The Identification and Function of Linc01615 on Influenza Virus Infection and Antiviral Response
Abstract
1. Introduction
2. Results
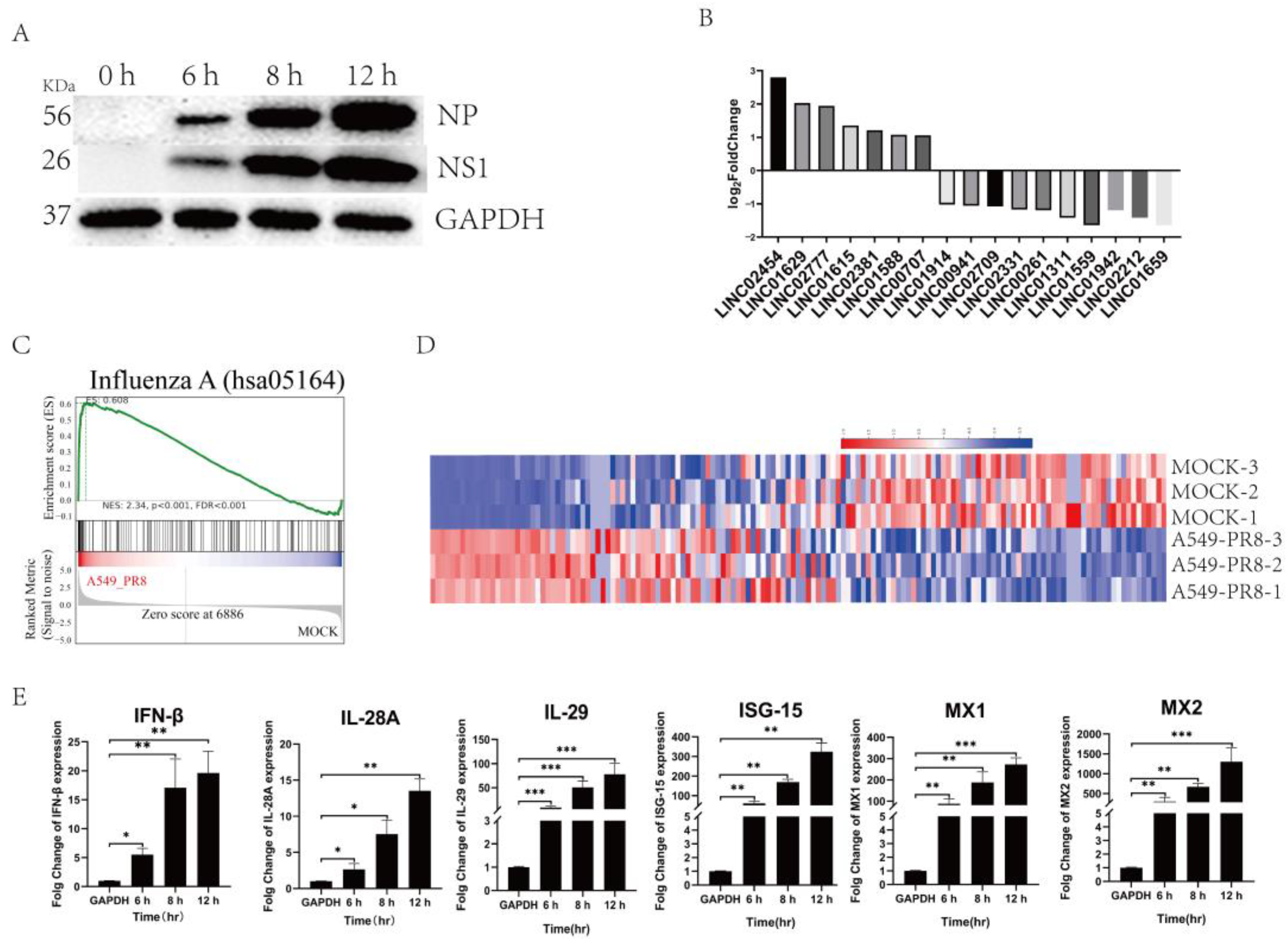
2.1. Intracellular LincRNA Profiles after Influenza Virus Infection
2.2. Influenza Virus Infection Activated the Gene Expressions of Immune-Related Molecular
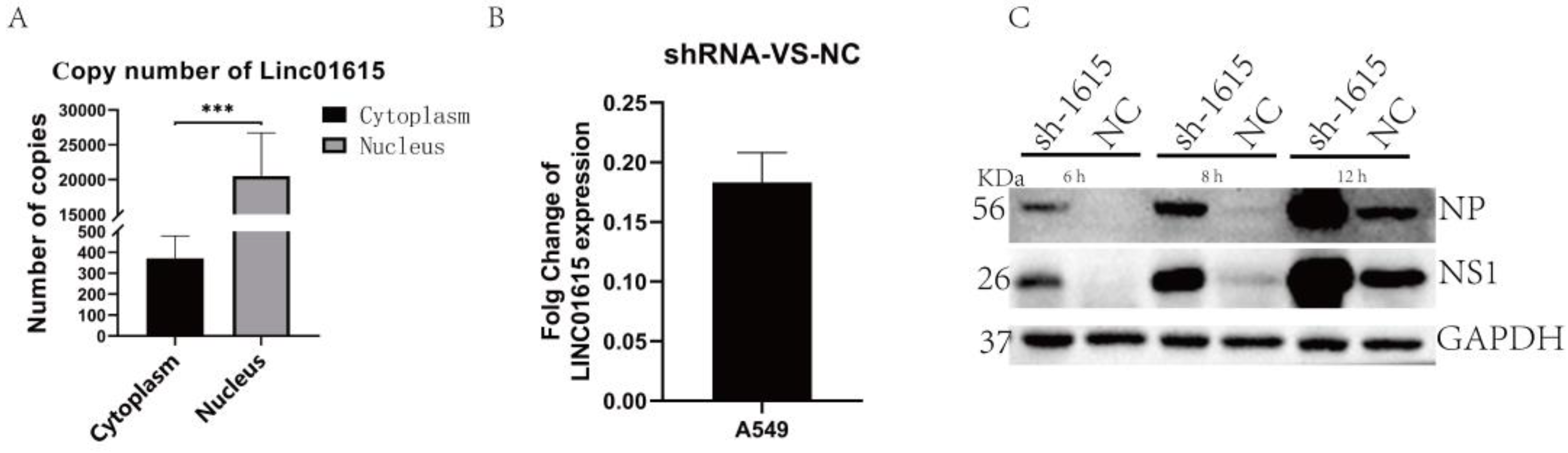
2.3. Knocking down Linc01615 Can Promote the Replication of the Influenza Virus
2.4. Interaction between DHX9 and Linc01615
2.5. Expression Profiles and Function Analysis of Differential Genes in Cells Expressing DHX9
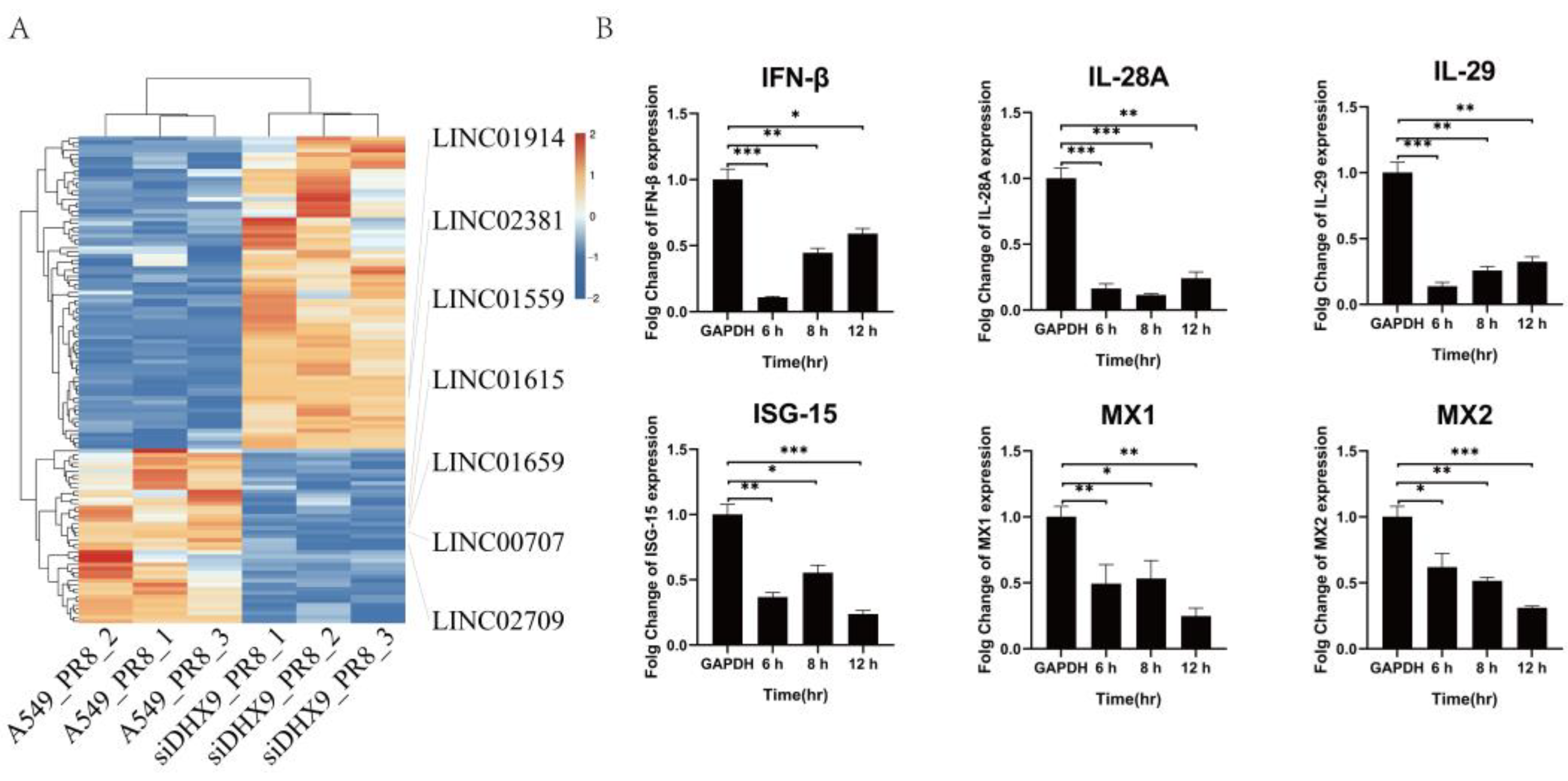
2.6. Immune Factor Related to Interferons and ISGs upon LINC01615 Knockdown
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Virus
4.2. RNA-Seq and Data Analysis
4.3. Transfection
4.4. Quantitative Real-Time PCR
4.5. Western Blotting
4.6. CLIP-Seq
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Influenza(Seasonal). Available online: https://www.who.int/zh/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 18 February 2024).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nature reviews. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.C.; Compans, R.W. Host cell dependence of viral morphology. Proc. Natl. Acad. Sci. USA 1998, 95, 5746–5751. [Google Scholar] [CrossRef]
- Lowen, A.C. It’s in the mix: Reassortment of segmented viral genomes. PLoS Pathog. 2018, 14, e1007200. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Rupérez, C.; Checa, R.; Gálvez, R.; Hernández, L.; García, M.; Canorea, I.; Marino, V.; Montoya, A. Current status of L. infantum infection in stray cats in the Madrid region (Spain): Implications for the recent outbreak of human leishmaniosis? Parasites Vectors 2014, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, E.; Melissaris, S.; Panagopoulos, D.; Kalloniati, E.; Sfakianos, G. Pathophysiology and neuroinflammation in COVID-19. Eur. J. Neurodegener. Dis. 2022, 11, 7–9. [Google Scholar]
- Anogeianakis, G. COVID-19: The Omicron B.1.1.529 Variant. Eur. J. Neurodegener. Dis. Sept. -Dec. 2023, 12, 91–93. [Google Scholar]
