Bidirectional Two-Sample Mendelian Randomization Study of Immunoglobulin G N-Glycosylation and Senescence-Associated Secretory Phenotype
Abstract
:1. Introduction
2. Results
2.1. Forward Associations of IgG N-Glycans with SASP
2.2. Reverse Associations of SASP with IgG N-Glycans
3. Discussion
4. Materials and Method
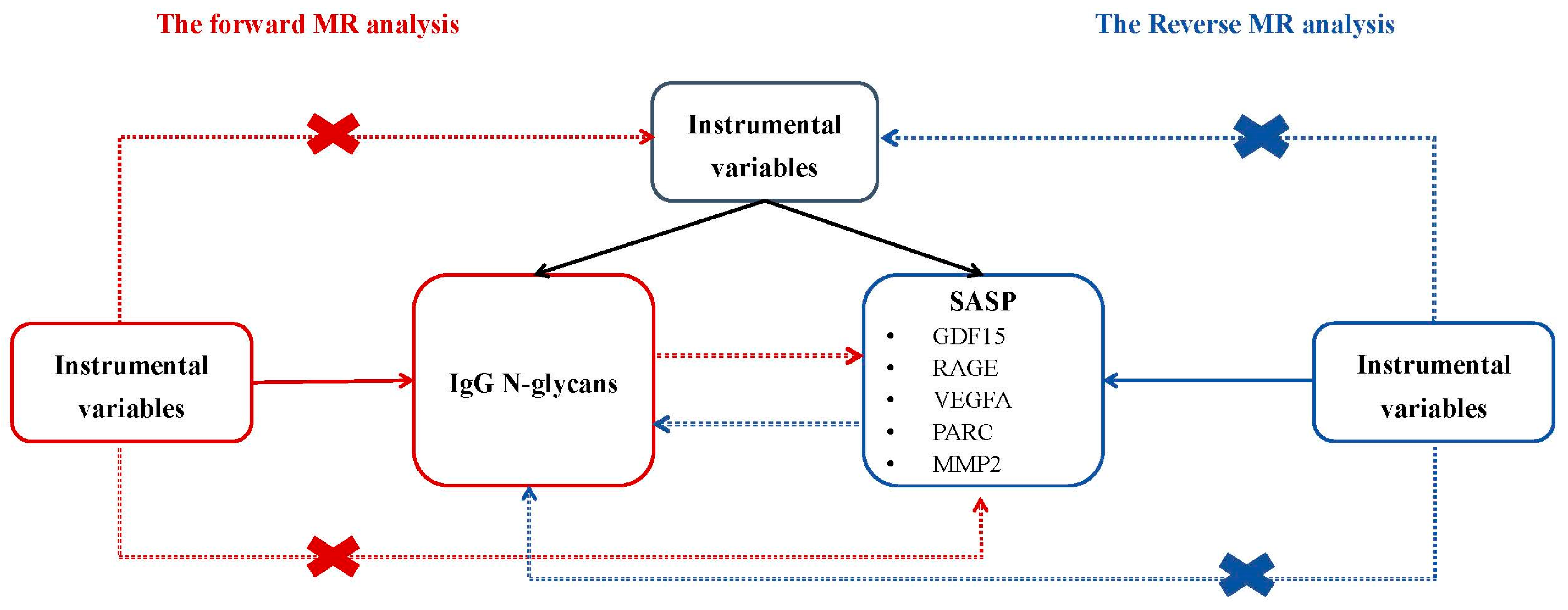
4.1. Bidirectional MR Design
4.2. Data Sources in Bidirectional MR Design
4.2.1. GWAS Data for IgG N-Glycans
4.2.2. GWAS Data for SASP
4.3. Instrumental Variables Selection
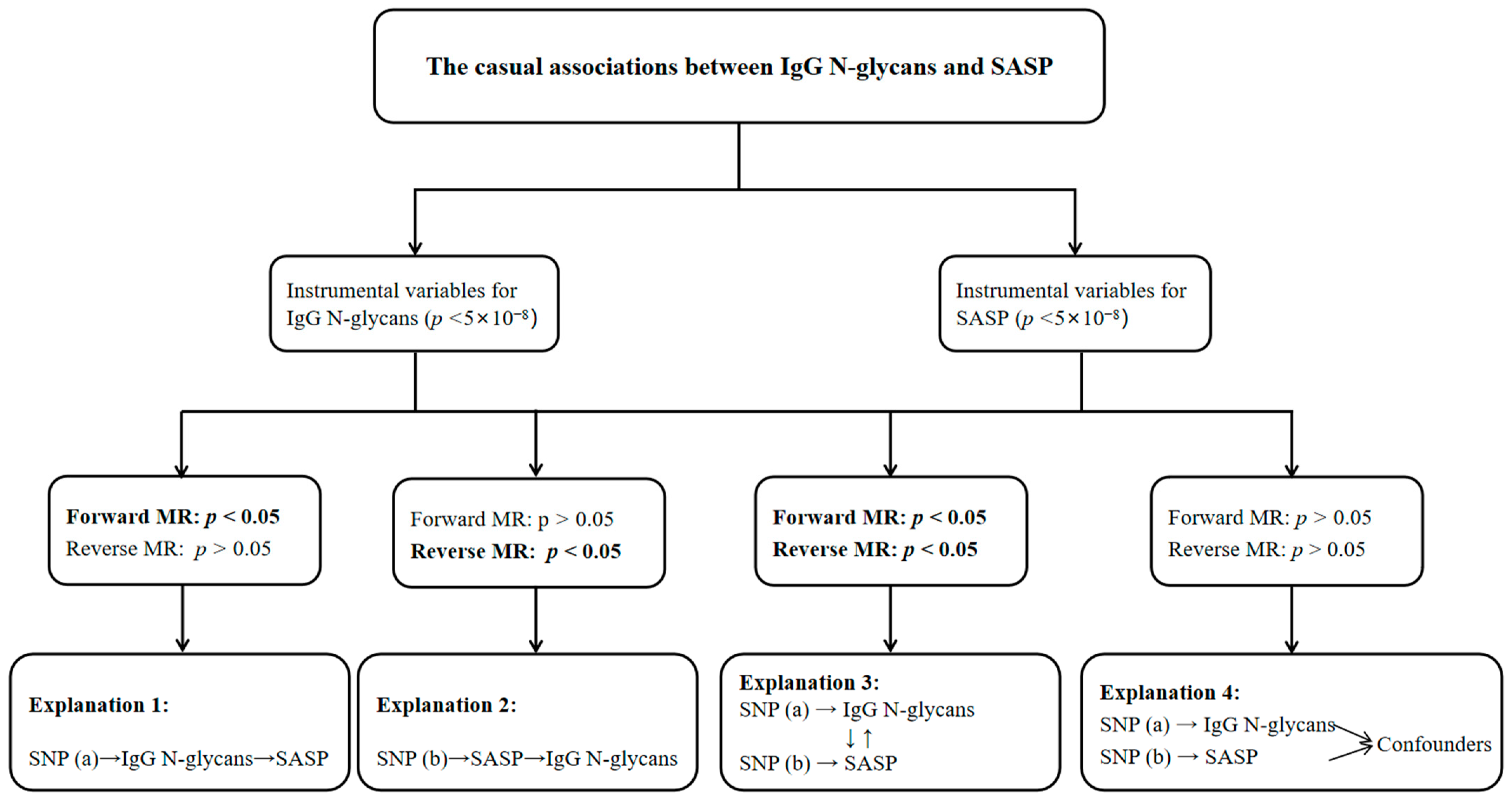
4.4. MR Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- St Sauver, J.L.; Weston, S.A.; Atkinson, E.J.; Mc Gree, M.E.; Mielke, M.M.; White, T.A.; Heeren, A.A.; Olson, J.E.; Rocca, W.A.; Palmer, A.K.; et al. Biomarkers of cellular senescence and risk of death in humans. Aging Cell 2023, 22, e14006. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J. Protein glycosylation. Curr. Biol. CB 2019, 29, R229–R231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, Y.; Ma, M.; Kapur, A.; Patankar, M.; Li, L. High-Throughput Quantitative Glycomics Enabled by 12-plex Isobaric Multiplex Labeling Reagents for Carbonyl-Containing Compound (SUGAR) Tags. J. Proteome Res. 2023, 22, 1557–1563. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Zhang, L.; Cai, Y.; Peng, Y.; Cao, T.; Zhang, Y.; Lu, H. Chemical labeling for fine mapping of IgG N-glycosylation by ETD-MS. Chem. Sci. 2019, 10, 9302–9307. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef]
- Seeling, M.; Brückner, C.; Nimmerjahn, F. Differential antibody glycosylation in autoimmunity: Sweet biomarker or modulator of disease activity? Nat. Reviews. Rheumatol. 2017, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Glycomedicine: The Current State of the Art. Engineering 2022, 26, 12–15. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Lyu, J.; Meng, X.; Tian, Q.; Li, Y.; Zhang, J.; Xu, X.; Su, J.; Hou, H.; et al. Association of dementia with immunoglobulin G N-glycans in a Chinese Han Population. NPJ Aging Mech. Dis. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, F.; Gao, X.; Wang, B.; Xu, X.; Wang, Y.; Wang, W.; Zeng, Q. Association of IgG N-glycomics with prevalent and incident type 2 diabetes mellitus from the paradigm of predictive, preventive, and personalized medicine standpoint. EPMA J 2023, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Klarić, L.; Yu, X.; Thaqi, K.; Dong, J.; Novokmet, M.; Wilson, J.; Polasek, O.; Liu, Y.; Krištić, J.; et al. The Association Between Glycosylation of Immunoglobulin G and Hypertension: A Multiple Ethnic Cross-Sectional Study. Medicine 2016, 95, e3379. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chu, X.; Wang, H.; Dong, J.; Ge, S.Q.; Zhao, Z.Y.; Peng, H.L.; Sun, M.; Wu, L.J.; Song, M.S.; et al. The changes of immunoglobulin G N-glycosylation in blood lipids and dyslipidaemia. J. Transl. Med. 2018, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Z.; Wang, A.; Ge, S.; Wang, H.; Zhang, X.; Sun, Q.; Cao, W.; Sun, M.; Wu, L.; et al. Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflamm. 2018, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Kristic, J.; Dong, J.; Chu, X.; Ge, S.; Wang, H.; Fang, H.; Gao, Q.; Liu, D.; et al. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: A community-based study in a Han Chinese population. Medicine 2016, 95, e4112. [Google Scholar] [CrossRef]
- Krištić, J.; Lauc, G.; Pezer, M. Immunoglobulin G glycans—Biomarkers and molecular effectors of aging. Clin. Chim. Acta Int. J. Clin. Chem. 2022, 535, 30–45. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cong, R.; Geng, T.; Zhang, J.; Liu, D.; Tian, Q.; Meng, X.; Song, M.; Wu, L.; Zheng, D.; et al. Assessment of the Causal Effect of IgG N-Glycosylation Level on Risk of Dementia: A 2-Sample Mendelian Randomization Study. J. Alzheimer’s Dis. 2022, 88, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, J.; Zhang, J.; Xu, X.; Tian, Q.; Meng, X.; Wu, L.; Zheng, D.; Chu, X.; Wang, W.; et al. Genome-Wide Mapping of Plasma IgG N-Glycan Quantitative Trait Loci Identifies a Potentially Causal Association between IgG N-Glycans and Rheumatoid Arthritis. J. Immunol. 2022, 208, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cao, W.; Liu, D.; Elijah, I.; Xing, W.; Hou, H.; Xu, X.; Song, M.; Wang, Y. Bidirectional Causality Between Immunoglobulin G N-Glycosylation and Metabolic Traits: A Mendelian Randomization Study. Engineering 2022, 26, 74–88. [Google Scholar] [CrossRef]
