Diversity and Pathogenicity of Fusarium Root Rot Fungi from Canola (Brassica napus) in Alberta, Canada
Abstract
1. Introduction
2. Results
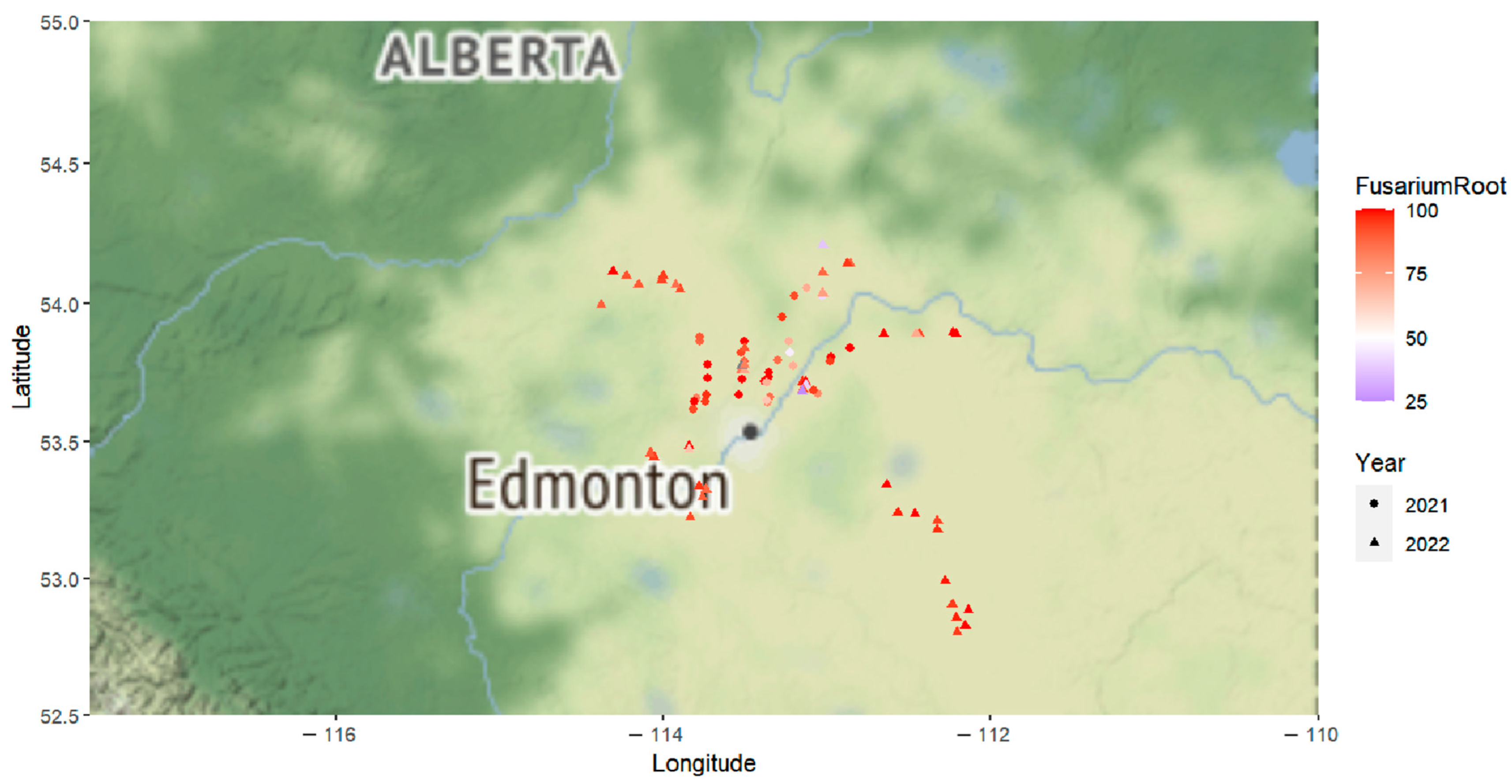
2.1. Root Rot Incidence and Pathogen Recovery
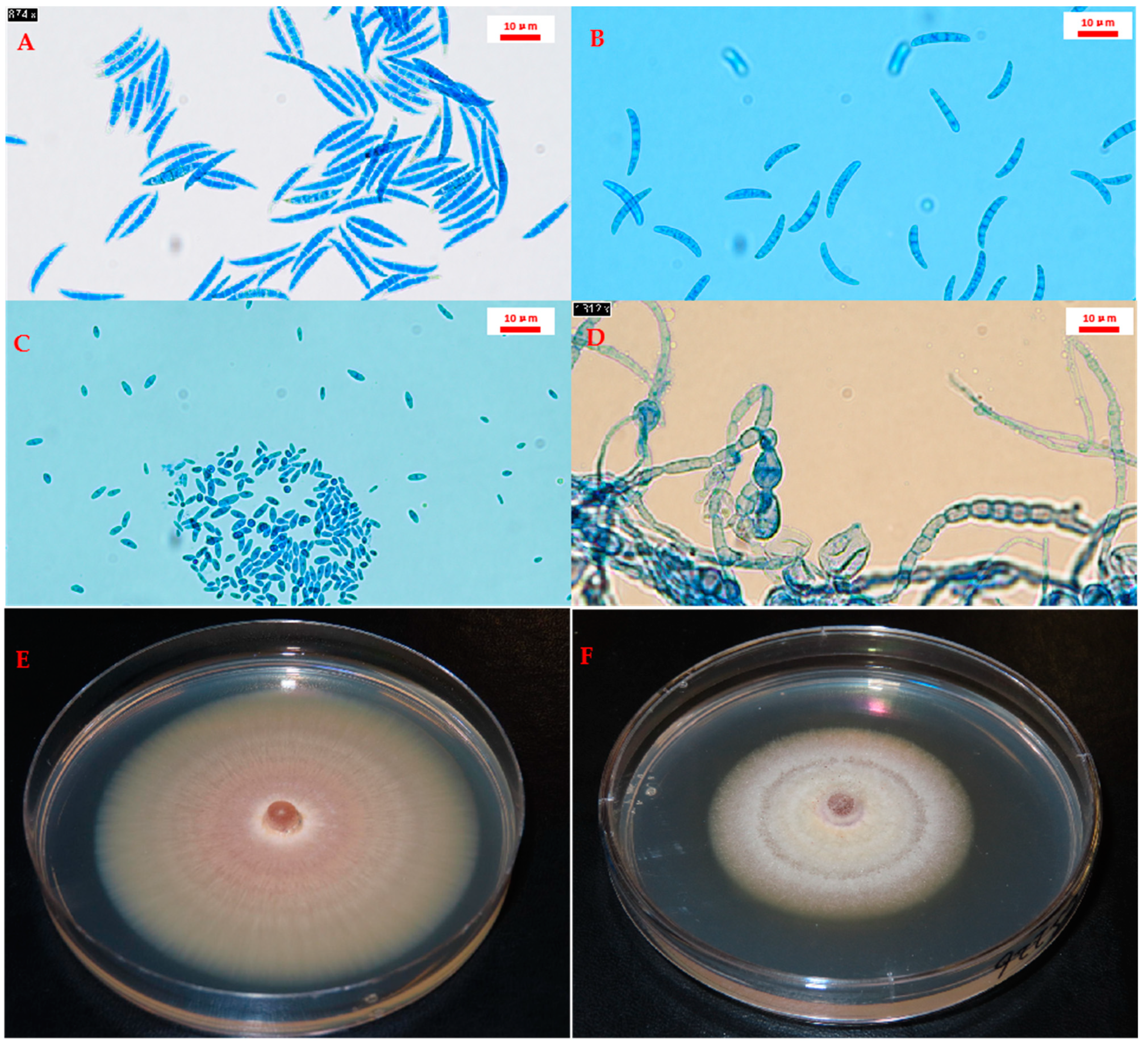
2.2. Fusarium Species Identification and Prevalence
2.3. Pathogenicity Test
2.3.1. Effect of Fusarium spp. on Disease Severity
2.3.2. Effect of Fusarium spp. on Seedling Emergence
2.3.3. Effect of Fusarium spp. on Plant Height
2.3.4. Effect of Fusarium spp. on Shoot Dry Weight
2.3.5. Effect of Fusarium spp. on Root Dry Weight
2.3.6. Comparison of Disease Severity between Fusarium avenaceum and other Fusarium Species
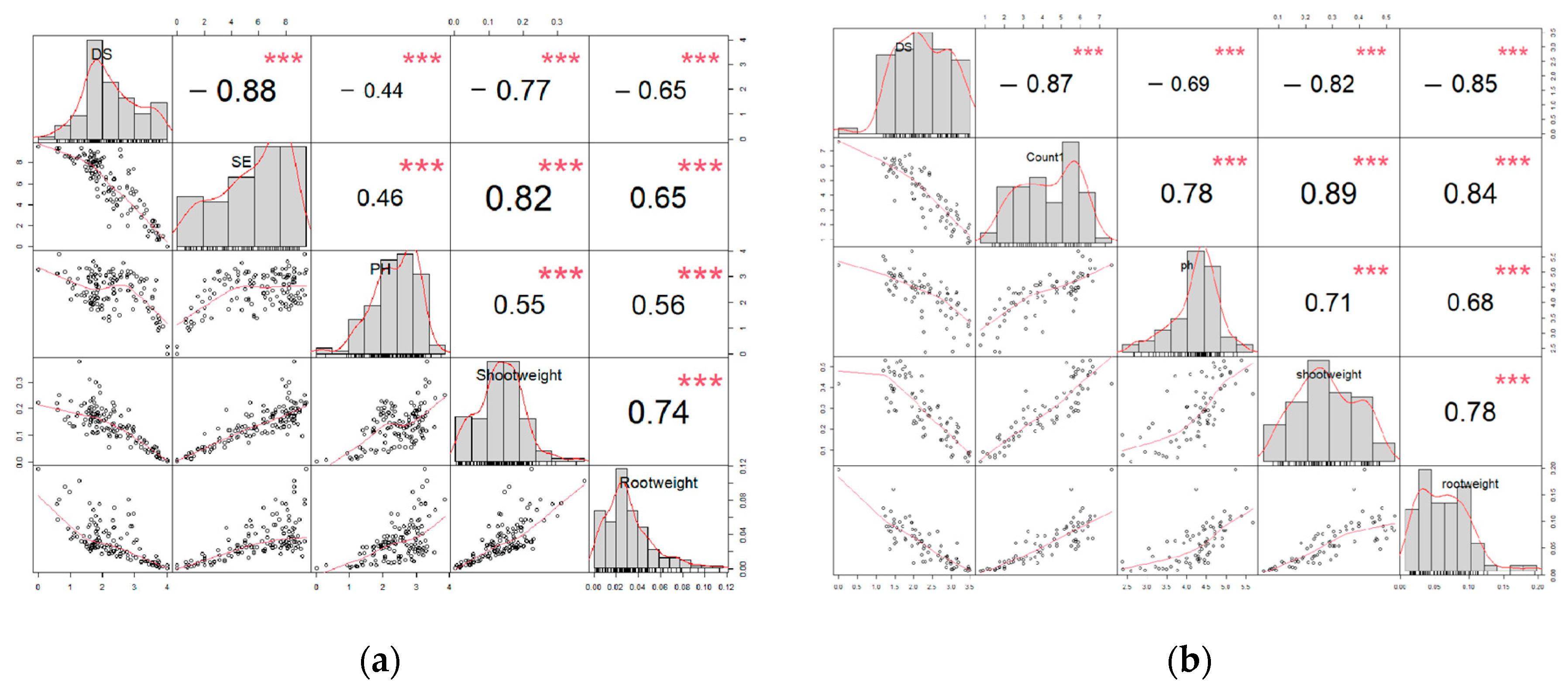
2.3.7. Correlation and Principal Component Analysis Based on Disease Severity and Growth Parameters
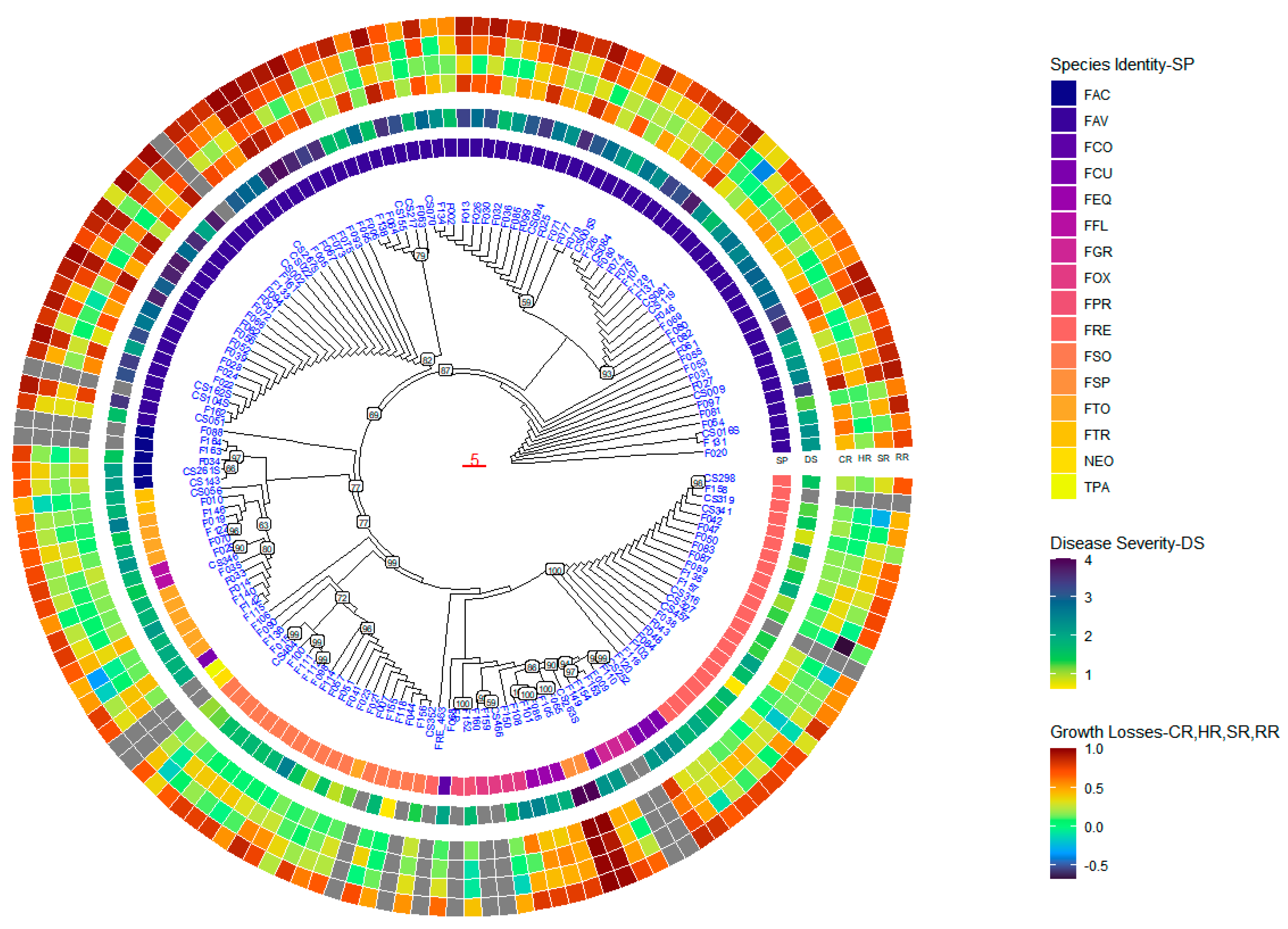
2.4. Phylogenetic Analysis
2.4.1. Phylogenetic Tree Based on ITS Factor Sequences
2.4.2. Phylogenetic Tree Based on TEF-1α Sequence
2.4.3. Phylogenetic Tree Based on the Concatenated TEF-1α and ITS Sequences
2.5. Geographic Origins and Phylogenetic Evolution
3. Discussion
4. Materials and Methods
4.1. Disease Surveys
4.1.1. Root Rot Surveys
4.1.2. Fungal Isolate Recovery
4.2. Isolation and Molecular Identification of Fusarium Species
Hyphal Tip Purification and Species Identification
4.3. Pathogenicity Test
4.4. Disease Ratings
4.5. Emergence, Plant Height, and Shoot and Root Dry Weights
4.6. Phylogenetic Tree Construction
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodah, E. Root Rot Diseases in Plants: A Review of Common Causal Agents and Management Strategies. Agric. Res. Technol. Open Access J. 2017, 5, 555661. [Google Scholar] [CrossRef]
- Wang, H.; Hwang, S.F.; Eudes, F.; Chang, K.F.; Howard, R.J.; Turnbull, G.D. Trichothecenes and Aggressiveness of Fusarium Graminearum Causing Seedling Blight and Root Rot in Cereals. Plant Pathol. 2006, 55, 224–230. [Google Scholar] [CrossRef]