- Kritas, S.K. COVID-19 and pain. Eur. J. Neurodegener. Dis. 2021, 10, 32–35. [Google Scholar]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Jeang, K.T.; Yedavalli, V. Role of RNA helicases in HIV-1 replication. Nucleic Acids Res. 2006, 34, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.R.; Wilson, G.M.; Coon, J.J.; Mehle, A. Post-Translation Regulation of Influenza Virus Replication. Annu. Rev. Virol. 2020, 7, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, F.; Wang, Q.; Xu, N.; Xie, Y.; Chen, S.; Qin, T.; Peng, D. Influenza a virus antagonizes type I and type II interferon responses via SOCS1-dependent ubiquitination and degradation of JAK1. Virol. J. 2020, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Pauli, E.K.; Schmolke, M.; Wolff, T.; Viemann, D.; Roth, J.; Bode, J.G.; Ludwig, S. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008, 4, e1000196. [Google Scholar] [CrossRef]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, E.G. ENCODE project writes eulogy for junk DNA. Science 2012, 337, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef]
- Morgado-Palacin, L.; Brown, J.A.; Martinez, T.F.; Garcia-Pedrero, J.M.; Forouhar, F.; Quinn, S.A.; Reglero, C.; Vaughan, J.; Heydary, Y.H.; Donaldson, C.; et al. The TINCR ubiquitin-like microprotein is a tumor suppressor in squamous cell carcinoma. Nat. Commun. 2023, 14, 1328. [Google Scholar] [CrossRef]
- Frankish, A.; Carbonell-Sala, S.; Diekhans, M.; Jungreis, I.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Arnan, C.; Barnes, I.; et al. GENCODE: Reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res 2023, 51, D942–D949. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2017, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Ballabio, A.; Rupert, J.L.; Lafreniere, R.G.; Grompe, M.; Tonlorenzi, R.; Willard, H.F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 1991, 349, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Davidow, L.S.; Warshawsky, D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat. Genet. 1999, 21, 400–404. [Google Scholar] [CrossRef]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell Biol. 1990, 10, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sotomaru, Y.; Katsuzawa, Y.; Hatada, I.; Obata, Y.; Sasaki, H.; Kono, T. Unregulated expression of the imprinted genes H19 and Igf2r in mouse uniparental fetuses. J. Biol. Chem. 2002, 277, 12474–12478. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Donaghey, J.; Carey, B.W.; Garber, M.; Grenier, J.K.; Munson, G.; Young, G.; Lucas, A.B.; Ach, R.; Bruhn, L.; et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 2011, 477, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sauvageau, M.; Goff, L.A.; Lodato, S.; Bonev, B.; Groff, A.F.; Gerhardinger, C.; Sanchez-Gomez, D.B.; Hacisuleyman, E.; Li, E.; Spence, M.; et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2013, 2, e01749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Bochenek, G.; Häsler, R.; El Mokhtari, N.E.; König, I.R.; Loos, B.G.; Jepsen, S.; Rosenstiel, P.; Schreiber, S.; Schaefer, A.S. