Novel Fibrillar and Non-Fibrillar Collagens Involved in Fibrotic Scar Formation after Myocardial Infarction
Abstract
1. Introduction
2. Results
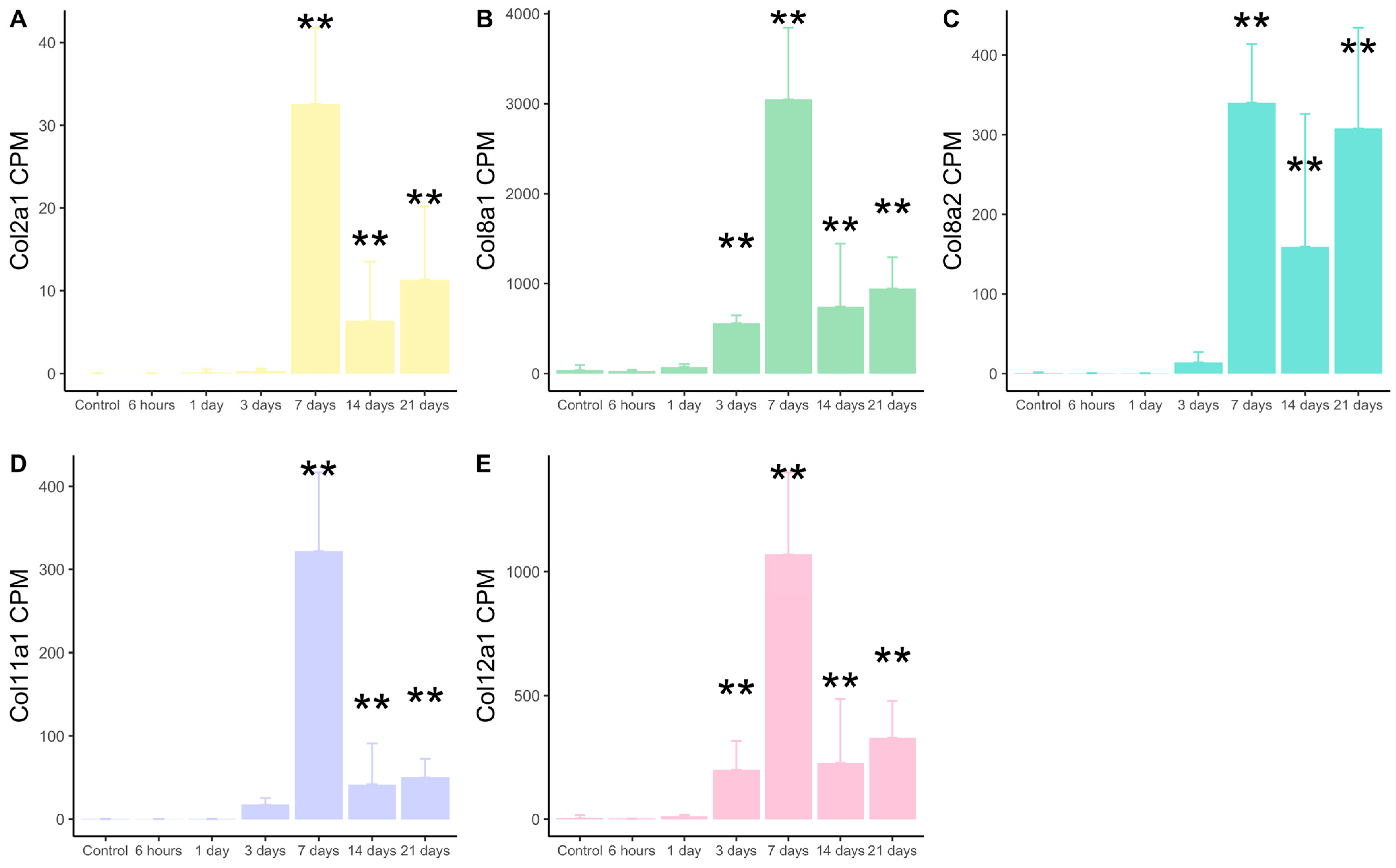
2.1. Transcriptomic Expression of New Collagen Subunits Based on RNA-Sequencing Datasets from Mice Submitted to Non-Reperfused MI
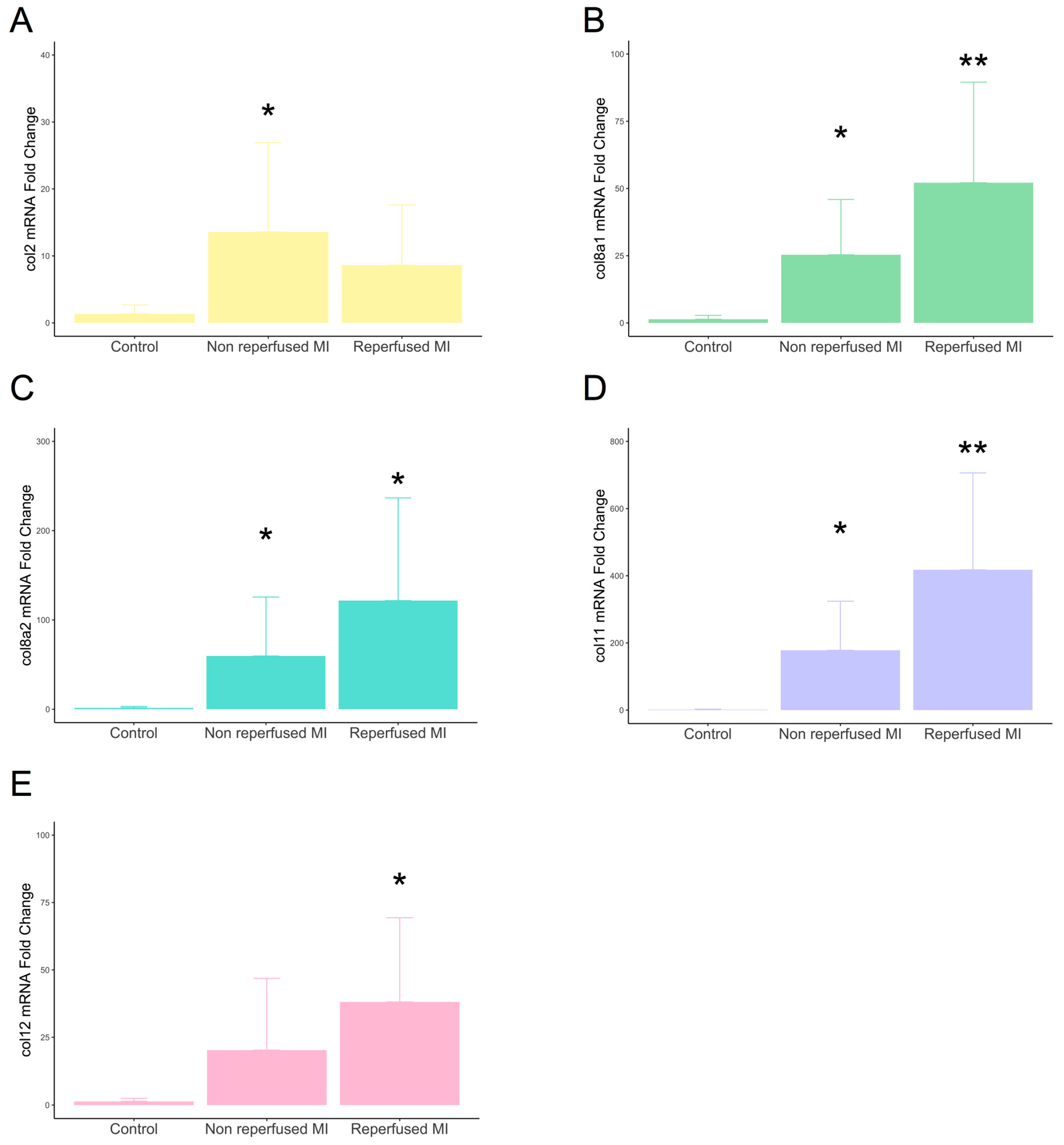
2.2. Involvement of New Collagen Subunits in Non-Reperfused and Reperfused Models of MI
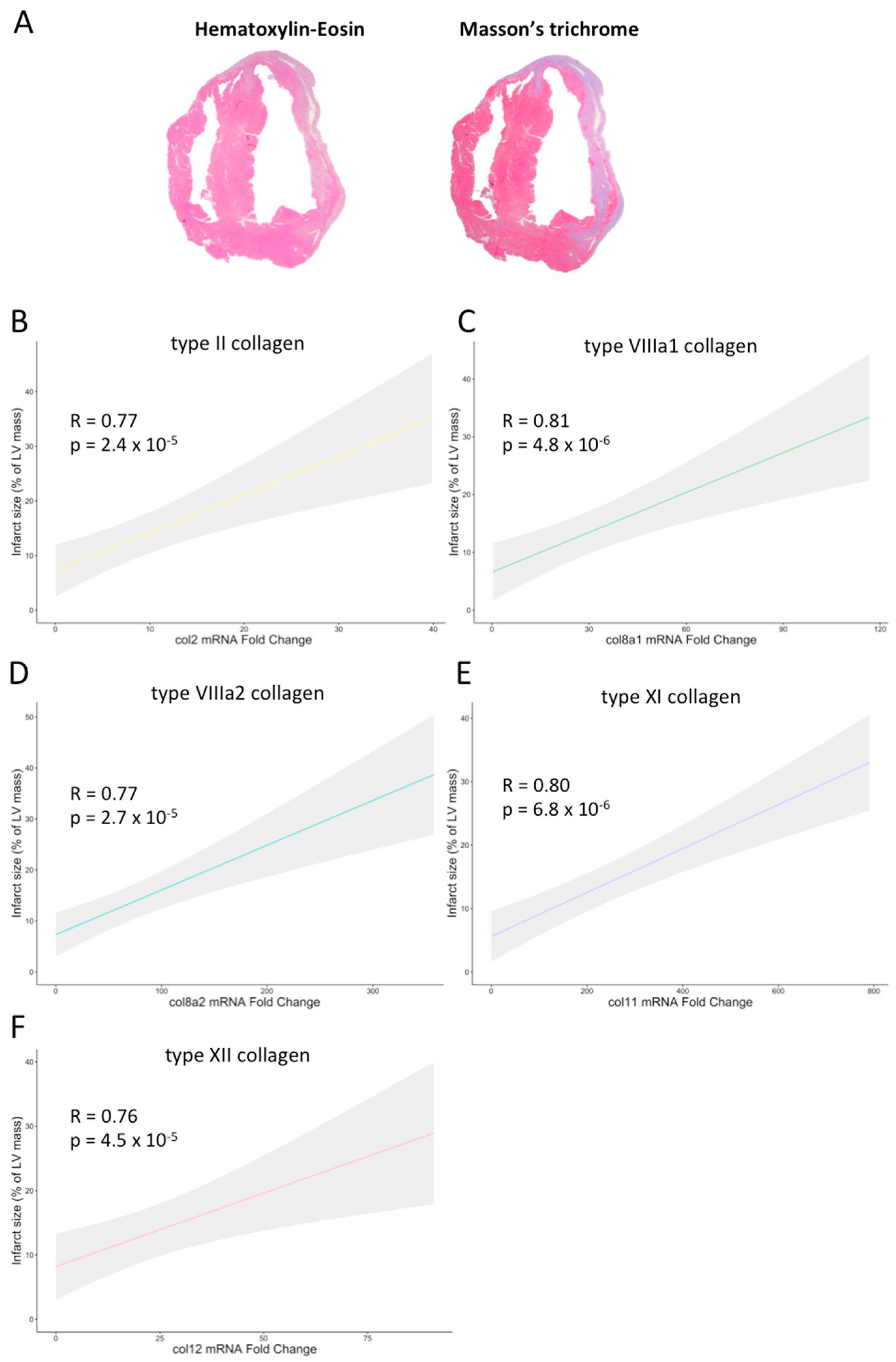
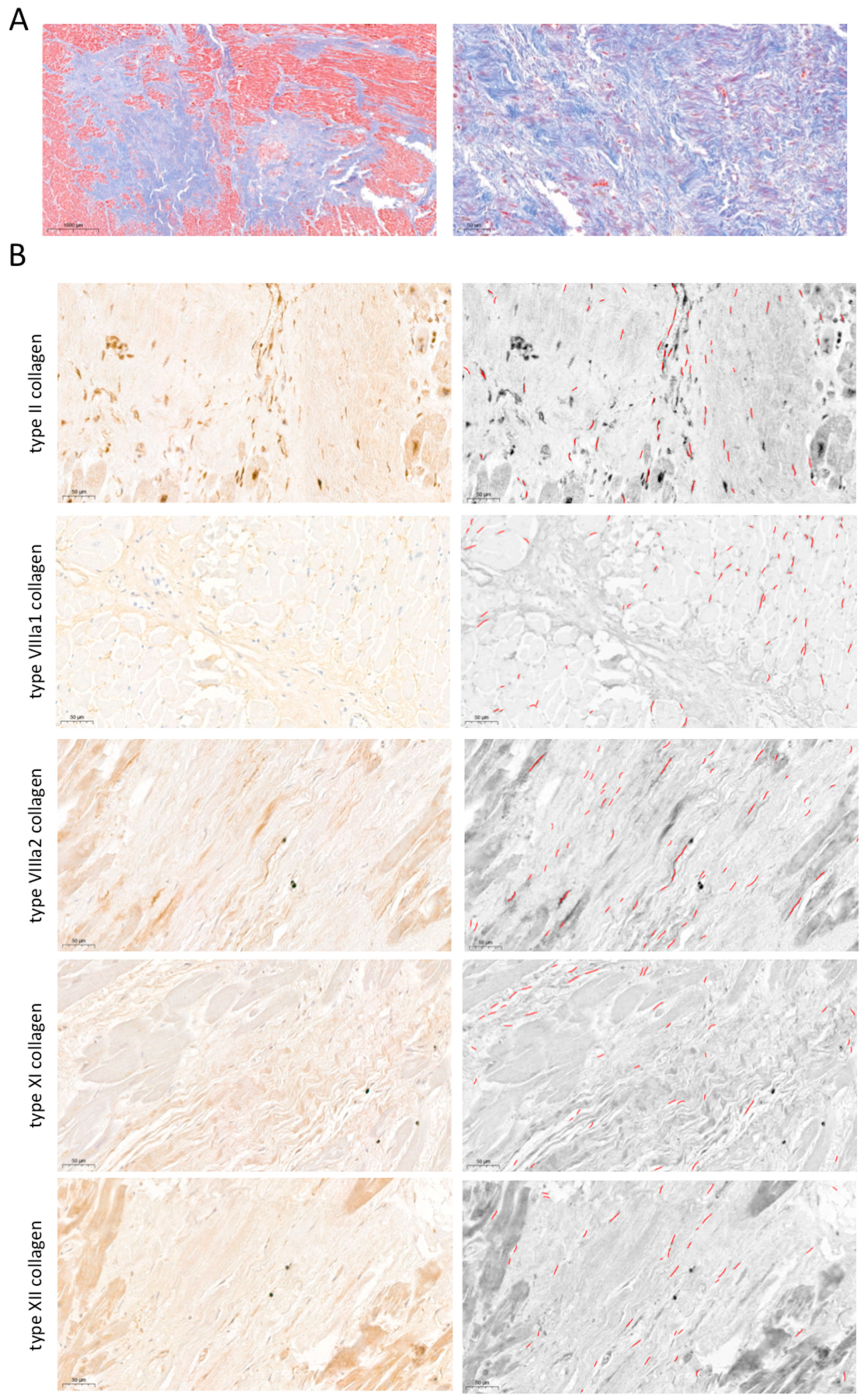
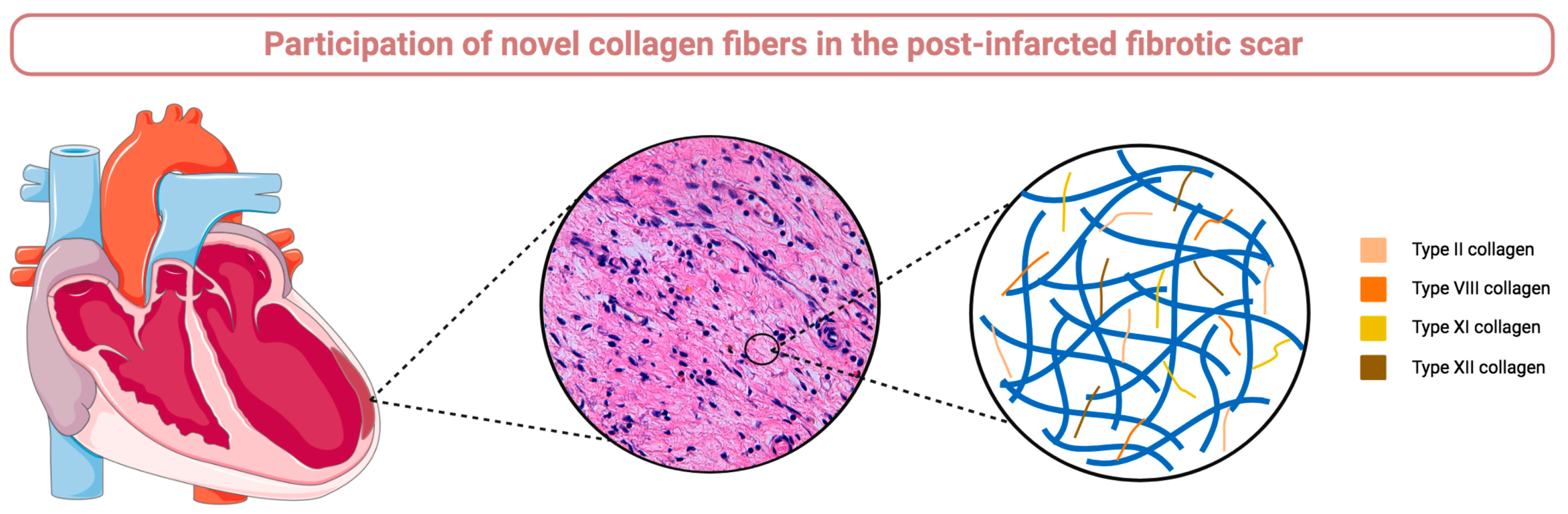
2.3. Participation of Novel Collagen Subunits in Fibrotic Scar from Patients with Chronic MI
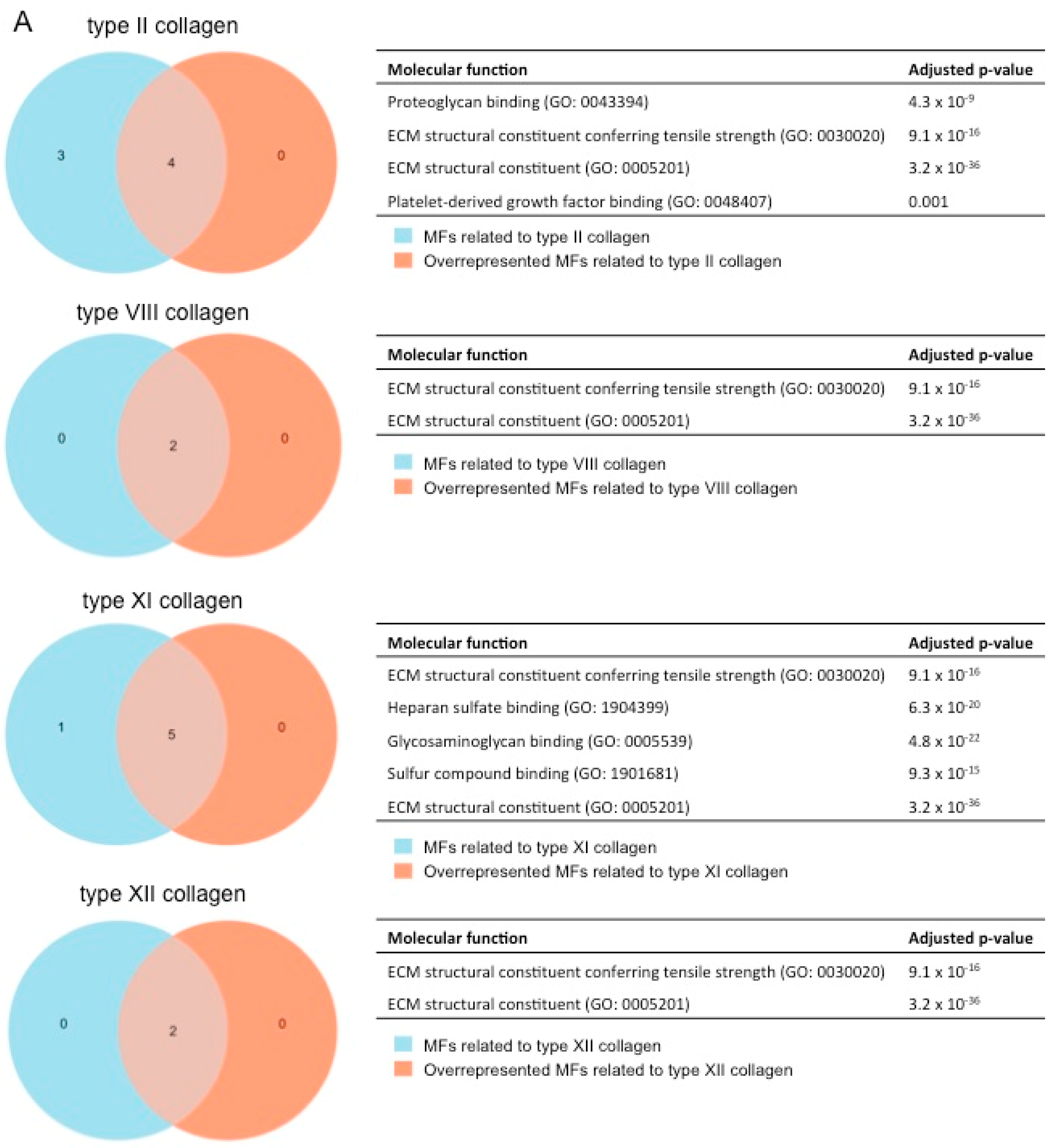
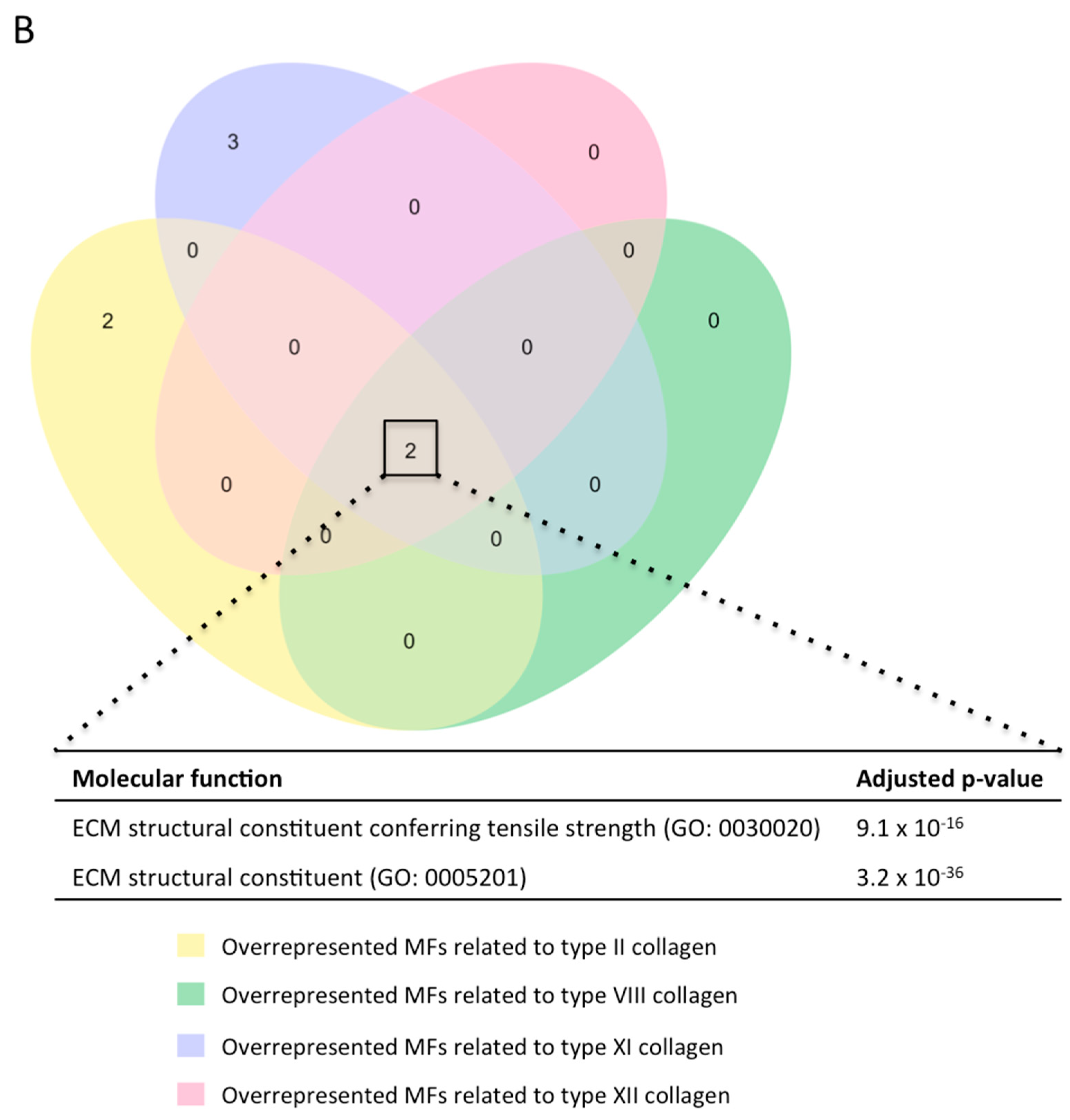
2.4. Gene Ontology Enrichment Analysis to Predict the Function of the Novel Subunits in the Context of MI
3. Discussion
3.1. The Role of the Fibrotic Process in MI Pathophysiology
3.2. Collagen Fibers Participating in Fibrotic Scar Formation following MI
3.3. Unraveling the Participation of Novel Collagen Subunits in the MI Scenario
3.4. The Role of New Collagen Subunits in the Post-Infarction Fibrosis
