The Influence of the Auxiliary Ligand in Monofunctional Pt(II) Anticancer Complexes on the DNA Backbone
Abstract
1. Introduction
2. Results and Discussion
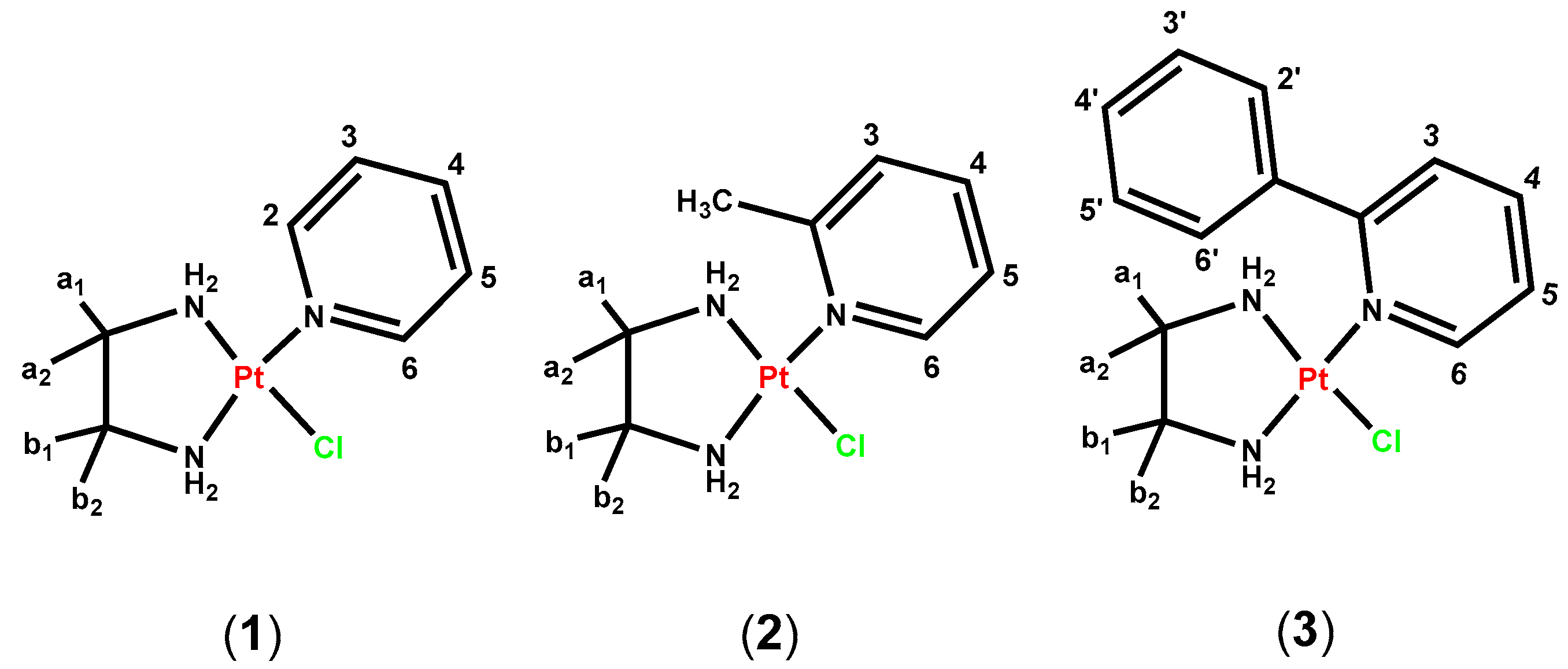
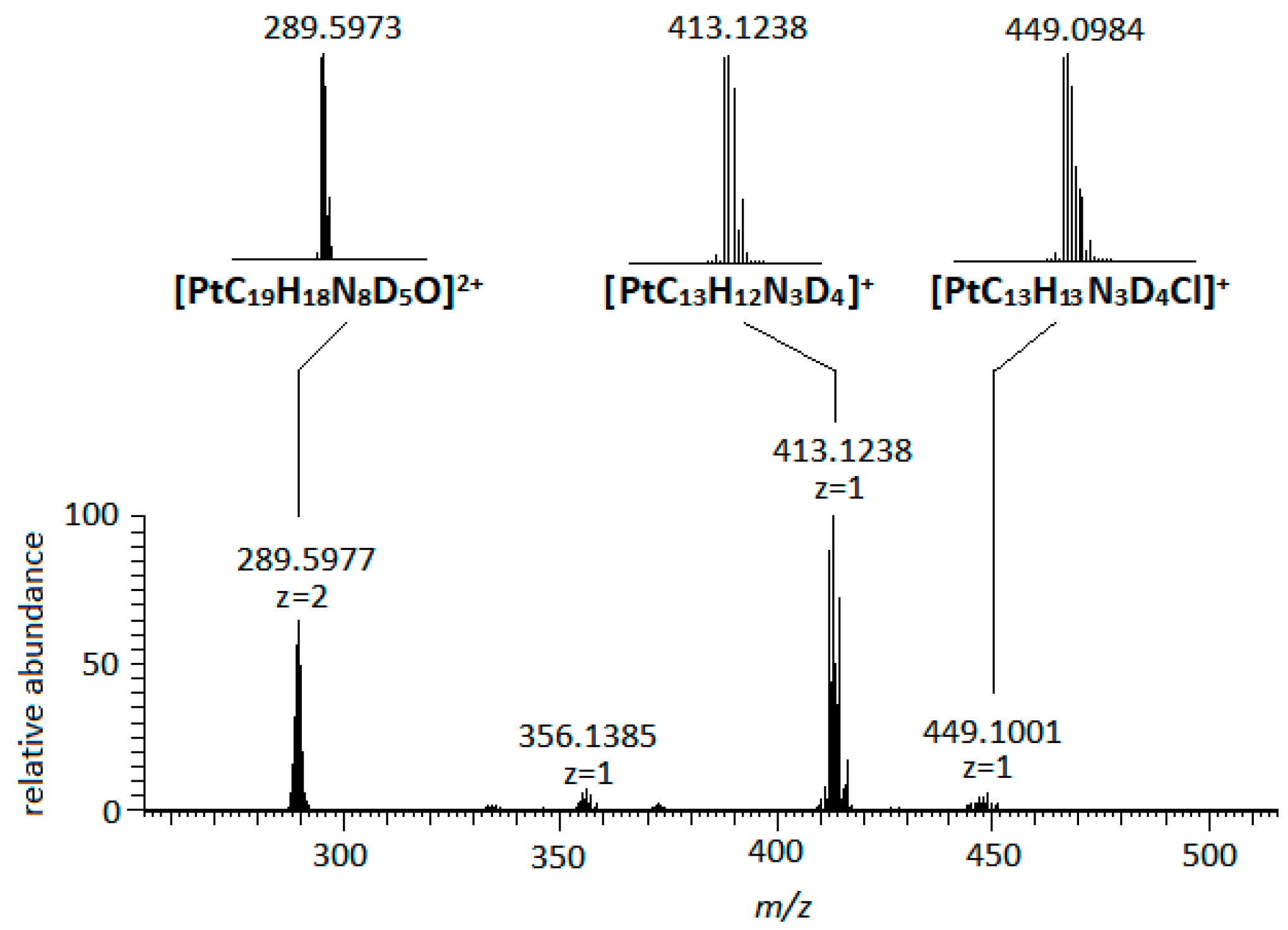
2.1. Synthesis and Characterization of the Complexes (1)–(3)
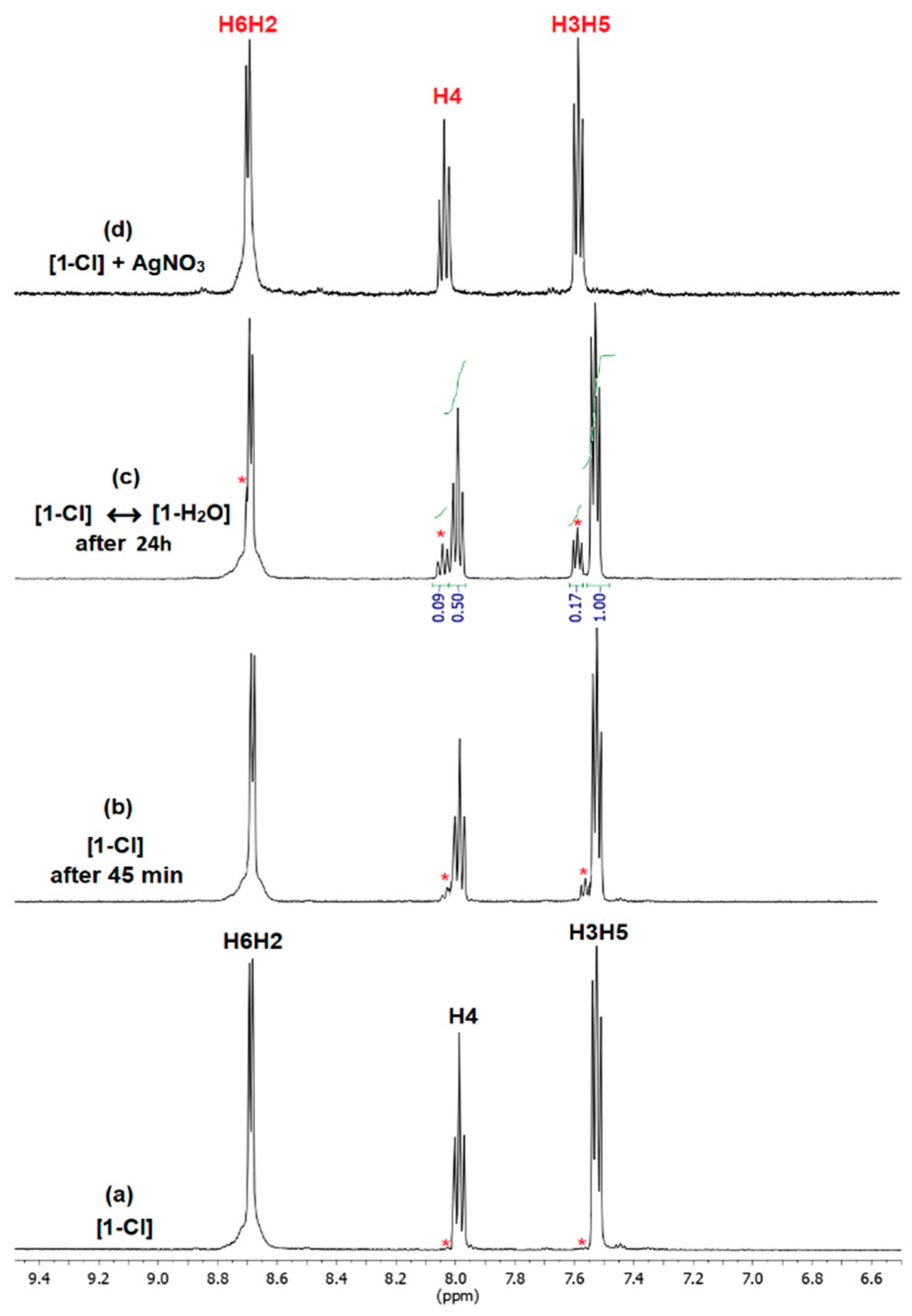
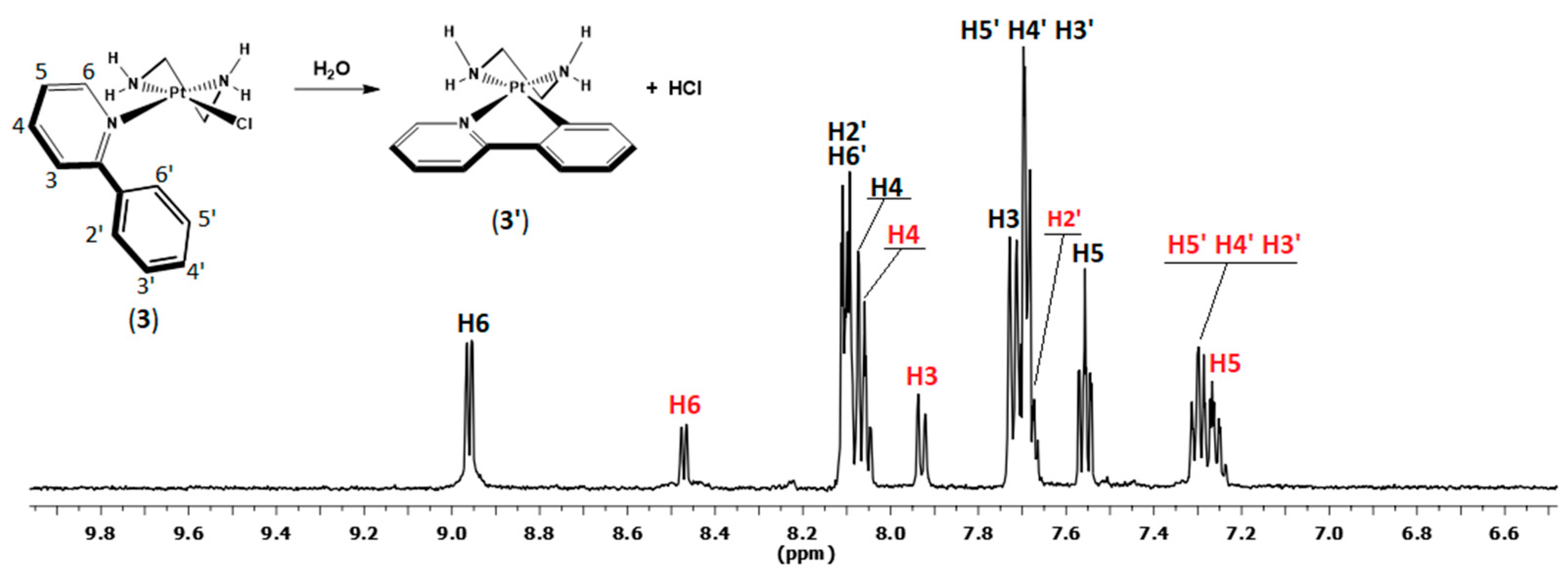
2.2. Hydrolysis of the Complexes (1)–(3)
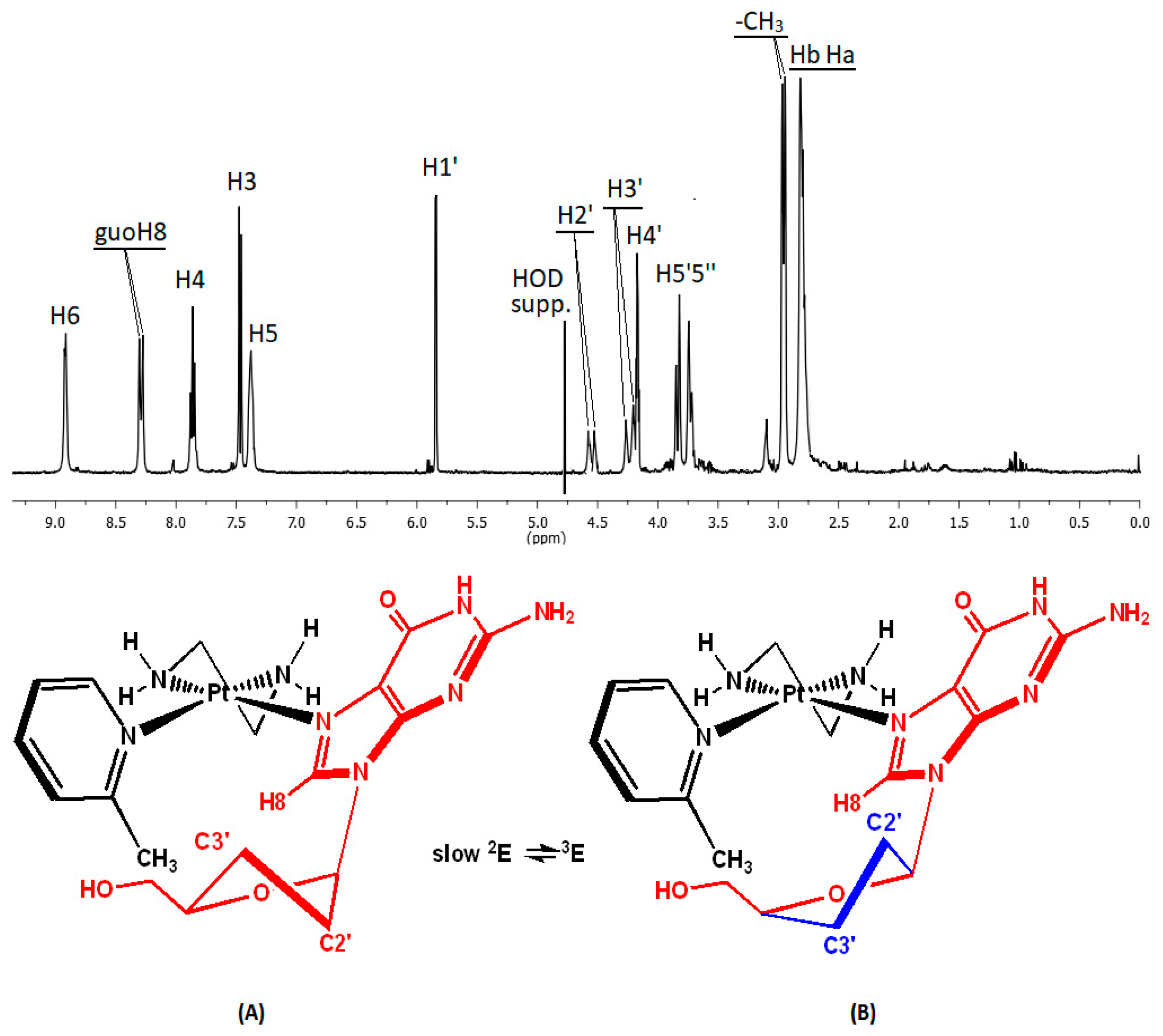
2.3. Interactions of (1) and (2) with Guanosine (Guo)
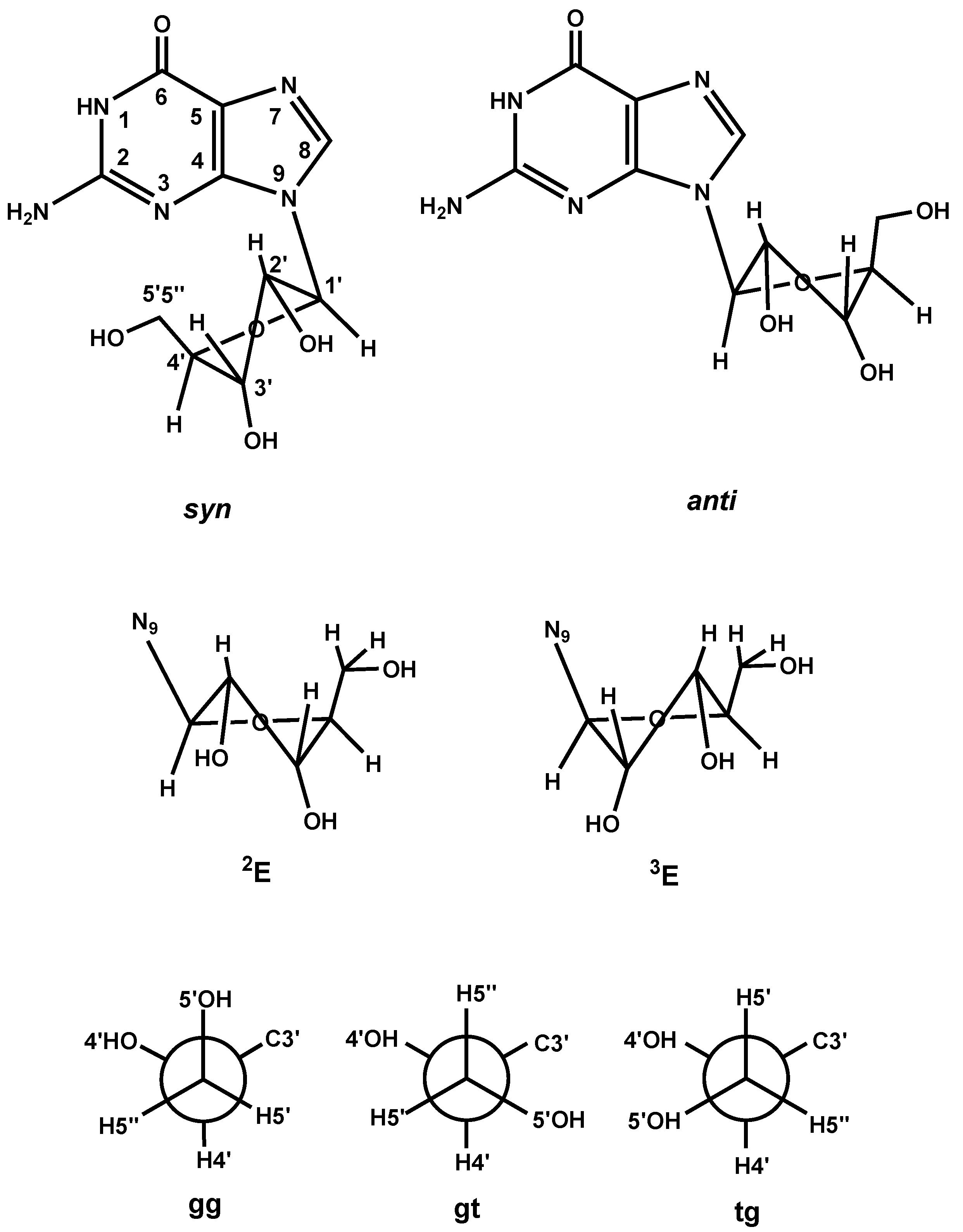
Sugar Conformation Analysis
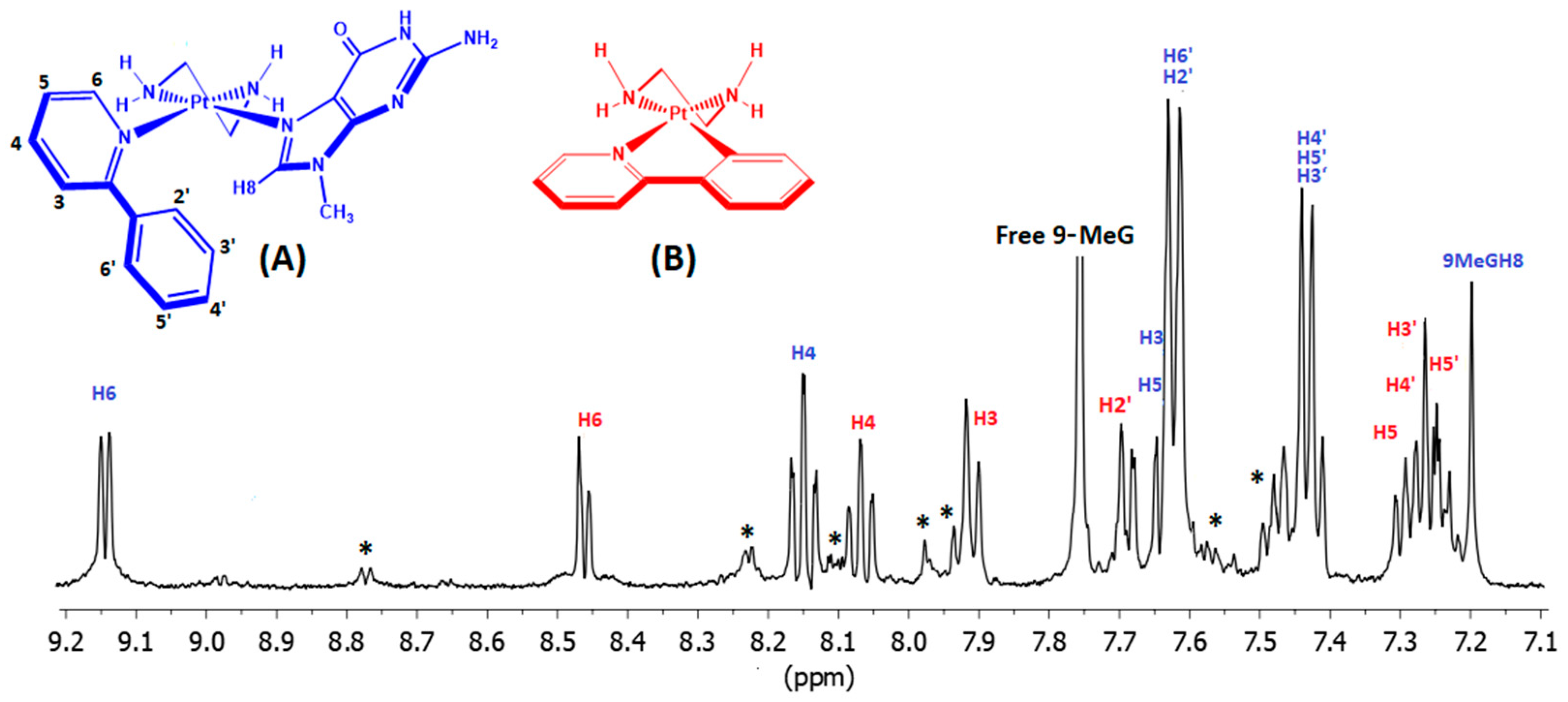
2.4. Interactions of (3) with 9-Methyl Guanine (9-MeG)
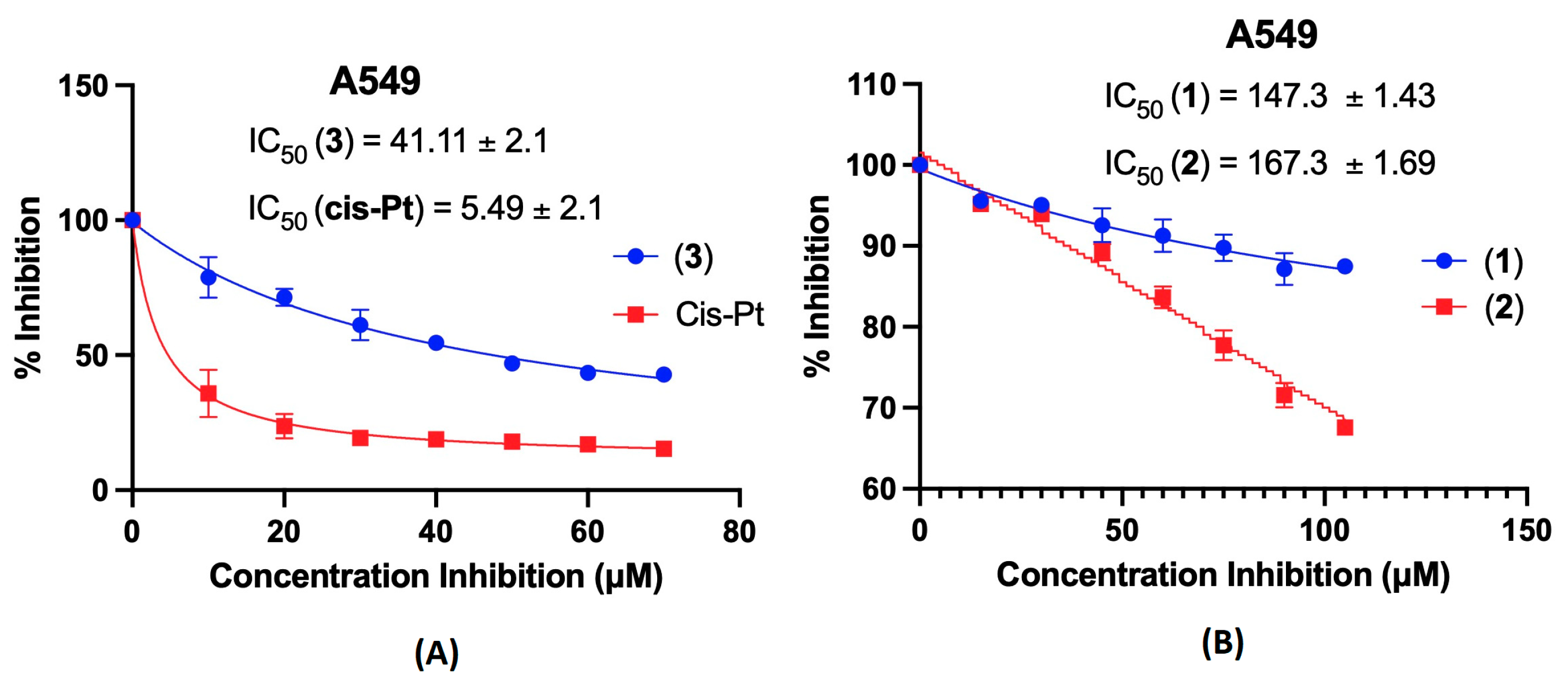
2.5. Cytotoxic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Methods
3.3. Cell Culture
3.4. Cell Growth Assay
3.5. Determination of Equilibrium Constants
3.6. Determination of Hydrolysis Constants
3.7. Synthesis of the Complexes (1)–(3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Lippard, S.J. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalt. Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalt. Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and Improving Platinum Anticancer Drugs—Phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar] [PubMed]
- Monroe, J.D.; Hruska, H.L.; Ruggles, H.K.; Williams, K.M.; Smith, M.E. Anti-Cancer Characteristics and Ototoxicity of Platinum(II) Amine Complexes with Only One Leaving Ligand. PLoS ONE 2019, 13, e0192505. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.L.; Lippard, S.J. Sequence-Dependent Termination of in Vitro DNA Synthesis by Cisand Trans-Diamminedichloroplatinum(II). Proc. Natl. Acad. Sci. USA 1985, 82, 4616–4619. [Google Scholar] [CrossRef] [PubMed]
- Cleare, M.J.; Hoeschele, J.D. Studies on the Antitumor Activity of Group VIII Transition Metal Complexes. Part I. Platinum (II) Complexes. Bioinorg. Chem. 1973, 2, 187–210. [Google Scholar] [CrossRef]
- Macquet, J.P.; Butour, J.L. Platinum-Amine Compounds: Importance of the Labile and Inert Ligands for Their Pharmacological Activities toward L1210 Leukemia Cells. J. Natl. Cancer Inst. 1983, 70, 899–905. [Google Scholar] [PubMed]