- Arora, H.; Sharma, A.; Sharma, S.; Haron, F.F.; Gafur, A.; Sayyed, R.Z.; Datta, R. Pythium Damping-Off and Root Rot of Capsicum annuum L.: Impacts, Diagnosis, and Management. Microorganisms 2021, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Rebollar-Alviter, A.; Silva-Rojas, H.V.; Fuentes-Aragón, D.; Acosta-González, U.; Martínez-Ruiz, M.; Parra-Robles, B.E. An Emerging Strawberry Fungal Disease Associated with Root Rot, Crown Rot and Leaf Spot Caused by Neopestalotiopsis Rosae in Mexico. Plant Dis. 2020, 104, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Hou, W.; Tang, X.; Zhao, Y. First Report of Root Rot Caused by Fusarium Solani on Roselle in Nanning, Guangxi, China. Plant Dis. 2023, 107, 959. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Monaim, M.F.; Ismail, M.E. The Use of Antioxidants to Control Root Rot and Wilt Diseases of Pepper. Not. Sci. Biol. 2010, 2, 46–55. [Google Scholar] [CrossRef]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding Root Rot Disease in Agricultural Crops. Horticulturae 2021, 7, 33. [Google Scholar] [CrossRef]
- Yu, H.; Chang, K.F.; Hwang, S.F.; Strelkov, S.E. Characterization of the Virulence and Yield Impact of Fusarium Species on Canola (Brassica napus). Plants 2023, 12, 3020. [Google Scholar] [CrossRef] [PubMed]
- Canola Concil of Canada Canola Industry in Canada, from Farm to Global Markets. The Canola Council of Canada 2023. Available online: https://www.canolacouncil.org/markets-stats/top-markets/ (accessed on 25 March 2024).
- Kataria, H.R.; Verma, P.R. Rhizoctonia Solani Damping-off and Root Rot in Oilseed Rape and Canola. Crop Prot. 1992, 11, 8–13. [Google Scholar] [CrossRef]
- Gugel, R.K.; Yitbarek, S.M.; Verma, P.R.; Morrall, R.A.A.; Sadasivaiah, R.S. Etiology of the Rhizoctonia Root Rot Complex of Canola in the Peace River Region of Alberta. Can. J. Plant Pathol. 1987, 9, 119–128. [Google Scholar] [CrossRef]
- Acharya, S.N.; Verma, P.R.; Dueck, J.; Downey, R.K. Screening Rapeseed/Canola for Resistance to Damping-off and Seedling Root Rot Caused by Rhizoctonia solani. Can. J. Plant Pathol. 1984, 6, 325–328. [Google Scholar] [CrossRef]
- Calman, A.I.; Tewari, J.P.; Mugala, M. Fusarium Avenaceum as One of the Causal Agents of Seedling Blight of Canola in Alberta. Plant Dis. 1986, 70, 694. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Strelkov, S.E.; Hwang, S.F. Genetic Diversity and Aggressiveness of Fusarium spp. Isolated from Canola in Alberta, Canada. Plant Dis. 2014, 98, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, Y.; Yang, Y.; Ahmed, H.; Hwang, S.F.; Strelkov, S.E. Effect of Inoculum Density and Quantitative PCR-Based Detection of Rhizoctonia Solani AG-2-1 and Fusarium Avenaceum on Canola. Crop Prot. 2014, 59, 71–77. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Gossen, B.D.; Kutcher, H.R.; Brandt, S.A.; Strelkov, S.E.; Chang, K.F.; Turnbull, G.D. Effect of Crop Rotation on the Soil Pathogen Population Dynamics and Canola Seedling Establishment. Plant Pathol. J. 2009, 8, 106–112. [Google Scholar] [CrossRef]
- Zhou, Q.; Hwang, S.F.; Fu, H.T.; Strelkov, S.E.; Gossen, B.D. Genetic Variation of Rhizoctonia Solani Isolates from Canola in Alberta, Canada. Can. J. Plant Sci. 2014, 94, 671–681. [Google Scholar] [CrossRef]
- Broders, K.D.; Parker, M.L.; Melzer, M.S.; Boland, G.J. Phylogenetic Diversity of Rhizoctonia Solani Associated with Canola and Wheat in Alberta, Manitoba, and Saskatchewan. Plant Dis. 2014, 98, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.R. Fusarium Populations in Roots of Oilseed and Pulse Crops Grown in Eastern Saskatchewan. Can. J. Plant Sci. 2007, 87, 945–952. [Google Scholar] [CrossRef]
- Li, M.; Murray, G.M.; Ash, G.J. New Root Diseases of Canola in Australia. Austral. Plant Dis. Notes 2007, 2, 93–94. [Google Scholar] [CrossRef]
- Okello, P.N.; Petrović, K.; Kontz, B.; Ali, S.; Marek, L.F.; Mathew, F.M. Root Rot Caused by Species of Fusarium on Brassica Carinata in South Dakota. Plant Health Prog. 2018, 19, 188–192. [Google Scholar] [CrossRef]
- Starzycki, M.; Starzycka, E.; Pszczola, J.; Solecka, D. Evaluation of Chosen Winter Rapeseed Genotypes Resistance to Fusarium spp. Using in Vitro Methods. In Proceedings of the 12th International Rapeseed Congress, Wuhan, China, 26–30 March 2007; Science Press USA Inc.: Wuhan, China, 2007; pp. 165–166. [Google Scholar]
- Heydari, S.; Raoufi, F.; Najafi Pour, G. Identification the Etiology of Root and Foot Rot’s Causal Agents of Brassica Napus in Marvdasht (Fars Province). J. Microb. World 2010, 2, 261–269. [Google Scholar]
- Larki, Z.; Farrokhi Nejad, R. Identification of the Fusarium Species Associated with Canola Crown and Root Rot in Khuzestan Province. J. Microb. World 2015, 8, 168–172. [Google Scholar]
- Nemati Mondanipour, O.; Farokhinejad, R.; Mehrabi-Koushki, M. Identification of Fusarium Species Associated with Root Rot Symptoms of the Rapeseed in Khuzestan Province. J. Appl. Res. Plant Prot. 2021, 10, 29–43. [Google Scholar] [CrossRef]