- Krištić, J.; Vučković, F.; Menni, C.; Klarić, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K.; et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Xie, S.; Lu, L.; Liu, L. Growth differentiation factor-15 and the risk of cardiovascular diseases and all-cause mortality: A meta-analysis of prospective studies. Clin. Cardiol. 2019, 42, 513–523. [Google Scholar] [CrossRef]
- Nakano, M.; Mishra, S.K.; Tokoro, Y.; Sato, K.; Nakajima, K.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. Bisecting GlcNAc Is a General Suppressor of Terminal Modification of N-glycan. Mol. Cell. Proteom. MCP 2019, 18, 2044–2057. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Spazzafumo, L.; Mensà, E.; Matacchione, G.; Galeazzi, T.; Zampini, L.; Recchioni, R.; Marcheselli, F.; Prattichizzo, F.; Testa, R.; Antonicelli, R.; et al. Age-related modulation of plasmatic beta-Galactosidase activity in healthy subjects and in patients affected by T2DM. Oncotarget 2017, 8, 93338–93348. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Matsuta, K.; Takeuchi, F.; Kosuge, E.; Miyamoto, T.; Kobata, A. Kinetic study of a galactosyltransferase in the B cells of patients with rheumatoid arthritis. Int. Immunol. 1990, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tijardović, M.; Marijančević, D.; Bok, D.; Kifer, D.; Lauc, G.; Gornik, O.; Keser, T. Intense Physical Exercise Induces an Anti-inflammatory Change in IgG N-Glycosylation Profile. Front. Physiol. 2019, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Klarić, L.; Tsepilov, Y.A.; Stanton, C.M.; Mangino, M.; Sikka, T.T.; Esko, T.; Pakhomov, E.; Salo, P.; Deelen, J.; McGurnaghan, S.J.; et al. Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci. Adv. 2020, 6, eaax0301. [Google Scholar] [CrossRef] [PubMed]
- Folkersen, L.; Fauman, E.; Sabater-Lleal, M.; Strawbridge, R.J.; Frånberg, M.; Sennblad, B.; Baldassarre, D.; Veglia, F.; Humphries, S.E.; Rauramaa, R.; et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 2017, 13, e1006706. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Stacey, D.; Eriksson, N.; Macdonald-Dunlop, E.; Hedman, Å.K.; Kalnapenkis, A.; Enroth, S.; Cozzetto, D.; Digby-Bell, J.; Marten, J.; et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat. Immunol. 2023, 24, 1540–1551. [Google Scholar] [CrossRef]
