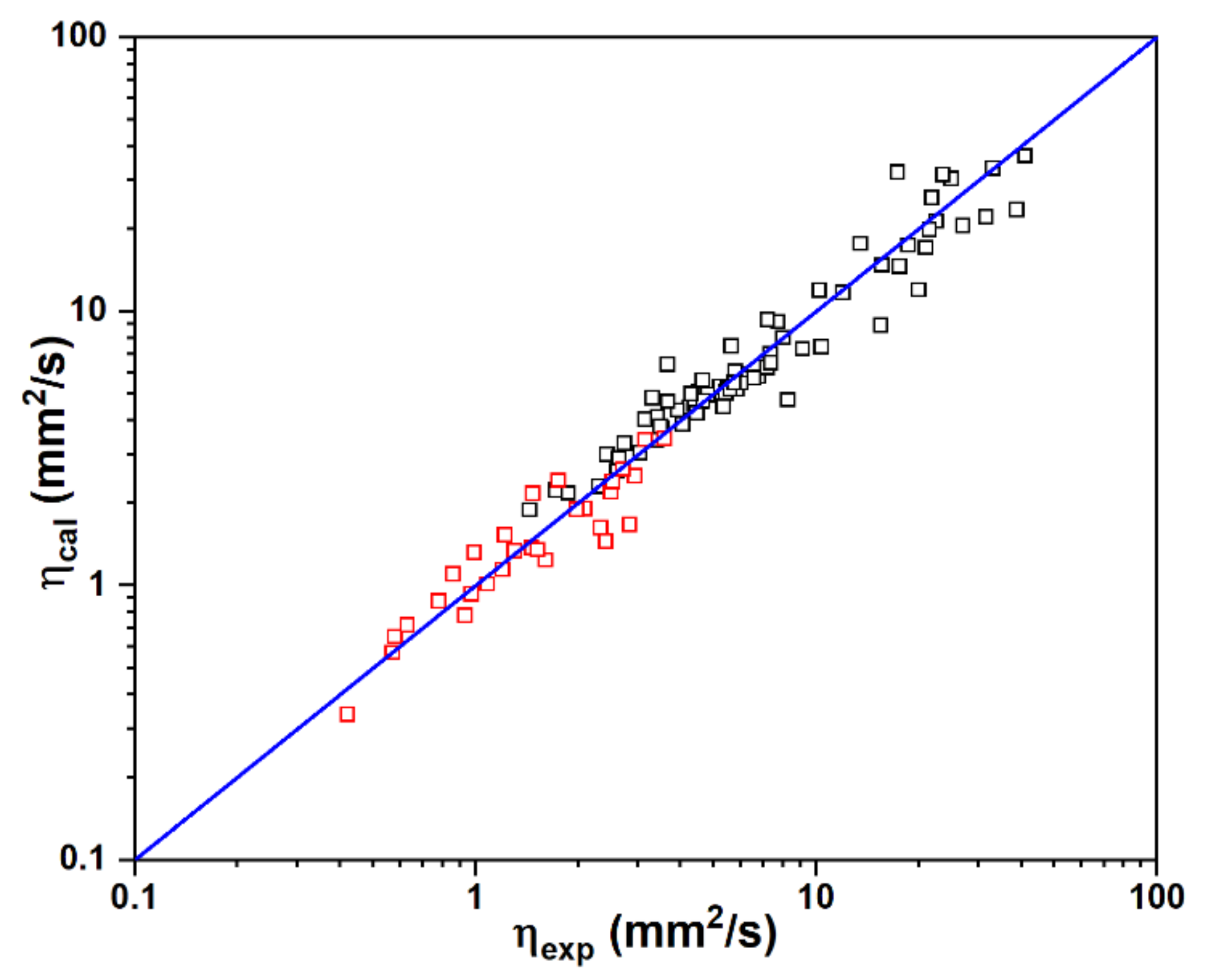
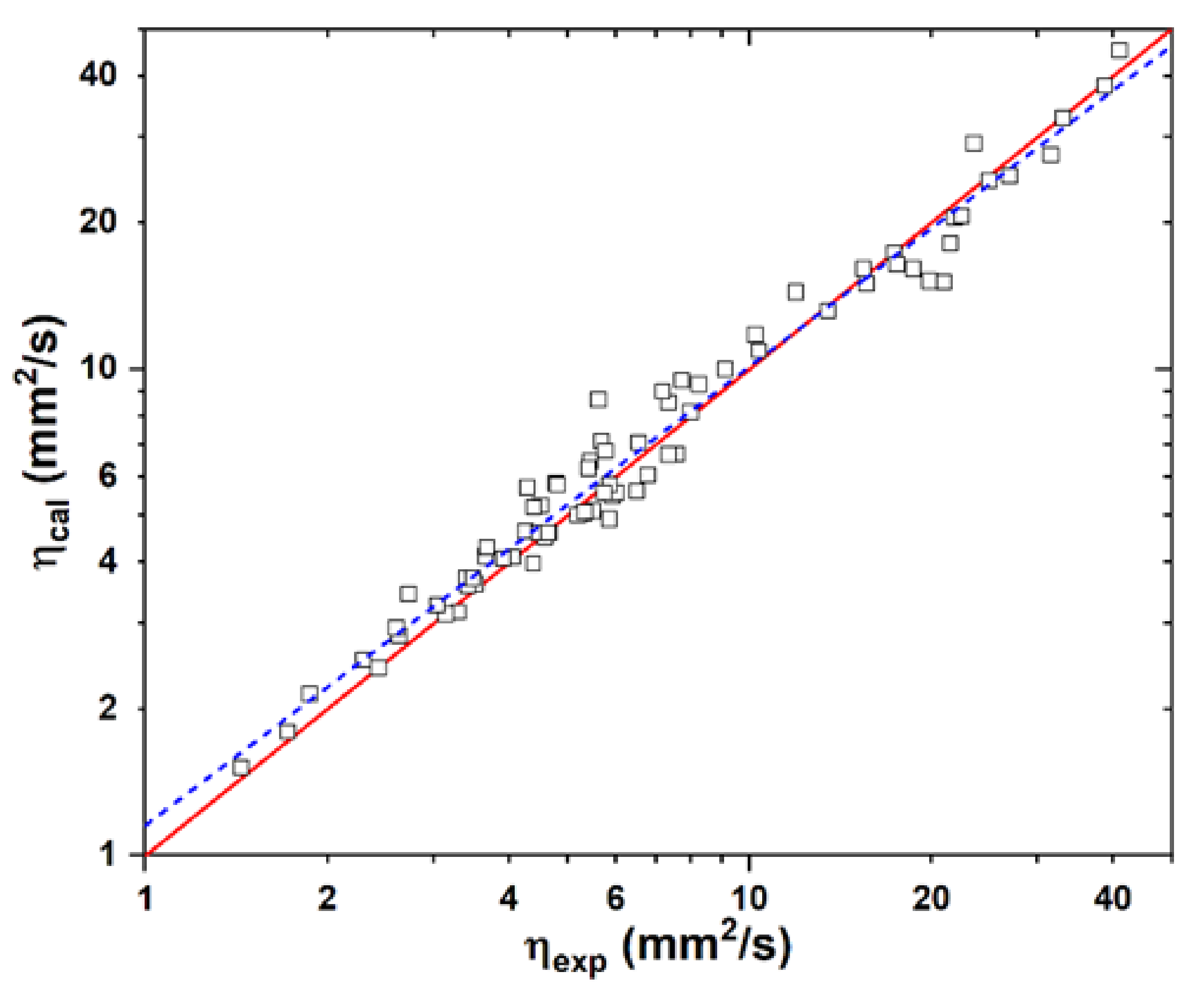
2.4. Kinematic Viscosity
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claiborne, C.C.; Pearce, H.A. Transformer fluids. IEEE Electr. Insul. Mag. 1989, 5, 16–19. [Google Scholar] [CrossRef]
- Wedin, P. Electrical breakdown in dielectric liquids—A short overview. IEEE Electr. Insul. 2014, 30, 20–25. [Google Scholar] [CrossRef]
- Fernández, I.; Ortiz, A.; Delgado, F.; Renedo, C.; Pérez, S. Comparative evaluation of alternative fluids for power transformers. Electr. Power Syst. Res. 2013, 98, 58–69. [Google Scholar] [CrossRef]
- Lashbrook, M.; Gyore, A.; Martin, R. A review of the fundamental dielectric characteristics of ester-based dielectric liquids. Procedia Eng. 2017, 202, 121–129. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative Dielectric Fluids for Transformer Insulation System: Progress, Challenges, and Future Prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar]
- Shen, Z.; Wang, F.; Wang, Z.; Li, J. A critical review of plant-based insulating fluids for transformer: 30-year development. Renew. Sustain. Energy Rev. 2021, 141, 110783. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Engineering Green Lubricants II: Thermal Transition and Flow Properties of Vegetable Oil-Derived Diesters. ACS Sustain. Chem. Eng. 2016, 4, 693–700. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Engineering Green Lubricants I: Optimizing Thermal and Flow Properties of Linear Diesters Derived from Vegetable Oils. ACS Sustain. Chem. Eng. 2016, 4, 686–692. [Google Scholar] [CrossRef]