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum. Mol. Genet. 2013, 22, 4516–4527. [Google Scholar] [CrossRef]
- Atianand, M.K.; Fitzgerald, K.A. Long non-coding RNAs and control of gene expression in the immune system. Trends Mol. Med. 2014, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Aiello, D.; Atianand, M.K.; Ricci, E.P.; Gandhi, P.; Hall, L.L.; Byron, M.; Monks, B.; Henry-Bezy, M.; Lawrence, J.B.; et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 2013, 341, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Rapicavoli, N.A.; Qu, K.; Zhang, J.; Mikhail, M.; Laberge, R.M.; Chang, H.Y. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife 2013, 2, e00762. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Emerson, B.M. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-κB complexes. Elife 2014, 3, e01776. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chao, T.C.; Chang, K.Y.; Lin, N.; Patil, V.S.; Shimizu, C.; Head, S.R.; Burns, J.C.; Rana, T.M. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. USA 2014, 111, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA activates Xist by evicting CTCF. Cell 2013, 153, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef]
- Koh, H.R.; Xing, L.; Kleiman, L.; Myong, S. Repetitive RNA unwinding by RNA helicase A facilitates RNA annealing. Nucleic Acids Res. 2014, 42, 8556–8564. [Google Scholar] [CrossRef]
- Su, C.; Tang, Y.D.; Zheng, C. DExD/H-box helicases: Multifunctional regulators in antiviral innate immunity. Cell Mol. Life Sci. 2021, 79, 2. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, B.; Lu, N.; Facchinetti, V.; Liu, Y.J. DHX9 pairs with IPS-1 to sense double-stranded RNA in myeloid dendritic cells. J. Immunol. 2011, 187, 4501–4508. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.R.; Qian, S.; Bolinger, C.; Fernandez, S.; Schoenberg, D.R.; Boris-Lawrie, K. RNA helicase A is necessary for translation of selected messenger RNAs. Nat. Struct. Mol. Biol. 2006, 13, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Y.; Pyo, H.M.; Lu, X.; Raman, S.N.; Liu, Q.; Brown, E.G.; Zhou, Y. Identification of RNA helicase A as a cellular factor that interacts with influenza A virus NS1 protein and its role in the virus life cycle. J. Virol. 