Arginine Biosynthesis Mediates Wulingzhi Extract Resistance to Busulfan-Induced Male Reproductive Toxicity
Abstract
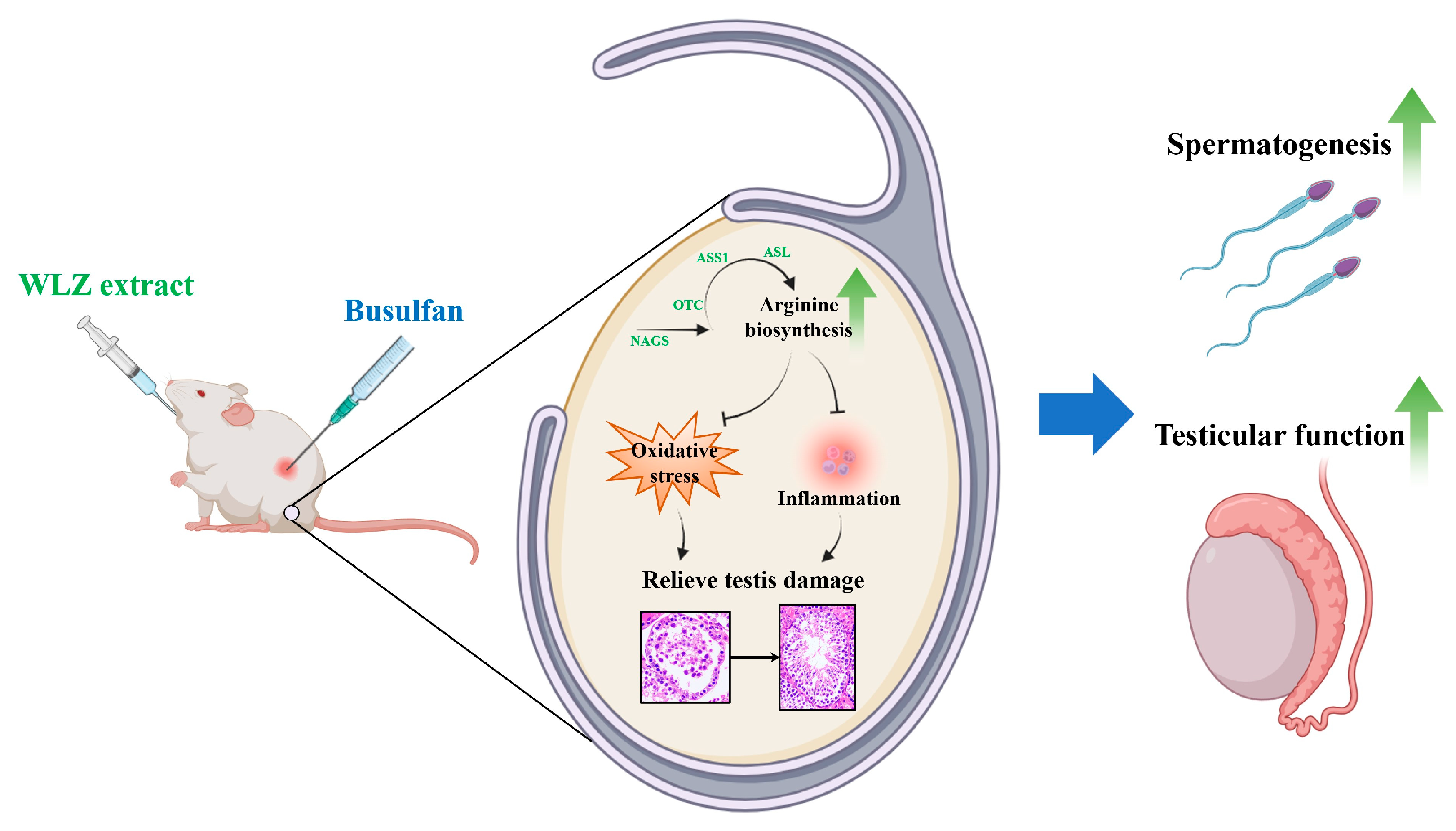
:1. Introduction
2. Results
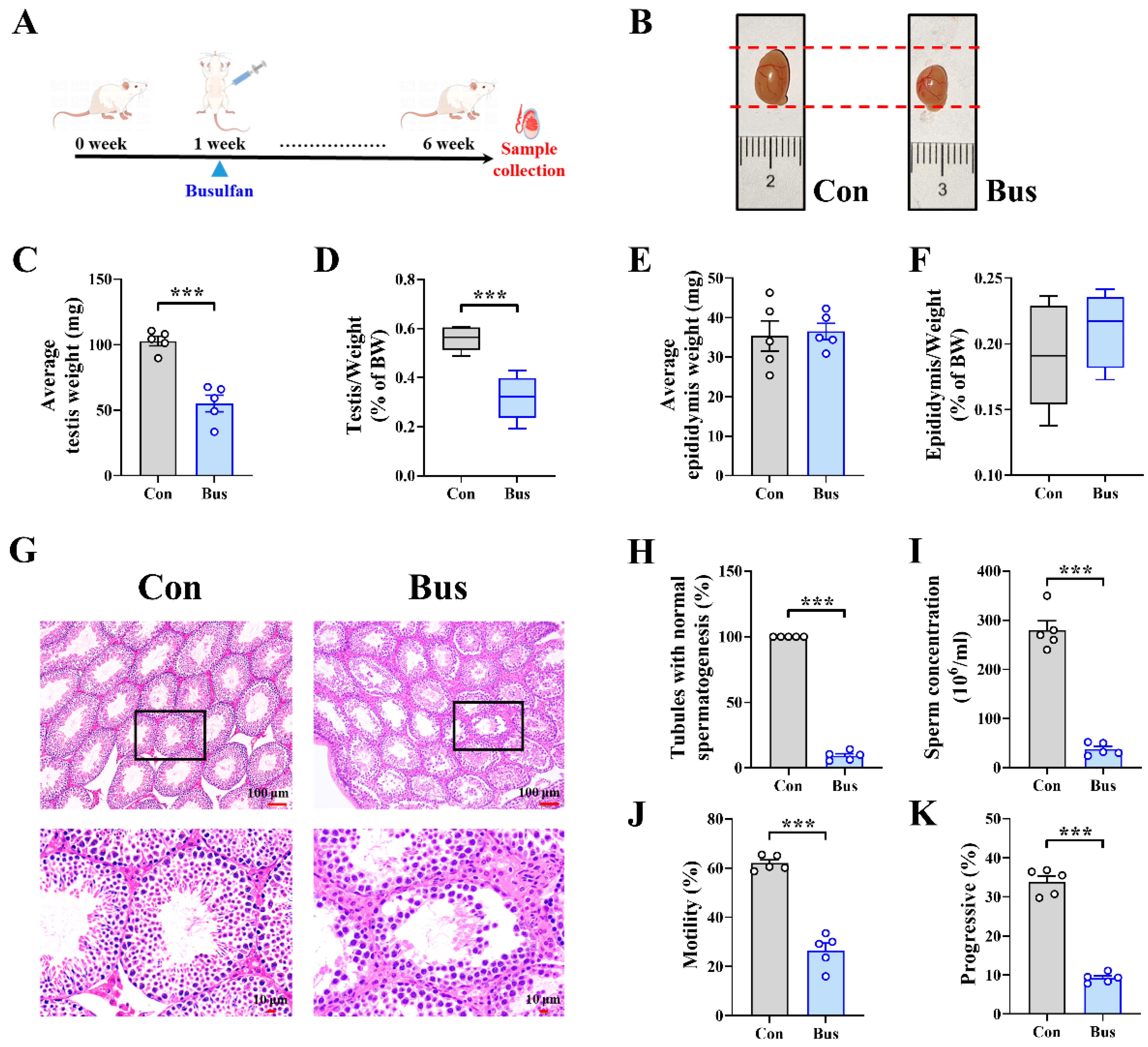
2.1. Busulfan Exposure Caused Testicular Dysfunction and Abnormal Spermatogenesis in Mice
2.2. The Identification of Components in Different WLZ Extracts
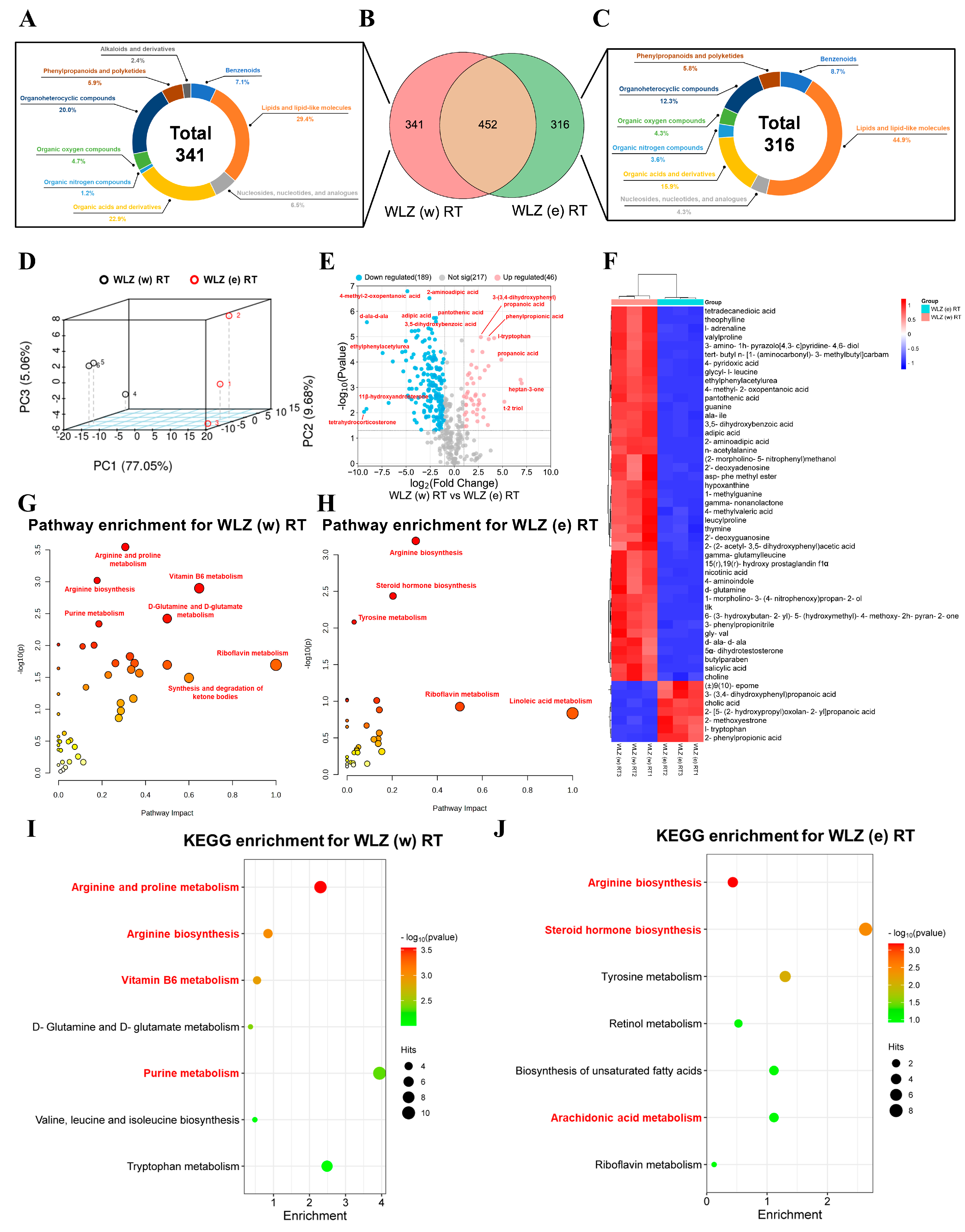
2.3. The Untargeted Metabolomics Analyses of Different WLZ Extracts
2.4. The Metabolic Changes in WLZ (w) RT and WLZ (e) RT
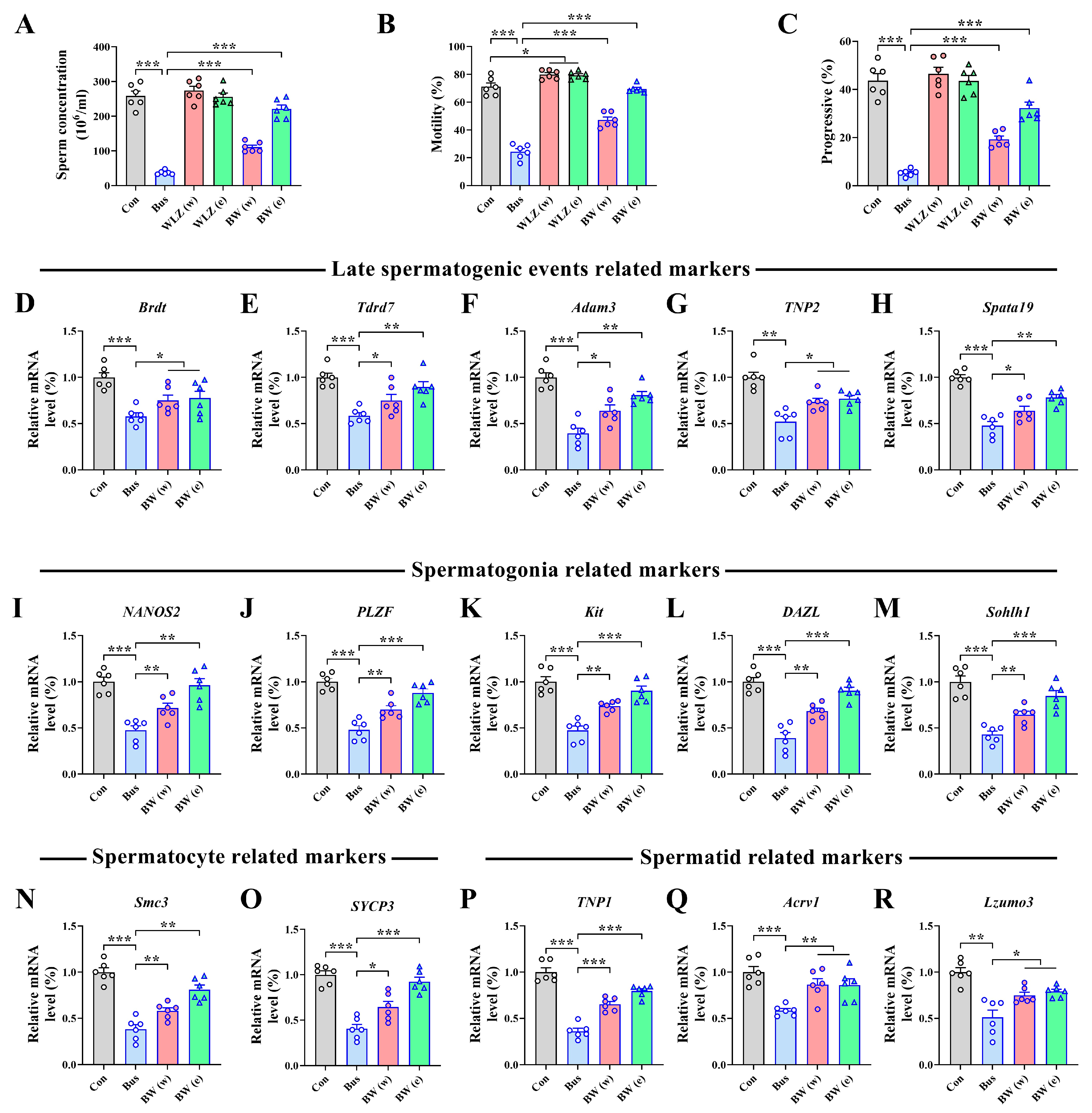
2.5. WLZ Extract Supplementation Alleviated Busulfan-Induced Testis Injury
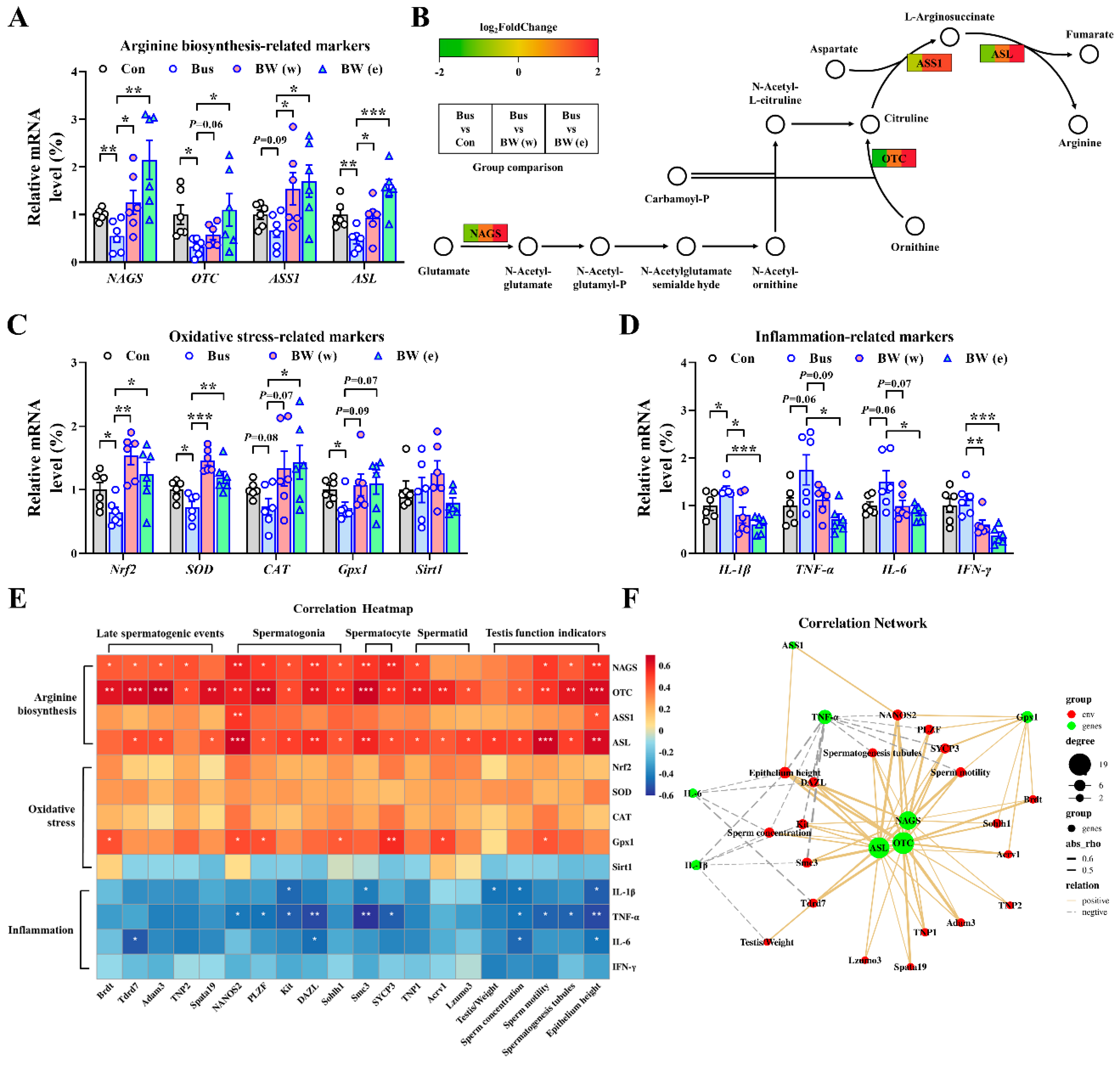
2.6. WLZ Extracts Alleviate the Oxidative Stress and Inflammation in Busulfan-Treated Testes via Arginine Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Preparation of Different WLZ Extracts
4.2. Animals and Experimental Design
4.3. Sperm Analysis
4.4. Histology Examination
4.5. Untargeted Metabolomics Study
4.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wallace, W.H.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef]
- Dohle, G.R. Male infertility in cancer patients: Review of the literature. Int. J. Urol. 2010, 17, 327–331. [Google Scholar] [CrossRef]
- Domingo-Echaburu, S.; Lopez de Torre-Querejazu, A.; Valcárcel, Y.; Orive, G.; Lertxundi, U. Hazardous drugs (NIOSH’s list-group 1) in healthcare settings: Also a hazard for the environment? Sci. Total Environ. 2022, 817, 152954. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Lalmohamed, A.; van Reij, E.M.; Dvorak, C.C.; Savic, R.M.; Zwaveling, J.; Bredius, R.G.; Egberts, A.C.; Bierings, M.; Kletzel, M.; et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: A multicentre, retrospective cohort analysis. Lancet Haematol. 2016, 3, e526–e536. [Google Scholar] [CrossRef]