4. Materials and Methods
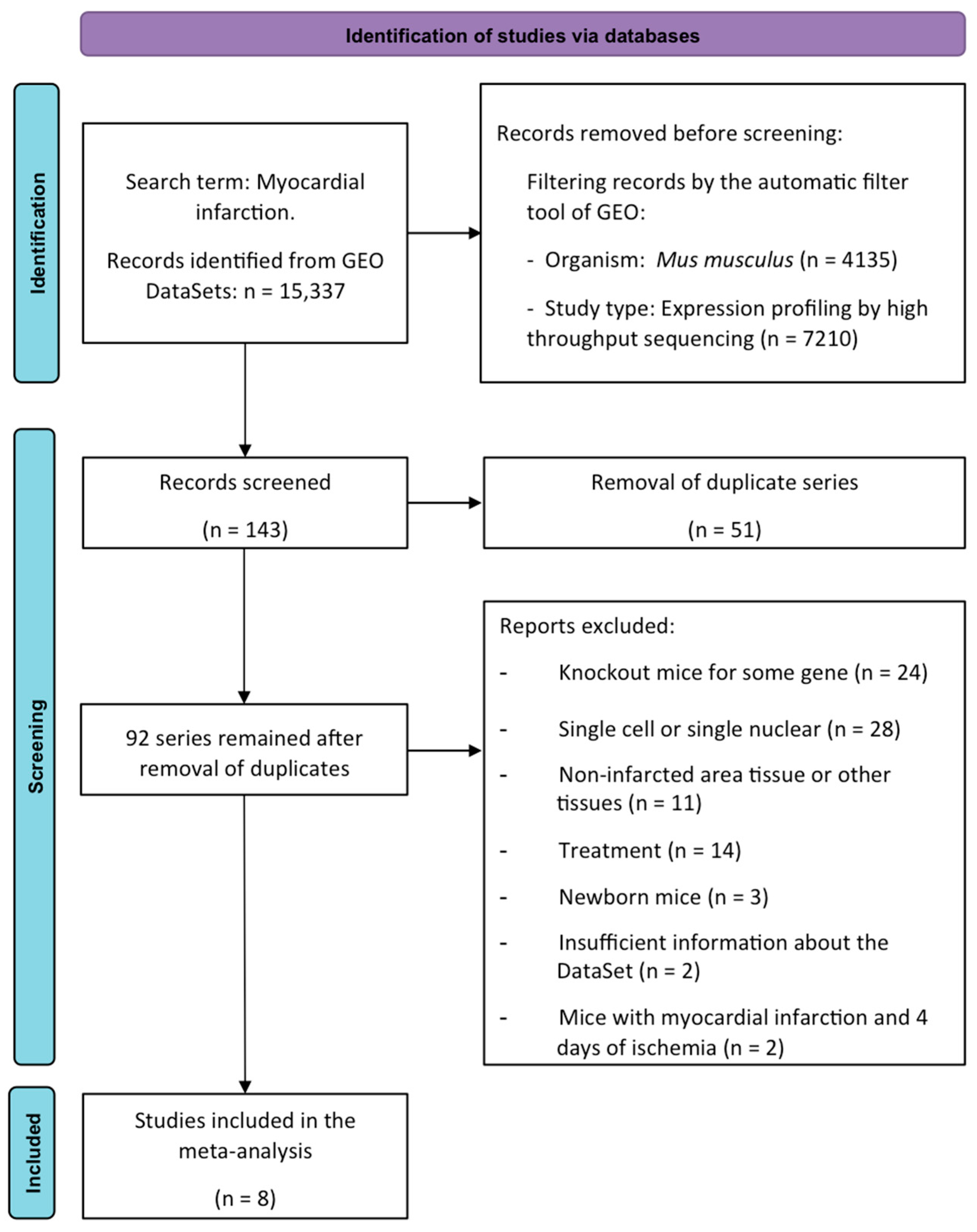
4.1. Meta-Analysis of RNA-Sequencing Data
4.2. Mouse MI Model
4.3. Human Sample Selection
4.4. Microscopic and Immunohistochemical Analysis
4.5. Morphometric Quantification in the Infarcted Myocardium
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rios-Navarro, C.; Ortega, M.; Marcos-Garces, V.; Gavara, J.; de Dios, E.; Perez-Sole, N.; Chorro, F.J.; Bodi, V.; Ruiz-Sauri, A. Interstitial changes after reperfused myocardial infarction in swine: Morphometric and genetic analysis. BMC Vet. Res. 2020, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Francis Stuart, S.D.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Hervas, A.; Ruiz-Sauri, A.; de Dios, E.; Forteza, M.J.; Minana, G.; Nunez, J.; Gomez, C.; Bonanad, C.; Perez-Sole, N.; Gavara, J.; et al. Inhomogeneity of collagen organization within the fibrotic scar after myocardial infarction: Results in a swine model and in human samples. J. Anat. 2016, 228, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- Ortega, M.; Ríos-Navarro, C.; Gavara, J.; de