- Tokunaga, J.; Nikaido, M.; Koide, H.; Hikosaka, T. Palm fatty acid ester as biodegradable dielectric fluid in transformers: A review. IEEE Elect. Insul. Mag. 2019, 35, 34–46. [Google Scholar] [CrossRef]
- Raof, N.A.; Rashid, U.; Yunus, R.; Azis, N.; Yaakub, Z. Development of palm-based neopentyl glycol diester as dielectric fluid and its thermal aging performance. IEEE Trans. Dielectr. Elect. Insul. 2016, 23, 2051–2058. [Google Scholar] [CrossRef]
- Idrus, N.F.; Yunus, R.; Zainal Abidin, Z.; Rashid, U.; Abd Rahman, N. High oleic pentaerythritol tetraester formation via transesterification: Effect of reaction conditions. Indones. J. Chem. 2020, 20, 887–898. [Google Scholar] [CrossRef]
- Dixon, J.A.; Webb, W.; Steele, W.A. Properties of Hydrocarbons of High Molecular Weight Synthesized by Research Project 42 of the American Petroleum Institute; Pennsylvania State University (American Petroleum Institute): Washington, DC, USA, 1962. [Google Scholar]
- Franck, C.M.; Chachereau, A.; Pachin, J. SF6-Free Gas-Insulated Switchgear: Current Status and Future Trends. IEEE Electr. Insul. Mag. 2021, 37, 7–16. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, K.; Hou, H.; Wang, B. Review on computational screening and molecular design of replacement gases for SF6. High Volt. Eng. 2022, 49, 2816–2831. [Google Scholar]