- Faraci, M.; Tinelli, C.; Lanino, E.; Giardino, S.; Leoni, M.; Ferretti, M.; Castagnola, E.; Broglia, M.; De Silvestri, A.; Di Martino, D.; et al. Monitoring of Busulphan Concentrations in Children Undergone Hematopoietic Stem Cell Transplantation: Unicentric Experience over 10 years. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 173–181. [Google Scholar] [CrossRef]
- Bashir, Q.; Thall, P.F.; Milton, D.R.; Fox, P.S.; Kawedia, J.D.; Kebriaei, P.; Shah, N.; Patel, K.; Andersson, B.S.; Nieto, Y.L.; et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: An open-label, randomised, phase 3 trial. Lancet Haematol. 2019, 6, e266–e275. [Google Scholar] [CrossRef]
- Ali, E.A.; Tayel, S.G.; Abbas, M.A. Sitagliptin ameliorates busulfan-induced pulmonary and testicular injury in rats through antioxidant, anti-inflammatory, antifibrotic, and antiapoptotic effects. Sci. Rep. 2023, 13, 9794. [Google Scholar] [CrossRef]
- Anand, S.; Bhartiya, D.; Sriraman, K.; Mallick, A. Underlying Mechanisms that Restore Spermatogenesis on Transplanting Healthy Niche Cells in Busulphan Treated Mouse Testis. Stem Cell Rev. Rep. 2016, 12, 682–697. [Google Scholar] [CrossRef]
- Bishop, J.B.; Wassom, J.S. Toxicological review of busulfan (Myleran). Mutat. Res. 1986, 168, 15–45. [Google Scholar]
- Xiao, X.; Zhou, S.H.; Jiang, N.; Tian, D.Z.; Zhou, Z.M.; Zhang, M.; Ke, H.; Jiang, X.C.; Lv, W.L.; Gao, Q.H. First record of Leptospira and Blastocystis infections in captive flying squirrels (Trogopterus xanthipes) from Enshi County, China. Acta Trop. 2019, 197, 105065. [Google Scholar] [CrossRef]
- Liu, H.; Fan, M.; Fu, X.; Chen, Y.; Ye, M.; Guo, H. Simultaneous Determination of Prostaglandin and Hormones in Excreta of Trogopterus xanthipes. J. Chromatogr. Sci. 2020, 58, 542–548. [Google Scholar] [CrossRef]
- Baek, S.; Xia, X.; Min, B.S.; Park, C.; Shim, S.H. Trogopterins A–C: Three new neolignans from feces of Trogopterus xanthipes. Beilstein J. Org. Chem. 2014, 10, 2955–2962. [Google Scholar] [CrossRef]
- Yang, N.Y.; Tao, W.W.; Duan, J.A.; Guo, J.M.; Cao, L.L. Four new fatty acid esters from the Feces of Trogopterus xanthipes. Lipids 2009, 44, 849–853. [Google Scholar] [CrossRef]