2012, 86, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Isken, O.; Baroth, M.; Grassmann, C.W.; Weinlich, S.; Ostareck, D.H.; Ostareck-Lederer, A.; Behrens, S.E. Nuclear factors are involved in hepatitis C virus RNA replication. RNA 2007, 13, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.; Rieder, E. Identification of RNA helicase A as a new host factor in the replication cycle of foot-and-mouth disease virus. J. Virol. 2009, 83, 11356–11366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, J.; Li, J.; Li, Y.; Lu, Z.; Che, Y.; Mao, S.; Lei, Y.; Zang, R.; Zheng, S.; Liu, C.; et al. Interferon-inducible lncRNA IRF1-AS represses esophageal squamous cell carcinoma by promoting interferon response. Cancer Lett. 2019, 459, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Cao, Y.; Cai, Z.; Nie, X.; Ruan, J.; Zhou, Z.; Ruan, G.; Zhu, Z.; Han, W.; Ding, C. The lncRNA PILA promotes NF-κB signaling in osteoarthritis by stimulating the activity of the protein arginine methyltransferase PRMT1. Sci. Signal. 2022, 15, eabm6265. [Google Scholar] [CrossRef] [PubMed]
- Koffler-Brill, T.; Noy, Y.; Avraham, K.B. The long and short: Non-coding RNAs in the mammalian inner ear. Hear. Res. 2023, 428, 108666. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Ren, Z.; Liu, X.; Song, J.; Zhang, P.; Zhang, C.; Gong, S.; Wu, N.; Zhang, X.; et al. LINC01615 maintains cell survival in adaptation to nutrient starvation through the pentose phosphate pathway and modulates chemosensitivity in colorectal cancer. Cell. Mol. Life Sci. 2022, 80, 20. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, K.; Tang, Z.; Feng, Y.; Cai, S.; Ye, J.; Xi, Y.; Li, J.; Liu, R.; Cai, C.; et al. Identification and experimental validation of a tumor-infiltrating lymphocytes-related long noncoding RNA signature for prognosis of clear cell renal cell carcinoma. Front. Immunol. 2022, 13, 1046790. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, W.; Liu, F.; Wang, W.; Su, C.; Zhu, H.; Liao, Z.; Zhang, B.; Chen, X. ALKBH5-mediated m6A modification of lincRNA LINC02551 enhances the stability of DDX24 to promote hepatocellular carcinoma growth and metastasis. Cell Death Dis. 2022, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Yi, J.; Jiang, J.; Zou, Z.; Mo, Y.; Ren, Q.; Lin, Z.; Lu, Y.; Zhang, J.; Liu, J. Identification