- Hollis, L.S.; Amundsen, A.R.; Stern, E.W. Chemical and Biological Properties of a New Series of Cis-Diammineplatinum(II) Antitumor Agents Containing Three Nitrogen Donors: Cis-[Pt(NH3)2(N-Donor)Cl]+. J. Med. Chem. 1989, 32, 128–136. [Google Scholar] [CrossRef]
- Lovejoy, K.S.; Todd, R.C.; Zhang, S.; McCormick, M.S.; D’Aquino, J.A.; Reardon, J.T.; Sancar, A.; Giacomini, K.M.; Lippard, S.J. Cis-Diammine(Pyridine)Chloroplatinum(II), a Monofunctional Platinum(II) Antitumor Agent: Uptake, Structure, Function, and Prospects. Proc. Natl. Acad. Sci. USA 2008, 105, 8902–8907. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, G.; Huang, X.; Lippard, S.J. X-ray Structure and Mechanism of RNA Polymerase II Stalled at an Antineoplastic Monofunctional Platinum-DNA Adduct. Proc. Natl. Acad. Sci. USA 2010, 107, 9584–9589. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Wilson, J.J.; Song, Y.; Lippard, S.J. Phenanthriplatin, a Monofunctional DNA-Binding Platinum Anticancer Drug Candidate with Unusual Potency and Cellular Activity Profile. Proc. Natl. Acad. Sci. USA 2012, 109, 11987–11992. [Google Scholar] [CrossRef] [PubMed]
- Almaqwashi, A.A.; Zhou, W.; Naufer, M.N.; Riddell, I.A.; Yilmaz, Ö.H.; Lippard, S.J.; Williams, M.C. DNA Intercalation Facilitates Efficient DNA-Targeted Covalent Binding of Phenanthriplatin. J. Am. Chem. Soc. 2019, 141, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Veclani, D.; Melchior, A.; Tolazzi, M.; Cerón-Carrasco, J.P. Using Theory to Reinterpret the Kinetics of Monofunctional Platinum Anticancer Drugs: Stacking Matters. J. Am. Chem. Soc. 2018, 140, 14024–14027. [Google Scholar] [CrossRef]
- Kellinger, M.W.; Park, G.Y.; Chong, J.; Lippard, S.J.; Wang, D. Effect of a Monofunctional Phenanthriplatin-DNA Adduct on RNA Polymerase II Transcriptional Fidelity and Translesion Synthesis. J. Am. Chem. Soc. 2013, 135, 13054–13061. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.T.; Park, G.Y.; Johnstone, T.C.; Lee, Y.S.; Yang, W.; Lippard, S.J. Structural and Mechanistic Studies of Polymerase η Bypass of Phenanthriplatin DNA Damage. Proc. Natl. Acad. Sci. USA 2014, 111, 9133–9138. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A Subset of Platinum-Containing Chemotherapeutic Agents Kills Cells by Inducing Ribosome Biogenesis Stress. Nat. Med. 2017, 23, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.D.; Moolani, S.A.; Irihamye, E.N.; Speed, J.S.; Gibert, Y.; Smith, M.E. Article RNA-Seq Analysis of Cisplatin and the Monofunctional Platinum(II) Complex, Phenanthriplatin, in A549 Non-Small Cell Lung Cancer and IMR90 Lung Fibroblast Cell Lines. Cells 2020, 9, 2637. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.T.; Baruah, H.; Kramarczyk, J.; Saluta, G.; Day, C.S.; Kucera, G.L.; Bierbach, U. Design, Synthesis, and Biological Activity of a Novel Non-Cisplatin-Type Platinum-Acridine Pharmacophore. J. Med. Chem. 2001, 44, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Smyre, C.L.; Saluta, G.; Kute, T.E.; Kucera, G.L.; Bierbach, U. Inhibition of DNA Synthesis by a Platinum-Acridine Hybrid Agent Leads to Potent Cell Kill in Nonsmall Cell Lung Cancer. ACS Med. Chem. Lett. 2011, 2, 870–874. [Google Scholar] [CrossRef]
- Ma, Z.; Choudhury, J.R.; Wright, M.W.; Day, C.S.; Saluta, G.; Kucera, G.L.; Bierbach, U. A Non-Cross-Linking Platinum-Acridine Agent with Potent Activity in Non-Small-Cell Lung Cancer. J. Med. Chem. 2008, 51, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Riddell, I.A.; Agama, K.; Park, G.Y.; Pommier, Y.; Lippard, S.J. Phenanthriplatin Acts as a Covalent Poison of Topoisomerase II Cleavage Complexes. ACS Chem. Biol. 2016, 11, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, W.; Wang, J.; Li, X.; Wu, Y.B.; Li, S.; Lu, L.; Zhu, M.; Xing, S.; Fu, X. Potent and Selective PTP1B Inhibition by a Platinum(Ii) Complex: Possible Implications for a New Antitumor Strategy. Chem. Commun. 2019, 56, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Zhang, C.; Wang, Y.; Zhang, H.; Gan, Z.; Guo, Z.; Wang, X. Mitochondrion-Targeted Platinum Complexes Suppressing Lung Cancer through Multiple Pathways Involving Energy Metabolism. Chem. Sci. 2019, 10, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Qian, C.; Fang, H.; Liu, H.K.; Yuan, H.; Guo, Z.; Bai, Y.; He, W. Photoactivated Lysosomal Escape of a Monofunctional PtII Complex Pt-BDPA for Nucleus Access. Angew. Chem. Int. Ed. 2019, 58, 12661–12666. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guo, Y.; Guo, Z.; Wang, X. Monofunctional Platinum(II) Anticancer Agents. Pharmaceiticals 2021, 14, 133. [Google Scholar] [CrossRef]
- Sifnaiou, E.; Garypidou, A.; Ypsilantis, K.; Plakatouras, J.C.; Garoufis, A. Synthesis, Characterization and Photophysical Properties of Mixed Ligand Cyclometalated Platinum(II) Complexes Containing 2-Phenylpyridine and Pyridine Carboxylic Acids. Polyhedron 2023, 231, 116252. [Google Scholar] [CrossRef]
- Rochon, F.D.; Morneau, A. 195Pt and 1H NMR Studies of Platinum(II) Complexes with Ethylenediamine Derivatives. Magn. Reson. Chem. 1991, 29, 120–126. [Google Scholar] [CrossRef]
- Marti, N.; Hoa, G.H.B.; Kozelka, J. Reversible Hydrolysis of [PtCl(Dien)]+ and [PtCl(NH3)3]+. Determination of the Rate Constants Using UV Spectrophotometry. Inorg. Chem. Commun. 1998, 1, 439–442. [Google Scholar] [CrossRef]
- Kozelka, J. Hydrolysis of Chlorido Complexes of D8 Metals: Old Models, New Facts. Inorganica Chim. Acta 2019, 495, 118946. [Google Scholar] [CrossRef]
- Qu, Y.; Farrell, N. Interaction of Bis(Platinum) Complexes with the Mononucleotide 5′-Guanosine Monophosphate. Effect of Diamine Linker and the Nature of the Bis(Platinum) Complex on Product Formation. J. Am. Chem. Soc. 1991, 113, 4851–4857. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Lippard, S.J. The Chiral Potential of Phenanthriplatin and Its Influence on Guanine Binding. J. Am. Chem. Soc. 2014, 136, 2126–2134. [Google Scholar] [CrossRef]
- Ma, Y.; Day, C.S.; Bierbach, U. Synthesis, Structure, and Reactivity of Monofunctional Platinum(II) and Palladium(II) Complexes Containing the Sterically Hindered Ligand 6-(Methylpyridin-2-Yl)Acetate. J. Inorg. Biochem. 2005, 99, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.S.; Scarcia, T.; Natile, G.; Marzilli, L.G. Factors Influencing Conformer Equilibria in Retro Models of Cisplatin-DNA Adducts as Revealed by Moderately Dynamic (N,N′-Dimethyl-2,3-Diaminobutane)PtG2 Retro Models (G = a Guanine Derivative). Inorg. Chem. 2002, 41, 4923–4935. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, C.A.G.; de Leeuw, F.A.A.M.; Altona, C. The Relationship between Proton-Proton NMR Coupling Constants and Substituent Electronegativities-I. An Empirical Generalization of the Karplus Equation. Tetrahedron 1980, 36, 2783–2792. [Google Scholar] [CrossRef]
- Kim, C.H.; Sarma, R.H. Spatial Configuration of the Bizarre 5′ Terminus of Mammalian MRNA1. J. Am. Chem. Soc. 1978, 100, 1571–1590. [Google Scholar] [CrossRef]
- Lee, C.H.; Sarma, R.H. Aqueous Solution Conformation of Rigid Nucleosides and Nucleotides. J. Am. Chem. Soc. 1976, 98, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sarma, R.H.; Ezra, F.S.; Danyluk, S.S.; Kondo, N.S. Conformational Properties of Dinucleoside Monophosphates in Solution: Dipurines and Dipyrimidines. Biochemistry 1976, 15, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, E.; Troganis, A.; Hadjiliadis, N. Binary and Ternary Complexes of Platinum(II) with the Dipeptide Esters Gly-GlyOEt, Gly-AlaOMe, Gly-2-AbaOMe, Gly-NvalOMe, Gly-NleuOMe, and the Nucleosides Guo (Guanosine) and Cyd (Cytidine). Inorganica Chim. Acta 1997, 256, 21–28. [Google Scholar] [CrossRef]
- Kasselouri, S.; Garoufis, A.; Hadjiliadis, N. Pd(II) and Pt(II) Ternary Complexes with Nucleosides and Amino Acids. Inorganica Chim. Acta 1987, 135, 23–25. [Google Scholar] [CrossRef]
- Garoufis, A.; Hatiris, J.; Hadjiliadis, N. Ternary Complexes of Pt(II) with Guanosine and Amino Acids of the Type Trans-[(Guo)2Pt(AmacH)2]Cl2, Where AmacH Is Glycine, L-Alanine, L-Valine, and L-Isoleucine. J. Inorg. Biochem. 1991, 41, 195–203. [Google Scholar] [CrossRef]
- Giorgi, T.; Lena, S.; Mariani, P.; Cremonini, M.A.; Masiero, S.; Pieraccini, S.; Rabe, J.P.; Samori, P.; Spada, G.P.; Gottarelli, G. Supramolecular Helices via Self-Assembly of 8-Oxoguanosines. J. Am. Chem. Soc. 2003, 125, 14741–14749. [Google Scholar] [CrossRef]
- Heflich, R.H.; Neft, R.E. Genetic Toxicity of 2-Acetylaminofluorene, 2-Aminofluorene and Some of Their Metabolites and Model Metabolites. Mutat. Res. Genet. Toxicol. 1994, 318, 73–174. [Google Scholar] [CrossRef]