- Zhou, W.; Su, S.L.; Duan, J.A.; Guo, J.M.; Qian, D.W.; Shang, E.X.; Zhang, J. Characterization of the active constituents in Shixiao San using bioactivity evaluation followed by UPLC-QTOF and Markerlynx analysis. Molecules 2010, 15, 6217–6230. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Dai, H.; Coleman, D.N.; Hu, L.; Martinez-Cortés, I.; Wang, M.; Parys, C.; Shen, X.; Loor, J.J. Methionine and arginine supplementation alter inflammatory and oxidative stress responses during lipopolysaccharide challenge in bovine mammary epithelial cells in vitro. J. Dairy. Sci. 2020, 103, 676–689. [Google Scholar] [CrossRef]
- de Andrade Bernal Fagiani, M.; Fluminhan, A.; de Azevedo Mello, F.; Yabuki, D.; Gonçalves, G.V.; Tsujigushi, L.K.; Pereira, L.G.; da Silva, K.A.; da Silva, S.B.B.; Santarem, C.L.; et al. L-arginine minimizes immunosuppression and prothrombin time and enhances the genotoxicity of 5-fluorouracil in rats. Nutrition 2019, 66, 94–100. [Google Scholar] [CrossRef]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta. Mol. Basis Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef]
- Alfano, M.; Tascini, A.S.; Pederzoli, F.; Locatelli, I.; Nebuloni, M.; Giannese, F.; Garcia-Manteiga, J.M.; Tonon, G.; Amodio, G.; Gregori, S.; et al. Aging, inflammation and DNA damage in the somatic testicular niche with idiopathic germ cell aplasia. Nat. Commun. 2021, 12, 5205. [Google Scholar] [CrossRef]
- Shen, P.; Ji, S.; Li, X.; Yang, Q.; Xu, B.; Wong, C.K.C.; Wang, L.; Li, L. LPS-Induced Systemic Inflammation Caused mPOA-FSH/LH Disturbance and Impaired Testicular Function. Front. Endocrinol. 2022, 13, 886085. [Google Scholar] [CrossRef]
- Ratner, P.A.; Spinelli, J.J.; Beking, K.; Lorenzi, M.; Chow, Y.; Teschke, K.; Le, N.D.; Gallagher, R.P.; Dimich-Ward, H. Cancer incidence and adverse pregnancy outcome in registered nurses potentially exposed to antineoplastic drugs. BMC Nurs. 2010, 9, 15. [Google Scholar] [CrossRef]