- Mehraj, H.; Akter, A.; Miyaji, N.; Miyazaki, J.; Shea, D.J.; Fujimoto, R.; Doullah, M.A. Genetics of Clubroot and Fusarium Wilt Disease Resistance in Brassica Vegetables: The Application of Marker Assisted Breeding for Disease Resistance. Plants 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Mourou, M.; Raimondo, M.L.; Lops, F.; Carlucci, A. Brassicaceae Fungi and Chromista Diseases: Molecular Detection and Host–Plant Interaction. Plants 2023, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Oksana, S.; Victoria, T.; Lyudmila, G. Breeding and Chemical Methods of Brown Mustard (Brassica juncea L.) Protection from Fusarium Blight; EDP Sciences: Hulis, France, 2022; Volume 43. [Google Scholar] [CrossRef]
- Arie, T. Fusarium Diseases of Cultivated Plants, Control, Diagnosis, and Molecular and Genetic Studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Windels, C. Current Status of Fusarium Taxonomy. Phytopathology 1991, 81, 1048–1051. [Google Scholar]
- Chandra, N.S.; Wulff, E.G.; Udayashankar, A.C.; Nandini, B.P.; Niranjana, S.R.; Mortensen, C.N.; Prakash, H.S. Prospects of Molecular Markers in Fusarium Species Diversity. Appl. Microbiol. Biotechnol. 2011, 90, 1625–1639. [Google Scholar] [CrossRef]
- Kistler, H.C. Genetic Diversity in the Plant-Pathogenic Fungus Fusarium Oxysporum. Phytopathology 1997, 87, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Magdama, F.; Monserrate-Maggi, L.; Serrano, L.; García Onofre, J.; Jiménez-Gasco, M.d.M. Genetic Diversity of Fusarium oxysporum f. sp. Cubense, the Fusarium Wilt Pathogen of Banana, in Ecuador. Plants 2020, 9, 1133. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA Sequence-Based Identification of Fusarium: Current Status and Future Directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Torbati, M.; Arzanlou, M.; da Silva Santos, A.C. Fungicolous Fusarium Species: Ecology, Diversity, Isolation, and Identification. Curr. Microbiol. 2021, 78, 2850–2859. [Google Scholar] [CrossRef]
- Yang, J.; Verma, P.R. Screening Genotypes for Resistance to Pre-Emergence Damping-off and Postemergence Seedling Root Rot of Oilseed Rape and Canola Caused by Rhizoctonia Solani AG-2-1. Crop Prot. 1992, 11, 443–448. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Turnbull, G.D.; Gossen, B.D.; Strelkov, S.E. Effect of Seeding Date and Depth, Seed Size and Fungicide Treatment on Fusarium and Pythium Seedling Blight of Canola. Can. J. Plant Sci. 2015, 95, 293–301. [Google Scholar] [CrossRef]