Dios, E.; Perez-Solé, N.; Marcos-Garcés, V.; Ferrández-Izquierdo, A.; Bodí, V.; Ruiz-Saurí, A. Meta-Analysis of Extracellular Matrix Dynamics after Myocardial Infarction Using RNA-Sequencing Transcriptomic Database. Int. J. Mol. Sci. 2022, 23, 15615. [Google Scholar] [CrossRef]
- Liu, W.; Shen, J.; Li, Y.; Wu, J.; Luo, X.; Yu, Y.; Zhang, Y.; Gu, L.; Zhang, X.; Jiang, C.; et al. Pyroptosis inhibition improves the symptom of acute myocardial infarction. Cell Death Dis. 2021, 12, 852. [Google Scholar] [CrossRef]
- Yokota, T.; McCourt, J.; Ma, F.; Ren, S.; Li, S.; Kim, T.-H.; Kurmangaliyev, Y.Z.; Nasiri, R.; Ahadian, S.; Nguyen, T.; et al. Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury. Cell 2020, 182, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-J.; Park, J.H.; Lee, G.S.; Lee, J.-Y.; Shin, J.H.; Kim, M.W.; Kim, Y.S.; Kim, J.-Y.; Oh, K.-J.; Han, B.-S.; et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Klevstig, M.; Benfeitas, R.; Doran, S.; Turkez, H.; Uhlén, M.; Clausen, M.; Wikström, J.; Etal, D.; Zhang, C.; et al. Integrative transcriptomic analysis of tissue-specific metabolic crosstalk after myocardial infarction. Elife 2021, 10, e66921. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Walton, C.B.; Pinell, B.; Khadka, V.S.; Dunn, B.; Lee, K.; Anagaran, M.C.T.; Avelar, A.; Shohet, R.V. Ischemic heart injury leads to HIF1-dependent differential splicing of CaMK2γ. Sci. Rep. 2021, 11, 13116. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.B.; Hildrestrand, G.A.; Scheffler, K.; Vinge, L.E.; Alfsnes, K.; Palibrk, V.; Wang, J.; Neurauter, C.G.; Luna, L.; Johansen, J.; et al. NEIL3-Dependent Regulation of Cardiac Fibroblast Proliferation Prevents Myocardial Rupture. Cell Rep. 2017, 18, 82–92. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Cleutjens, J.P.M.; Creemers, E.E.J.M. Integration of concepts: Cardiac extracellular matrix remodeling after myocardial infarction. J. Card. Fail. 2002, 8, S344–S348. [Google Scholar] [CrossRef]
- Khan, R.; Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006, 118, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J.S. Fibrillar Collagens. Subcell Biochem. 2017, 82, 457–490. [Google Scholar]
- Yin, X.; Yin, X.; Pan, X.; Zhang, J.; Fan, X.; Li, J.; Zhai, X.; Jiang, L.; Hao, P.; Wang, J.; et al. Post-myocardial infarction fibrosis: Pathophysiology, examination, and intervention. Front. Pharmacol. 2023, 14, 1070973. [Google Scholar] [CrossRef] [PubMed]
- Shamhart, P.E.; Meszaros, J.G. Non-fibrillar collagens: Key mediators of post-infarction cardiac remodeling? J. Mol. Cell Cardiol. 2010, 48, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Didangelos, A.; Schoendube, F.A.; Drozdov, I.; Yin, X.; Fernández-Caggiano, M.; Willeit, P.; Puntmann, V.O.; Aldama-López, G.; Shah, A.M.; et al. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation 2012, 125, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Skrbic, B.; Engebretsen, K.V.; Strand, M.E.; Lunde, I.G.; Herum, K.M.; Marstein, H.S.; Sjaastad, I.; Lunde, P.K.; Carlson, C.R.; Christensen, G.; et al. Lack of collagen VIII reduces fibrosis and promotes early mortality and cardiac dilatation in pressure overload in mice. Cardiovasc. Res. 2015, 106, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Mohabeer, A.L.; Kroetsch, J.T.; McFadden, M.; Khosraviani, N.; Broekelmann, T.J.; Hou, G.; Zhang, H.; Zhou, Y.