- Pless, R.; Dudycz, L.; Shugar, D.; Stolarski, R. Purine Nucleosides and Nucleotides Unequivocally in the Syn Conformation: Guanosine and 5′-GMP with 8-Tert-Butyl and 8-(α-Hydroxyisopropyl) Substituents. Z. Fur Naturforsch. Sect. C J. Biosci. 1978, 33, 902–907. [Google Scholar] [CrossRef]
- Polissiou, M.; Theophanides, T. NMR and FT-IR Conformational Studies of 8-Substituted Guanine Nucleosides and Nucleotides and Their Metal Adducts and Cancer. Inorganica Chim. Acta 1987, 137, 195–201. [Google Scholar] [CrossRef]
- Tavale, S.S.; Sobell, H.M. Crystal and Molecular Structure of 8-Bromoguanosine and 8-Bromoadenosine, Two Purine Nucleosides in the Syn Conformation. J. Mol. Biol. 1970, 48, 109–123. [Google Scholar] [CrossRef]
- den Hartog, J.H.J.; Altona, C.; van Boom, J.H.; Marcelis, A.T.M.; van der Marel, G.A.; Rinkel, L.J.; Wille-Hazeleger, G.; Reedijk, J. Cis-Platinum Induced Distortions in DNA. Eur. J. Biochem. 1983, 134, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Polissiou, M.; Viet, M.T.P.; St-Jacques, M.; Theophanides, T. High Resolution Nuclear Magnetic Resonance Studies of Platinum(II)—Guanosine-5′-Monophosphate Interactions in Aqueous Solutions. Can. J. Chem. 1981, 59, 3297–3302. [Google Scholar] [CrossRef]
- Ezra, F.S.; Lee, C.H.; Kondo, N.S.; Danyluk, S.S.; Sarma, R.H. Conformational Properties of Purine-Pyrimidine and Pyrimidine-Purine Dinucleoside Monophosphates. Biochemistry 1977, 16, 1977–1987. [Google Scholar] [CrossRef]
- Kim, S.-G.; Leh, L.-J.; Reid, B.R. Determination of Nucleic Acid Backbone Conformation by 1H NMR. Biochemistry 1992, 31, 3564–3574. [Google Scholar] [CrossRef]
- Graziotto, M.E.; Akerfeldt, M.C.; Gunn, A.P.; Yang, K.; Somerville, M.V.; Coleman, N.V.; Roberts, B.R.; Hambley, T.W.; New, E.J. The Influence of the Ethane-1,2-Diamine Ligand on the Activity of a Monofunctional Platinum Complex. J. Inorg. Biochem. 2017, 177, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.K.; Gautam, S.; Howlader, P.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Pyriplatin-Boron-Dipyrromethene Conjugates for Imaging and Mitochondria-Targeted Photodynamic Therapy. Inorg. Chem. 2018, 57, 14374–14385. [Google Scholar] [CrossRef]