- Town, J.R.; Dumonceaux, T.; Tidemann, B.; Helgason, B.L. Crop Rotation Significantly Influences the Composition of Soil, Rhizosphere, and Root Microbiota in Canola (Brassica napus L.). Environ. Microbiome 2023, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cordero-Elvia, J.; Chang, K.F.; Yang, C.X.; Fredua-Agyeman, R.; Turnbull, G.D.; Hwang, S.F.; Strelkov, S.E. Canola Disease Survey in Central-Northern Alberta in 2021. Canadian Plant Disease Survey 2022 Vol. 102: Disease Highlights 2022. Can. J. Plant Pathol. 2022, 44, 114–116. [Google Scholar] [CrossRef]
- Yu, H.; Chang, K.F.; Wu, L.; Manolii, V.; Turnbull, G.D.; Brinkman, A.; Kirk, B.; Oh, S.; Cordero-Elvia, J.; Yang, C.X.; et al. Canola Disease Survey in North-Central Alberta in 2022. Canadian Plant Disease Survey 2023 Vol. 103: Disease Highlights 2022. Can. J. Plant Pathol. 2023, 45, 113–116. [Google Scholar] [CrossRef]
- Hwang, S.F.; Howard, R.J.; Chang, K.F.; Park, B.; Burnett, P.A. Etiology and Severity of Fusarium Root Rot of Lentil in Alberta. Can. J. Plant Pathol. 1994, 16, 295–303. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and Genetic Diversity of the Banana Fusarium Wilt Pathogen Fusarium oxysporum f. Sp. Cubense in the Indonesian Centre of Origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, Pathogenicity, and Management of Verticillium Species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Berkenkamp, B.; Vaartnou, H. Fungi Associated with Rape Root Rot in Alberta. Can. J. Plant Sci. 1972, 52, 973–976. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, N.; Chang, K.F.; Hwang, S.F.; Strelkov, S.E.; Conner, R.L.; McLaren, D.L.; Fu, H.; Harding, M.W.; Turnbull, G.D. Genetic Diversity and Aggressiveness of Fusarium Species Isolated from Soybean in Alberta, Canada. Crop Prot. 2018, 105, 49–58. [Google Scholar] [CrossRef]
- Nyandoro, R. Fusarium Root Rot of Soybean in Southern Alberta: Pathogen Aggressiveness and Disease Management. Available online: https://era.library.ualberta.ca/items/62651227-1fd4-45c1-8438-36b0936a5f04 (accessed on 30 August 2023).