- Pacey, A.A.; Eiser, C. The importance of fertility preservation in cancer patients. Expert. Rev. Anticancer Ther. 2014, 14, 487–489. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Gao, J.; Li, H.; Wang, X.; Li, Y.; Sun, F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489. [Google Scholar] [CrossRef]
- Iskandar, K.; Rezlan, M.; Yadav, S.K.; Foo, C.H.; Sethi, G.; Qiang, Y.; Bellot, G.L.; Pervaiz, S. Synthetic Lethality of a Novel Small Molecule against Mutant KRAS-Expressing Cancer Cells Involves AKT-Dependent ROS Production. Antioxid. Redox Signal. 2016, 24, 781–794. [Google Scholar] [CrossRef]
- Peer, C.J.; Younis, I.R.; Leonard, S.S.; Gannett, P.M.; Minarchick, V.C.; Kenyon, A.J.; Rojanasakul, Y.; Callery, P.S. Glutathione conjugation of busulfan produces a hydroxyl radical-trapping dehydroalanine metabolite. Xenobiotica 2012, 42, 1170–1177. [Google Scholar] [CrossRef]
- Panahi, M.; Keshavarz, S.; Rahmanifar, F.; Tamadon, A.; Mehrabani, D.; Karimaghai, N.; Sepehrimanesh, M.; Aqababa, H. Busulfan induced azoospermia: Stereological evaluation of testes in rat. Vet. Res. Forum 2015, 6, 273–278. [Google Scholar]
- Jung, S.W.; Kim, H.J.; Lee, B.H.; Choi, S.H.; Kim, H.S.; Choi, Y.K.; Kim, J.Y.; Kim, E.S.; Hwang, S.H.; Lim, K.Y.; et al. Effects of Korean Red Ginseng extract on busulfan-induced dysfunction of the male reproductive system. J. Ginseng Res. 2015, 39, 243–249. [Google Scholar] [CrossRef]
- Liu, F.J.; Dong, W.Y.; Zhao, H.; Shi, X.H.; Zhang, Y.L. Effect of molybdenum on reproductive function of male mice treated with busulfan. Theriogenology 2019, 126, 49–54. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure-Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Tesi, E.P.; Ben-Azu, B.; Mega, O.O.; Mordi, J.; Knowledge, O.O.; Awele, E.D.; Rotu, R.A.; Emojevwe, V.; Adebayo, O.G.; Eneni, O.A. Kolaviron, a flavonoid-rich extract ameliorates busulfan-induced chemo-brain and testicular damage in male rats through inhibition of oxidative stress, inflammatory, and apoptotic pathways. J. Food Biochem. 2022, 46, e14071. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Mgbudom-Okah, C.J.; Onuah, C.L.; Ogunlaja, A. Fluted pumpkin seeds protect against busulfan-induced oxidative stress and testicular injuries in adult mice. Drug Chem. Toxicol. 2022, 45, 22–32. [Google Scholar] [CrossRef]