- Drew, H.D.K. 332. The Behaviour of Chelate Groupings Attached to Platinum and to Palladium. J. Chem. Soc. 1932, 332, 2328–2331. [Google Scholar] [CrossRef]
- Galanski, M.; Keppler, B.K. Synthesis and Characterization of New Ethylenediamine Platinum(IV) Complexes Containing Lipophilic Carboxylate Ligands. Met. Based. Drugs 1995, 2, 57–63. [Google Scholar] [CrossRef]
- Tsolis, T.; Papavasileiou, K.D.; Divanis, S.A.; Melissas, V.S.; Garoufis, A. How Half Sandwich Ruthenium Compounds Interact with DNA While Not Being Hydrolyzed; A Comparative Study. J. Inorg. Biochem. 2016, 160, 12–23. [Google Scholar] [CrossRef]
- Tsolis, T.; Manos, M.J.; Karkabounas, S.; Zelovitis, I.; Garoufis, A. Synthesis, X-Ray Structure Determination, Cytotoxicity and Interactions with 9-Methylguanine, of Ruthenium(II) H6-Arene Complexes. J. Organomet. Chem. 2014, 768, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Hara, R.; Singh, G.; Sancar, A.; Lippard, S.J. Nucleotide Excision Repair from Site-Specifically. Biochemistry 2003, 42, 6747–6753. [Google Scholar] [CrossRef]




| Compound | Pyridine/2-Methylpyridine | en | Guanosine/9-Methylguanine | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | H3 | H4 | H5 | H6 | CH3 | Ha1a2/b1b2 | H8 | CH3 | H1′ (J1′2′) | H2′ (J2′3′) | H3′ (J3′4′) | H4′ | H5′ (J4′5′) | H5″ (J4′5′’) | |
| guo | 8.03 | 5.91 (5.95) | 4.74 (5.40) | 4.41 (3.80) | 4.18 | 3.89 (3.05) | 3.74 (4.05) | ||||||||
| [1-D2O] | 8.71 | 7.56 | 8.03 | 7.56 | 8.71 | - | 2.72–2.67 | ||||||||
| [1-guo] | 8.66 | 7.52 | 8.00 | 8.66 | 7.52 | - | 2.84–2.80 | 8.44 | 5.89 (4.85) | 4.61 (4.95) | 4.29 (5.60) | 4.21 | 3.86 (2.75) | 3.77 (4.15) | |
| [2-D2O] | - | 7.54 | 7.83 | 7.34 | 8.83 | 3.09 | 2.71–2.66 | ||||||||
| [2-guo]A | - | 7.48 | 7.87 | 7.39 | 8.93b | 2.96 | 2.82–2.80 | 8.31 | 5.84 (4.70) | 4.58 (4.30) | 4.28 (5.60) | 4.18 | 3.85 (2.75) | 3.74 (4.95) | |
| [2-guo]B | - | 7.48 | 7.87 | 7.39b | 8.93b | 2.98 | 2.82–2.80 | 8.29 | 5.84 (4.70) | 4.53 (4.60) | 4.22 (4.90) | 4.18 | 3.85 (2.75) | 3.74 (4.05) | |