-Q.; Wang, M.; Gramolini, A.O.; et al. Deletion of type VIII collagen reduces blood pressure, increases carotid artery functional distensibility and promotes elastin deposition. Matrix Biol. Plus. 2021, 12, 100085. [Google Scholar] [CrossRef] [PubMed]
- Berg, N.W.v.D.; Neefs, J.; Kawasaki, M.; Nariswari, F.A.; Wesselink, R.; Fabrizi, B.; Jongejan, A.; Klaver, M.N.; Havenaar, H.; Hulsman, E.L.; et al. Extracellular matrix remodeling precedes atrial fibrillation: Results of the PREDICT-AF trial. Heart Rhythm. 2021, 18, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Marro, J.; Pfefferli, C.; de Preux Charles, A.S.; Bise, T.; Jaźwińska, A. Collagen XII Contributes to Epicardial and Connective Tissues in the Zebrafish Heart during Ontogenesis and Regeneration. PLoS ONE 2016, 11, e0165497. [Google Scholar] [CrossRef] [PubMed]
- Tzortzaki, E.G.; Tischfield, J.A.; Sahota, A.; Siafakas, N.M.; Gordon, M.K.; Gerecke, D.R. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1073–1080. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 57–359. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Published Online. 2016. Available online: https://ggplot2.tidyverse.org (accessed on 1 February 2024).