- Xu, G.; Shu, Y.; Xu, Y. Metabolomics analyses of traditional Chinese medicine formula Shuang Huang Lian by UHPLC-QTOF-MS/MS. Chin. Med. 2022, 17, 62. [Google Scholar] [CrossRef]
- Wang, A.; Li, Z.; Wang, J.; Liu, H.; Fu, X.; Chen, Y.; Guo, H. Quantification and holistic quality evaluation of Wulingzhi extract by UHPLC-Q-Orbitrap-HRMS coupled with chemometric approaches. Biomed. Chromatogr. 2023, 37, e5726. [Google Scholar] [CrossRef]
- Ye, J.X.; Wei, W.; Quan, L.H.; Liu, C.Y.; Chang, Q.; Liao, Y.H. An LC-MS/MS method for the simultaneous determination of chlorogenic acid, forsythiaside A and baicalin in rat plasma and its application to pharmacokinetic study of shuang-huang-lian in rats. J. Pharm. Biomed. Anal. 2010, 52, 625–630. [Google Scholar] [CrossRef]
- Duan, K.; Yuan, Z.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.; Zhang, L.; Wang, N. LC-MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Rarani, F.Z.; Golshan-Iranpour, F.; Dashti, G.R. Correlation between sperm motility and sperm chromatin/DNA damage before and after cryopreservation and the effect of folic acid and nicotinic acid on post-thaw sperm quality in normozoospermic men. Cell Tissue Bank. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Karim, S.M.; Hillier, K. Prostaglandins in the control of animal and human reproduction. Br. Med. Bull. 1979, 35, 173–180. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Spitzer, T.L.; Trussell, J.C.; Coward, R.M.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Diamond, M.P.; Krawetz, S.A.; Sun, F.; Zhang, H.; et al. Biomarkers of Stress and Male Fertility. Reprod. Sci. 2022, 29, 1262–1270. [Google Scholar] [CrossRef]
- Heráček, J.; Sobotka, V.; Kolátorová, L.; Kočárek, J.; Hampl, R. Serum and intratesticular sex steroids in azoospermic men: How do they correlate? Physiol. Res. 2018, 67 (Suppl. S3), S521–S524. [Google Scholar] [CrossRef]