| 9-MeG | 7.76 | 3.65 | |||||||||||||
| [2-(9-MeG)] | 7.46 | 7.86 | 7.37 | 8.91 | 2.96 | 2.81 2.79 | 7.99 | 3.58 | |||||||
| Compound | anti (%) | syn (%) | 3E (%) | 2E (%) | gg (%) | (gt + tg) (%) |
|---|---|---|---|---|---|---|
| guo | 57 | 43 | 39 | 61 | 59 | 41 |
| [1-guo] | 79 | 21 | 54 | 46 | 61 | 39 |
| [2-guo]A | 85 | 15 | - | - | 62 | 38 |
| [2-guo]B | 93 | 7 | - | - | 53 | 47 |
| Pt-N7Gp5′ a | 51 | 49 | 36 | 64 | ||
| Me-N7Gp5′ b | 54 | 46 | 97 | 3 | ||
| H+-N7Gp5′ a | 57 | 43 | 87 | 13 | ||
| Gp5′ a | 36 | 64 | 65 | 35 |
| Compound | 2-phpy | 9-MeG | en | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H3 | H4 | H5 | H6 | H2′ | H3′ | H4′ | H5′ | H6′ | H8 | -CH3 | Ha1a2/b1b2 | |
| 9-MeG in D2O | 7.76 | 3.58 | ||||||||||
| (3) | 7.72 | 8.07 | 7.55 | 8.96 | 8.10 | 7.68 | 7.68 | 7.68 | 8.10 | - | - | 2.41–2.46 |
| (3′) | 7.92 | 8.07 | 7.25 | 8.46 | 7.69 | 7.32 | 7.32 | 7.31 | - | - | - | 2.58–2.56 |
| [3-(9-MeG)] | 8.23 | 8.15 | 7.65 | 9.12 | 7.64 | 7.44 | 7.44 | 7.44 | 7.64 | 7.21 | 3.62 | 2.60–2.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagari, E.-V.; Sifnaiou, E.; Tsolis, T.; Garoufis, A. The Influence of the Auxiliary Ligand in Monofunctional Pt(II) Anticancer Complexes on the DNA Backbone. Int. J. Mol. Sci. 2024, 25, 6526. https://doi.org/10.3390/ijms25126526
Tagari E-V, Sifnaiou E, Tsolis T, Garoufis A. The Influence of the Auxiliary Ligand in Monofunctional Pt(II) Anticancer Complexes on the DNA Backbone. International Journal of Molecular Sciences. 2024; 25(12):6526. https://doi.org/10.3390/ijms25126526
Chicago/Turabian StyleTagari, Evanthia-Vasiliki, Evangelia Sifnaiou, Theodoros Tsolis, and Achilleas Garoufis. 2024. "The Influence of the Auxiliary Ligand in Monofunctional Pt(II) Anticancer Complexes on the DNA Backbone" International Journal of Molecular Sciences 25, no. 12: 6526. https://doi.org/10.3390/ijms25126526
APA StyleTagari, E.-V., Sifnaiou, E., Tsolis, T., & Garoufis, A. (2024). The Influence of the Auxiliary Ligand in Monofunctional Pt(II) Anticancer Complexes on the DNA Backbone. International Journal of Molecular Sciences, 25(12), 6526. https://doi.org/10.3390/ijms25126526