- Ríos-Navarro, C.; Hueso, L.; Díaz, A.; Marcos-Garcés, V.; Bonanad, C.; Ruiz-Sauri, A.; Vila, J.M.; Sanz, M.J.; Chorro, F.J.; Piqueras, L.; et al. Role of antiangiogenic VEGF-A165b in angiogenesis and systolic function after reperfused myocardial infarction. Rev. Esp. Cardiol. 2021, 74, 131–139. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Rios-Navarro, C.; Gómez-Torres, F.; Gavara, J.; de Dios, E.; Diaz, A.; Miñana, G.; Chorro, F.J.; Bodi, V.; Ruiz-Sauri, A. Fourier analysis of collagen bundle orientation in myocardial infarction scars. Histochem. Cell Biol. 2022, 158, 471–483. [Google Scholar] [CrossRef]
- Ríos-Navarro, C.; Gavara, J.; de Dios, E.; Pérez-Solé, N.; Molina-García, T.; Marcos-Garcés, V.; Ruiz-Saurí, A.; Bayés-Genís, A.; Carrión-Valero, F.; Chorro, F.J.; et al. Effect of serum from patients with ST-segment elevation myocardial infarction on endothelial cells. Rev. Esp. Cardiol. 2024, 77, 254–264. [Google Scholar] [CrossRef]
| GEO DataSet | Reference | Number of Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 6 h Ischemia | 1 Day Ischemia | 3 Days Ischemia | 7 Days Ischemia | 14 Days Ischemia | 21 Days Ischemia | ||