- Anderson, R.A.; Wallace, A.M.; Kicman, A.T.; Wu, F.C. Comparison between testosterone oenanthate-induced azoospermia and oligozoospermia in a male contraceptive study. IV. Suppression of endogenous testicular and adrenal androgens. Hum. Reprod. 1997, 12, 1657–1662. [Google Scholar] [CrossRef]
- Schiffer, L.; Arlt, W.; Storbeck, K.H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell Endocrinol. 2018, 465, 4–26. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Kuroishi, T. Regulation of immunological and inflammatory functions by biotin. Can. J. Physiol. Pharmacol. 2015, 93, 1091–1096. [Google Scholar] [CrossRef]
- Salazar-Anzures, T.; Pastén-Hidalgo, K.; Sicilia-Argumedo, G.; Riverón-Negrete, L.; Hernández-Vázquez, A.J.; Fernanadez-Mejia, C. Dietary biotin supplementation increases proliferation pathways in mice testes without affecting serum follicle-stimulating hormone levels and stem cell factor expression. Toxicol. Appl. Pharmacol. 2021, 433, 115774. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Pedraza-Chaverri, J.; Tapia, E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020, 29, 101395. [Google Scholar] [CrossRef]
- Kuchkuntla, A.R.; Shah, M.; Velapati, S.; Gershuni, V.M.; Rajjo, T.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Ketogenic Diet: An Endocrinologist Perspective. Curr. Nutr. Rep. 2019, 8, 402–410. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Ge, W.; Feng, Y.; Li, L.; Sun, Z.; Zhang, H.; Shen, W. Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics 2020, 10, 3308–3324. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Zhuang, M.; Niu, B.; Wu, C.; Mu, H.; Tang, F.; Cui, Y.; Liu, W.; Zhao, B.; et al. Melatonin Ameliorates Busulfan-Induced Spermatogonial Stem Cell Oxidative Apoptosis in Mouse Testes. Antioxid. Redox Signal 2018, 28, 385–400. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Njoku, R.C.; John, I.G.; Amadi, B.A.; Mgbudom-Okah, C.J.; Onuah, C.L. Antioxidant and anti-inflammatory protective effects of rutin and kolaviron against busulfan-induced testicular injuries in rats. Syst. Biol. Reprod. Med. 2022, 68, 151–161. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine Inhibited Inflammatory Response and Oxidative Stress Induced by Lipopolysaccharide via Arginase-1 Signaling in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef]
- Caldovic, L.; Abdikarim, I.; Narain, S.; Tuchman, M.; Morizono, H. Genotype-Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update. J. Genet. Genom. 2015, 42, 181–194. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, A.; Yu, Y.; Guo, S.; Wang, M.; Wang, H. L-Arginine Protects Ovine Intestinal Epithelial Cells from Lipopolysaccharide-Induced Apoptosis through Alleviating Oxidative Stress. J. Agric. Food Chem. 2019, 67, 1683–1690. [Google Scholar] [CrossRef]
- Mo, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Tang, J.; Wu, C.; Cai, J.; Tian, G.; Wang, J.; et al. Roles of dietary supplementation with arginine or N-carbamylglutamate in modulating the inflammation, antioxidant property, and mRNA expression of antioxidant-relative signaling molecules in the spleen of rats under oxidative stress. Anim. Nutr. 2018, 4, 322–328. [Google Scholar] [CrossRef]
- Xie, W.; Wu, C.J.; Li, Y.; Lu, Q.L.; Gan, X.W.; Li, X.G.; Tang, J. Effect analysis of sacral canal therapy combined with Fufang Wulingzhi Tangjiang in the treatment of residual root pain after lumbar surgery. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9212–9220. [Google Scholar]
- Shi, L.; Wang, J.; Yang, Q.; Shi, L.; Liu, L.; Feng, X.; Chai, S.; Gou, J.; Zang, F.; He, S. Effect of Yang-activating and stasis-eliminating decoction from Traditional Chinese Medicine on intestinal mucosal permeability in rats with ulcerative colitis induced by dextran sulfate sodium. J. Tradit. Chin. Med. 2017, 37, 452–460. [Google Scholar]