- Rabie, M.; Franck, C.M. Computational Screening of New High Voltage Insulation Gases with Low Global Warming Potential. IEEE Trans. Dielectr. Elect. Insul. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Forster, E.O.; Mazzetti, C.; Pompili, M.; Cecere, R. The effect of molecular structure on the properties of dielectric fluids. IEEE Trans. Elect. Insul. 1991, 26, 749–754. [Google Scholar] [CrossRef]
- Casserly, E. The relationship between the physical, chemical, and functional properties of insulating liquids. In Proceedings of the 2019 IEEE 20th International Conference on Dielectric Liquids (ICDL), Rome, Italy, 23–27 June 2019. [Google Scholar]
- Sharbaugh, A.H.; Crowe, R.W.; Cox, E.B. Influence of Molecular Structure upon the Electric Strengths of Liquid Hydrocarbons. J. Appl. Phys. 1956, 27, 806–808. [Google Scholar] [CrossRef]
- Crowe, R.W.; Sharbaugh, A.H.; Bragg, J.K. Electric Strength and Molecular Structure of Saturated Hydrocarbon Liquids. J. Appl. Phys. 1954, 25, 1480–1484. [Google Scholar] [CrossRef]
- Kao, K.C. Theory of High-Field Electric Conduction and Breakdown in Dielectric Liquids. IEEE Trans. Electr. Insul. 1976, EI-11, 121–128. [Google Scholar] [CrossRef]
- Nelson, J.K.; McGrath, P.B.; Hashad, I.F.M. A comparison of the electrical breakdown characteristics of hydro- and fluorocarbon liquids. J. Electrost. 1979, 7, 67–74. [Google Scholar] [CrossRef]
- Sharbaugh, A.H.; Devins, J.C.; Rzed, S.J. Progress in the field of electric breakdown in dielectric liquids. IEEE Trans. Electr. Insul. 1978, EI-13, 249–275. [Google Scholar]
- IEC 60156; Insulating Liquids—Determination of the Breakdown Voltage at Power Frequency—Test Method. Swedish Institute for Standards: Stockholm, Sweden, 1995.