- Zhou, Q.; Yang, Y.; Wang, Y.; Jones, C.; Feindel, D.; Harding, M.; Feng, J. Phylogenetic, Phenotypic and Host Range Characterization of Five Fusarium Species Isolated from Chickpea in Alberta, Canada. Can. J. Plant Pathol. 2021, 43, 651–657. [Google Scholar] [CrossRef]
- Holtz, M.D.; Chang, K.F.; Hwang, S.F.; Gossen, B.D.; Strelkov, S.E. Characterization of Fusarium Avenaceum from Lupin in Central Alberta: Genetic Diversity, Mating Type and Aggressiveness. Can. J. Plant Pathol. 2011, 33, 61–76. [Google Scholar] [CrossRef]
- Holtz, M.D.; Chang, K.F.; Hwang, S.F.; Gossen, B.D.; Strelkov, S.E. Characterization of Fusarium Spp. Associated with Lupin in Central Alberta, Canada. Can. J. Plant Pathol. 2013, 35, 56–67. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Gossen, B.D.; Turnbull, G.D.; Blade, S.F. Disease Reaction to Fusarium Avenaceum and Yield Losses in Narrow-Leafed Lupin Lines. Can. J. Plant Sci. 2014, 94, 1211–1218. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Rew, L.J.; Jacobsen, B.J.; Hogg, A.C.; Dyer, A.T. Distribution and Prevalence of Fusarium Crown Rot and Common Root Rot Pathogens of Wheat in Montana. Plant Dis. 2011, 95, 1099–1108. [Google Scholar] [CrossRef]
- Gentosh, D.T.; Kyryk, M.M.; Gentosh, I.D.; Pikovskyi, M.Y.; Polozhenets, V.M.; Stankevych, S.V.; Nemerytska, L.V.; Zhuravska, I.A.; Zabrodina, I.V.; Zhukova, L.V. Species Compositions of Root Rot Agents of Spring Barley. Ukr. J. Ecol. 2020, 10, 106–109. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Holzgang, G.; Turkington, T.K. Common Root Rot of Barley in Saskatchewan and North-Central Alberta. Can. J. Plant Pathol. 2009, 31, 96–102. [Google Scholar] [CrossRef]
- Chatterton, S.; Harding, M.W.; Bowness, R.; Mclaren, D.L.; Banniza, S.; Gossen, B.D. Importance and Causal Agents of Root Rot on Field Pea and Lentil on the Canadian Prairies, 2014–2017. Can. J. Plant Pathol. 2019, 41, 98–114. [Google Scholar] [CrossRef]
- Gill, K.S. Crop Rotations Compared with Continuous Canola and Wheat for Crop Production and Fertilizer Use over 6 Yr. Can. J. Plant Sci. 2018, 98, 1139–1149. [Google Scholar] [CrossRef]
- Soon, Y.K.; Klein-Gebbinck, H.W.; Arshad, M.A. Residue Management and Crop Sequence Effects on the Yield and Brown Girdling Root Rot of Canola. Can. J. Plant Sci. 2005, 85, 67–72. [Google Scholar] [CrossRef]
- Safarieskandari, S.; Chatterton, S.; Hall, L.M. Pathogenicity and Host Range of Fusarium Species Associated with Pea Root Rot in Alberta, Canada. Can. J. Plant Pathol. 2021, 43, 162–171. [Google Scholar] [CrossRef]
- Feng, J.; Hwang, R.; Chang, K.F.; Hwang, S.F.; Strelkov, S.E.; Gossen, B.D.; Conner, R.L.; Turnbull, G.D. Genetic Variation in Fusarium Avenaceum Causing Root Rot on Field Pea. Plant Pathol. 2010, 59, 845–852. [Google Scholar] [CrossRef]
- Bock, C.H.; Chiang, K.S.; Del Ponte, E.M. Plant Disease Severity Estimated Visually: A Century of Research, Best Practices, and Opportunities for Improving Methods and Practices to Maximize Accuracy. Trop. Plant Pathol. 2022, 47, 25–42. [Google Scholar] [CrossRef]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium Species Associated with Soybean Root Rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Pappas, M.L.; Liapoura, M.; Papantoniou, D.; Avramidou, M.; Kavroulakis, N.; Weinhold, A.; Broufas, G.D.; Papadopoulou, K.K. The Beneficial Endophytic Fungus Fusariumsolani Strain K Alters Tomato Responses against Spider Mites to the Benefit of the Plant. Front. Plant Sci. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.T.; Dong, X.Y.; Li, Z.H.; Yan, H.; He, J.; Liu, J.K.; Feng, T. Antibacterial Metabolites from Kiwi Endophytic Fungus Fusarium tricinctum, a Potential Biocontrol Strain for Kiwi Canker Disease. J. Agric. Food Chem. 2023, 71, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.; Pennypacker, S.P. Principal Component Analysis of Tomato Early Blight Epidemics. J. Phytopathol. 1979, 95, 364–369. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Bulat, S.A.; Alekhina, I.A.; Nirenberg, H.I. Molecular, Morphological and Phylogenetic Analysis of the Fusarium avenaceum/F. arthrosporioides/F. tricinctum Species Complex—A Polyphasic Approach. Mycol. Res. 2002, 106, 655–669. [Google Scholar] [CrossRef]
- Chehri, K.; Salleh, B.; Zakaria, L. Morphological and Phylogenetic Analysis of Fusarium Solani Species Complex in Malaysia. Microb. Ecol. 2015, 69, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Laraba, I.; Busman, M.; Geiser, D.M.; O’Donnell, K. Phylogenetic Diversity and Mycotoxin Potential of Emergent Phytopathogens Within the Fusarium Tricinctum Species Complex. Phytopathology 2022, 112, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin Production in Fusarium According to Contemporary Species Concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Achari, S.R.; Kaur, J.; Dinh, Q.; Mann, R.; Sawbridge, T.; Summerell, B.A.; Edwards, J. Phylogenetic Relationship between Australian Fusarium Oxysporum Isolates and Resolving the Species Complex Using the Multispecies Coalescent Model. BMC Genom. 2020, 21, 248. [Google Scholar] [CrossRef] [PubMed]
- Root Rot|Canola Encyclopedia. Canola Council of Canada. Available online: https://www.canolacouncil.org/canola-encyclopedia/diseases/root-rot/ (accessed on 25 May 2021).
- Zhou, X.; Zhu, H.; Liu, L.; Lin, J.; Tang, K. A Review: Recent Advances and Future Prospects of Taxol-Producing Endophytic Fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, Z.; Barriuso, J.; Keshavarz, T.; Zheng, A. Molecular, Physiological, and Biochemical Properties of Sclerotia Metamorphosis in Rhizoctonia Solani. Fungal Biol. Rev. 2024, 48, 100351. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Q.; Chang, K.-F.; Yu, H.; Hwang, S.-F.; Conner, R.L.; Strelkov, S.E.; McLaren, D.L.; Turnbull, G.D. Occurrence, Pathogenicity and Species Identification of Pythium Causing Root Rot of Soybean in Alberta and Manitoba, Canada. Crop Prot. 2019, 118, 36–43. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 31, 315–322. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- You, J.M.; Lin, X.M.; Guo, J.; Zhang, M.D.; Liao, C.L.; He, M.J.; You, J.W.; Sun, Y.L. First Report of Root Rot on Atractylodes Macrocephala (Largehead Atractylodes Rhizome) Caused by Ceratobasidium sp. in China. Plant Dis. 2013, 97, 139. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- RStudio Team RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. USA. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 22 August 2023).
- Lin, S.R.; Lin, Y.H.; Ariyawansa, H.A.; Chang, Y.C.; Yu, S.Y.; Tsai, I.; Chung, C.L.; Hung, T.H. Analysis of the Pathogenicity and Phylogeny of Colletotrichum Species Associated with Brown Blight of Tea (Camellia sinensis) in Taiwan. Plant Dis. 2023, 107, 91–106. [Google Scholar] [CrossRef]

| Species a | Disease Severity (0–4) b | Reduction in Emergence c (%) | Reduction in Height d (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | 0–1.0 | 1.01–2.0 | 2.01–3.0 | 3.01–4.0 | Range | <25 | 25.1–50 | 50.1–75 | >75 | Range | <25 | 25.1–50 | 50.1–75 | >75 | |
| FAC | 1.50–2.41 | 0 | 3 | 3 | 0 | 19.7–37.7 | 4 | 2 | 0 | 0 | −1.9–17.7 | 6 | 0 | 0 | 0 |
| FAV | 1.22–3.88 | 0 | 17 | 51 | 37 | 10.5–93.4 | 11 | 31 | 39 | 24 | −8.6–71.6 | 66 | 28 | 11 | 0 |