- van der Horst, G. Computer Aided Sperm Analysis (CASA) in domestic animals: Current status, three D tracking and flagellar analysis. Anim. Reprod. Sci. 2020, 220, 106350. [Google Scholar] [CrossRef]
- Wu, Z.; Xiang, Q.; Feng, L.; Wu, D.; Huang, S.; Zhang, L.; Rao, S.; Luo, J.; Xiong, W.; Deng, J.; et al. Adenosine-ADORA2A Promotes Ang-Induced Angiogenesis in Intrauterine Growth Restriction Placenta via the Stat3/Akt Pathway. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e190–e209. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, C.; Xin, Z.; Huang, S.; Ma, J.; Zhang, M.; Shu, G.; Luo, H.; Deng, B.; Jiang, Q.; et al. UPLC-Orbitrap-MS/MS Combined with Biochemical Analysis to Determine the Growth and Development of Mothers and Fetuses in Different Gestation Periods on Tibetan Sow Model. Front. Nutr. 2022, 9, 836938. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, S.; Ren, D.; Liu, W.; Han, B.; Zhang, Y.; Xiao, J.; Yi, L.; Deng, B. UPLC-Orbitrap-MS/MS combined with chemometrics establishes variations in chemical components in green tea from Yunnan and Hunan origins. Food Chem. 2018, 266, 534–544. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Ma, Y.; Chen, S.; Liu, Y.; Liu, X.; Cao, H.; Jin, T.; Li, L.; Huang, M.; Yang, F.; et al. Arginine Biosynthesis Mediates Wulingzhi Extract Resistance to Busulfan-Induced Male Reproductive Toxicity. Int. J. Mol. Sci. 2024, 25, 6320. https://doi.org/10.3390/ijms25126320
Wu Z, Ma Y, Chen S, Liu Y, Liu X, Cao H, Jin T, Li L, Huang M, Yang F, et al. Arginine Biosynthesis Mediates Wulingzhi Extract Resistance to Busulfan-Induced Male Reproductive Toxicity. International Journal of Molecular Sciences. 2024; 25(12):6320. https://doi.org/10.3390/ijms25126320
Chicago/Turabian StyleWu, Zifang, Yuxuan Ma, Shaoxian Chen, Yuyan Liu, Xianglin Liu, Heran Cao, Tianqi Jin, Long Li, Mengqi Huang, Fangxia Yang, and et al. 2024. "Arginine Biosynthesis Mediates Wulingzhi Extract Resistance to Busulfan-Induced Male Reproductive Toxicity" International Journal of Molecular Sciences 25, no. 12: 6320. https://doi.org/10.3390/ijms25126320