and validation of a fatty acid metabolism-related lncRNA signature as a predictor for prognosis and immunotherapy in patients with liver cancer. BMC Cancer 2022, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Zhang, J.; Ray, R.; Ray, R.B. Hepatitis C Virus Evades Interferon Signaling by Suppressing Long Noncoding RNA Linc-Pint Involving C/EBP-β. J. Virol. 2021, 95, e0095221. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Y.; Zhan, F.; Zhang, H.; Han, B.; Hu, G.; Zhao, K.; Yang, N.; Yu, Y.; Mao, L.; et al. The conservation and signatures of lincRNAs in Marek’s disease of chicken. Sci. Rep. 2015, 5, 15184. [Google Scholar] [CrossRef]
- You, Z.; Zhang, Q.; Liu, C.; Song, J.; Yang, N.; Lian, L. Integrated analysis of lncRNA and mRNA repertoires in Marek’s disease infected spleens identifies genes relevant to resistance. BMC Genom. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, K.S.; Hu, B.; Li, S.G.; Li, Q.; Luo, Y.P.; Wang, Y.; Deng, Z.F. Systematic Analysis of RNA Regulatory Network in Rat Brain after Ischemic Stroke. BioMed Res. Int. 2018, 8354350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Song, X.; Li, F.; Ma, Y.; Wang, X.; Jin, F.; Liu, P.; et al. A novel lncRNA linc-AhRA negatively regulates innate antiviral response in murine microglia upon neurotropic herpesvirus infection. Theranostics 2021, 11, 9623–9651. [Google Scholar] [CrossRef]
- Fuller-Pace, F.V. DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006, 34, 4206–4215. [Google Scholar] [CrossRef]
- Chakraborty, P.; Hiom, K. DHX9-dependent recruitment of BRCA1 to RNA promotes DNA end resection in homologous recombination. Nat. Commun. 2021, 12, 4126. [Google Scholar] [CrossRef]
- Lee, C.G.; da Costa Soares, V.; Newberger, C.; Manova, K.; Lacy, E.; Hurwitz, J. RNA helicase A is essential for normal gastrulation. Proc. Natl. Acad. Sci. USA 1998, 95, 13709–13713. [Google Scholar] [CrossRef] [PubMed]


 : Cellular Processes,
: Cellular Processes,  : Environmental Information Processing,
: Environmental Information Processing,  : Genetic Information Processing,
: Genetic Information Processing,  : Human Diseases, and
: Human Diseases, and  : Organismal Systems. (D) GO analysis results of differential genes,
: Organismal Systems. (D) GO analysis results of differential genes,  : Biological Processes,
: Biological Processes,  : Cellular Components, and
: Cellular Components, and  : Molecular Functions.