- Gudjonsson, A.; Gudmundsdottir, V.; Axelsson, G.T.; Gudmundsson, E.F.; Jonsson, B.G.; Launer, L.J.; Lamb, J.R.; Jennings, L.L.; Aspelund, T.; Emilsson, V.; et al. A genome-wide association study of serum proteins reveals shared loci with common diseases. Nat. Commun. 2022, 13, 480. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, Y.; Wang, J.; Small, D.S. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int. J. Epidemiol. 2019, 48, 1478–1492. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]


| Phenotype | No. of Participants | SNP (N) | Author | Year of Publication | PMID |
|---|---|---|---|---|---|
| IgG N-glycans | 8090 | 2,574,846 | Klarić et al. [36] | 2020 | 32128391 |
| GDF15 | 3394 | 5,270,646 | Folkersen et al. [37] | 2017 | 28369058 |
| RAGE | 3301 | 10,534,735 | Sun et al. [38] | 2018 | 29875488 |
| VEGFA | 14,744 | 12,958,278 | Zhao et al. [39] | 2023 | 37563310 |
| PARC | 5368 | 7,506,463 | Gudjonsson et al. [40] | 2022 | 35078996 |
| MMP2 | 5368 | 7,506,463 | Gudjonsson et al. [40] | 2022 | 35078996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, D.; Meng, X.; Sun, W.; Li, C.; Lu, H.; Zheng, D.; Wu, L.; Sun, S.; Wang, Y. Bidirectional Two-Sample Mendelian Randomization Study of Immunoglobulin G N-Glycosylation and Senescence-Associated Secretory Phenotype. Int. J. Mol. Sci. 2024, 25, 6337. https://doi.org/10.3390/ijms25126337
Wang H, Liu D, Meng X, Sun W, Li C, Lu H, Zheng D, Wu L, Sun S, Wang Y. Bidirectional Two-Sample Mendelian Randomization Study of Immunoglobulin G N-Glycosylation and Senescence-Associated Secretory Phenotype. International Journal of Molecular Sciences. 2024; 25(12):6337. https://doi.org/10.3390/ijms25126337
Chicago/Turabian StyleWang, Haotian, Di Liu, Xiaoni Meng, Wenxin Sun, Cancan Li, Huimin Lu, Deqiang Zheng, Lijuan Wu, Shengzhi Sun, and Youxin Wang. 2024. "Bidirectional Two-Sample Mendelian Randomization Study of Immunoglobulin G N-Glycosylation and Senescence-Associated Secretory Phenotype" International Journal of Molecular Sciences 25, no. 12: 6337. https://doi.org/10.3390/ijms25126337
APA StyleWang, H., Liu, D., Meng, X., Sun, W., Li, C., Lu, H., Zheng, D., Wu, L., Sun, S., & Wang, Y. (2024). Bidirectional Two-Sample Mendelian Randomization Study of Immunoglobulin G N-Glycosylation and Senescence-Associated Secretory Phenotype. International Journal of Molecular Sciences, 25(12), 6337. https://doi.org/10.3390/ijms25126337