- IEC 61099; Insulating Liquids—Specifications for Unused Synthetic Organic Esters for Electrical Purposes—Test Method. Swedish Institute for Standards: Stockholm, Sweden, 2010.
- Raof, N.A.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effects of molecular structure on the physical, chemical, and electrical properties of ester-based transformer insulating liquids. J. Amer. Oil Chem. Soc. 2019, 96, 607–616. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Wang, C.; Shu, Y.; Ouyang, L.; Wang, Q.; Huang, Z.; Li, J. Optimizing Molecular Structure for Trimethylolpropane Ester-Insulating Oil: Achieving High Fluidity and Stability. IEEE Trans. Dielectr. Elect. Insul. 2023, 30, 1432–1440. [Google Scholar] [CrossRef]
- Yu, X.; Hou, H.; Wang, B. Prediction on dielectric strength and boiling point of gaseous molecules for replacement of SF6. J. Comput. Chem. 2017, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Yu, X.; Zhou, W.; Luo, Y.; Wang, B. Theoretical investigations on the structure-activity relationship to the dielectric strength of the insulation gases. Chem. J. Chin. Univ. 2018, 39, 2477–2484. [Google Scholar]
- ASTM D93; Standard Test Method for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D445-04e2; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids. ASTM International: West Conshohocken, PA, USA, 2002.
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods. 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Computational prediction of condensed phase properties from statistical characterization of molecular surface electrostatic potentials. Fluid Phase Equilibria 2001, 185, 129–137. [Google Scholar] [CrossRef]

| Descriptors | Description | ηi |
|---|---|---|
| C | Group >C< with 4 single bonds | 0.490 |
| CH | Group >CH- with 3 single bonds | −0.013 |
| CH2 | Group -CH2- with 2 single bonds | 0.109 |
| CH3 | Group CH3- with 1 single bond | 0.226 |
| CC | Group >C=C< double bond | −0.0422 |
| CO2 | -C(=O)O- carbonyl group | −0.0002 |
| OH | Hydroxyl group | 1.842 |
| nC | Number of C atoms in the R group of -C(=O)O-R | 0.0145 |
| mC | Number of C atoms between -C=O/OH and C=C | 0.0483 |
| trans | Number of trans C=C conformations | 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Hou, H.; Wang, B. Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System. Int. J. Mol. Sci. 2024, 25, 6654. https://doi.org/10.3390/ijms25126654
Zhang M, Hou H, Wang B. Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System. International Journal of Molecular Sciences. 2024; 25(12):6654. https://doi.org/10.3390/ijms25126654
Chicago/Turabian StyleZhang, Mi, Hua Hou, and Baoshan Wang. 2024. "Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System" International Journal of Molecular Sciences 25, no. 12: 6654. https://doi.org/10.3390/ijms25126654
APA StyleZhang, M., Hou, H., & Wang, B. (2024). Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System. International Journal of Molecular Sciences, 25(12), 6654. https://doi.org/10.3390/ijms25126654