: Molecular Functions.
 : Cellular Processes,
: Cellular Processes,  : Environmental Information Processing,
: Environmental Information Processing,  : Genetic Information Processing,
: Genetic Information Processing,  : Human Diseases, and
: Human Diseases, and  : Organismal Systems. (D) GO analysis results of differential genes,
: Organismal Systems. (D) GO analysis results of differential genes,  : Biological Processes,
: Biological Processes,  : Cellular Components, and
: Cellular Components, and  : Molecular Functions.
: Molecular Functions.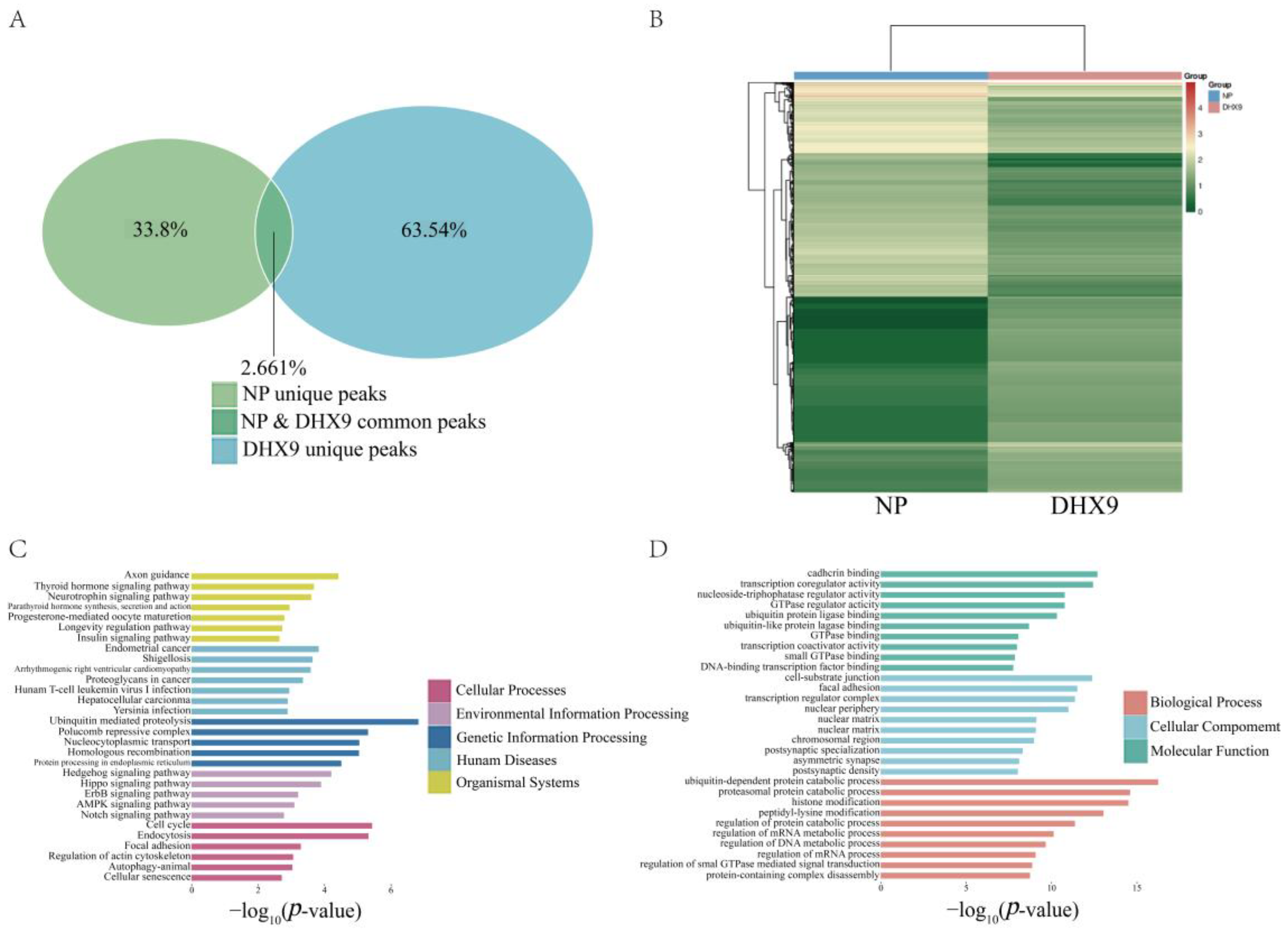
| Primer | Sequence |
|---|---|
| siDHX9-as | 5′-AUCAAUGUUGCUACUAGUCTT-3′ |
| siDHX9-ss | 5′-GACUAGUAGCAACAUUGAUTT-3′ |
| siNC-as | 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| siNC-ss | 5′-ACGUGACACGUUCGGAGAATT-3′ |
| Primer | Sequence |
|---|---|
| IFN-β-sense | 5′-AATTGCTCTCCTGTTGTGCTTCTCC-3′ |
| IFN-β-antisense | 5′-GTCAATGCGGCGTCCTCCTTC-3′ |
| IL-28A-sense | 5′-GCCCTGACGCTGAAGGTTCTG-3′ |
| IL-28A-antisense | 5′-GCGGAAGAGGTTGAAGGTGACAG-3′ |
| IL-29-sense | 5′-CCGTGGTGCTGGTGACTTTGG-3′ |
| IL-29-antisense | 5′-GTTGTGGTGGGCTTGGAAGTGG-3′ |
| ISG-15-sense | 5′- AATGCGACGAACCTCTGAACATCC-3′ |
| ISG-15-antisense | 5′-CGAAGGTCAGCCAGAACAGGTC-3′ |
| MX1-sense | 5′-CTCCGACACGAGTTCCACAA-3′ |
| MX1-antisense | 5′-GGCTCTTCCAGTGCCTTGAT-3′ |