| FCO | 2.04 | 0 | 0 | 1 | 0 | 10.5 | 1 | 0 | 0 | 0 | 14.70 | 1 | 0 | 0 | 0 |
| FCU | 1.46–2.37 | 0 | 5 | 5 | 0 | 13.1–80.3 | 4 | 5 | 0 | 1 | 10.1–60.0 | 3 | 6 | 1 | 0 |
| FEQ | 1.86–2.52 | 0 | 1 | 3 | 0 | 38.2–50.0 | 0 | 4 | 0 | 0 | 47.9–57.5 | 0 | 1 | 3 | 0 |
| FFL | 1.42–1.72 | 0 | 2 | 0 | 0 | 16.4–17.1 | 2 | 0 | 0 | 0 | 6.9–8.9 | 2 | 0 | 0 | 0 |
| FGR | 2.19 | 0 | 0 | 1 | 0 | 44.70 | 0 | 1 | 0 | 0 | 2.40 | 1 | 0 | 0 | 0 |
| FOX | 1.48–2.45 | 0 | 4 | 4 | 0 | 14.8–59.2 | 1 | 4 | 3 | 0 | 2.5–33.1 | 7 | 1 | 0 | 0 |
| FPR | 1.71 | 0 | 1 | 0 | 0 | 14.5 | 1 | 0 | 0 | 0 | −9.7 | 1 | 0 | 0 | 0 |
| FRE | 0.58–2.04 | 3 | 21 | 1 | 0 | 1.3–52.5 | 19 | 5 | 1 | 0 | −18.6–47.2 | 14 | 11 | 0 | 0 |
| FSO | 0.61–2.54 | 3 | 16 | 2 | 0 | 2.6–77.0 | 18 | 2 | 0 | 1 | −5.3–54.9 | 13 | 7 | 1 | 0 |
| FSP | 4 | 0 | 0 | 0 | 2 | 100 | 0 | 0 | 0 | 2 | 91.2–100 | 0 | 0 | 0 | 2 |
| FTO | 1.19–2.58 | 0 | 10 | 3 | 0 | 6.6–44.7 | 10 | 3 | 0 | 0 | −4.3–40.7 | 8 | 5 | 0 | 0 |
| FTR | 1.78–1.96 | 0 | 3 | 0 | 0 | 11.5–23.0 | 3 | 0 | 0 | 0 | −8.6–17.1 | 3 | 0 | 0 | 0 |
| Fsp | 2.41–3.48 | 0 | 0 | 2 | 1 | 29.5–73.8 | 0 | 1 | 2 | 0 | 17.6–35.0 | 2 | 1 | 0 | 0 |
| Total | 0.58–4.0 | 6 | 83 | 76 | 40 | 1.3–100 | 74 | 58 | 45 | 28 | −18.6–100 | 127 | 60 | 16 | 2 |
| Species a | Shoot Dry Weight Reduction b (%) | Root Dry Weight Reduction c (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | <25 | 25.1–50 | 50.1–75 | >75 | Range | <25 | 25.1–50 | 50.1–75 | >75 | |
| FAC | −26.5–37.3 | 3 | 3 | 0 | 0 | 55.8–77.7 | 0 | 0 | 5 | 1 |
| FAV | −38.9–94.9 | 15 | 37 | 28 | 25 | 30.6–98.2 | 0 | 6 | 24 | 75 |
| FCO | 30.5 | 0 | 1 | 0 | 0 | 63.5 | 0 | 0 | 1 | 0 |
| FCU | −19.6–75.2 | 5 | 3 | 1 | 1 | 18.8–84.9 | 1 | 0 | 5 | 4 |
| FEQ | 42.5–54.2 | 0 | 2 | 2 | 0 | 78.0–84.1 | 0 | 0 | 0 | 4 |
| FFL | 5.3–24.9 | 2 | 0 | 0 | 0 | 54.5–75.1 | 0 | 0 | 1 | 1 |
| FGR | 24.7 | 1 | 0 | 0 | 0 | 64.7 | 0 | 0 | 1 | 0 |
| FOX | −13.7–47.2 | 4 | 4 | 0 | 0 | 45.5–79.7 | 0 | 2 | 5 | 1 |
| FPR | −6.7 | 1 | 0 | 0 | 0 | 41.2 | 0 | 1 | 0 | 0 |
| FRE | −67.9–52.2 | 15 | 9 | 1 | 0 | 11.9–82.2 | 2 | 3 | 15 | 5 |
| FSO | −24.3–58.8 | 15 | 3 | 3 | 0 | 23.3–87.7 | 1 | 2 | 11 | 7 |
| FSP | 96.4–98.6 | 0 | 0 | 0 | 2 | 96.6–99.6 | 0 | 0 | 0 | 2 |
| FTO | −33.7–43.9 | 10 | 3 | 0 | 0 | 46.9–79.1 | 0 | 2 | 6 | 5 |
| FTR | −12.8–20.3 | 3 | 0 | 0 | 0 | 43.9–62.6 | 0 | 1 | 2 | 0 |
| Fsp | 27.3–82.5 | 0 | 2 | 0 | 1 | 61.2–93.9 | 0 | 0 | 2 | 1 |
| Total | −67.9–98.6 | 74 | 67 | 35 | 29 | 11.9–99.6 | 4 | 17 | 78 | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Chang, K.-F.; Fredua-Agyeman, R.; Hwang, S.-F.; Strelkov, S.E. Diversity and Pathogenicity of Fusarium Root Rot Fungi from Canola (Brassica napus) in Alberta, Canada. Int. J. Mol. Sci. 2024, 25, 6244. https://doi.org/10.3390/ijms25116244
Yu H, Chang K-F, Fredua-Agyeman R, Hwang S-F, Strelkov SE. Diversity and Pathogenicity of Fusarium Root Rot Fungi from Canola (Brassica napus) in Alberta, Canada. International Journal of Molecular Sciences. 2024; 25(11):6244. https://doi.org/10.3390/ijms25116244
Chicago/Turabian StyleYu, Haitian, Kan-Fa Chang, Rudolph Fredua-Agyeman, Sheau-Fang Hwang, and Stephen E. Strelkov. 2024. "Diversity and Pathogenicity of Fusarium Root Rot Fungi from Canola (Brassica napus) in Alberta, Canada" International Journal of Molecular Sciences 25, no. 11: 6244. https://doi.org/10.3390/ijms25116244
APA StyleYu, H., Chang, K.-F., Fredua-Agyeman, R., Hwang, S.-F., & Strelkov, S. E. (2024). Diversity and Pathogenicity of Fusarium Root Rot Fungi from Canola (Brassica napus) in Alberta, Canada. International Journal of Molecular Sciences, 25(11), 6244. https://doi.org/10.3390/ijms25116244