| MX2-sense | 5′-AGGTTCCAGACCTGACCATC-3′ |
| MX2-antisense | 5′-GTCTGCTGCCTCTGGATGTA-3′ |
| GAPDH-sense | 5′-GAGTCAACGGATTTGGTCGT-3′ |
| GAPDH-antisense | 5′-GACAAGCTTCCCGTTCTCAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Hu, J.; Huang, X.; Cai, Y.; Gao, Z.; Guo, X.; Feng, X. The Identification and Function of Linc01615 on Influenza Virus Infection and Antiviral Response. Int. J. Mol. Sci. 2024, 25, 6584. https://doi.org/10.3390/ijms25126584
Yin G, Hu J, Huang X, Cai Y, Gao Z, Guo X, Feng X. The Identification and Function of Linc01615 on Influenza Virus Infection and Antiviral Response. International Journal of Molecular Sciences. 2024; 25(12):6584. https://doi.org/10.3390/ijms25126584
Chicago/Turabian StyleYin, Guihu, Jianing Hu, Xiangyu Huang, Yiqin Cai, Zichen Gao, Xinyu Guo, and Xiuli Feng. 2024. "The Identification and Function of Linc01615 on Influenza Virus Infection and Antiviral Response" International Journal of Molecular Sciences 25, no. 12: 6584. https://doi.org/10.3390/ijms25126584
APA StyleYin, G., Hu, J., Huang, X., Cai, Y., Gao, Z., Guo, X., & Feng, X. (2024). The Identification and Function of Linc01615 on Influenza Virus Infection and Antiviral Response. International Journal of Molecular Sciences, 25(12), 6584. https://doi.org/10.3390/ijms25126584