| GSE153494 | [10] | 3 | 3 | 3 | 3 | |||
| GSE153493 | N/A | 3 | 3 | 3 | ||||
| GSE151834 | [11] | 4 | 4 | 4 | 8 | |||
| GSE114695 | [12] | 9 | 3 | 3 | 3 | |||
| GSE153485 | [13] | 10 | 5 | 5 | ||||
| GSE154072 | N/A | 3 | ||||||
| GSE104187 | [14] | 4 | 2 | 2 | ||||
| GSE83350 | [15] | 1 | 1 | |||||
| Total | 30 | 8 | 16 | 10 | 7 | 10 | 11 | |
| Counts per Million | |||||||
|---|---|---|---|---|---|---|---|
| Genes | Control | 6 h Ischemia | 1 Day Ischemia | 3 Days Ischemia | 7 Days Ischemia | 14 Days Ischemia | 21 Days Ischemia |
| col1a1 | 44.9 ± 32.0 | 20.0 ± 3.6 | 61.5 ± 32.8 | 2227.2 ± 1068.5 ** | 7995.1 ± 3871.1 ** | 1065.4 ± 1017.4 * | 1167.8 ± 710.5 ** |
| col3a1 | 144.0 ± 88.2 | 46.7 ± 16.4 | 181.0 ± 96.1 | 3696.8 ± 1365.6 ** | 13,659.5 ± 673.2 ** | 3965.3 ± 4010.0 * | 42,433.6 ± 1973.6 ** |
| tgfb1 | 12.4 ± 9.9 | 22.5 ± 18.6 | 44.2 ± 44.6 * | 96.9 ± 61.6 ** | 59.6 ± 34.5 * | 28.5 ± 21.8 | 29.3 ± 12.2 ** |
| ccn2 | 91.7 ± 86.0 | 647.6 ± 407.8 * | 562.6 ± 310.8 ** | 581.5 ± 233.7 ** | 2243.5 ± 614.7 ** | 957.9 ± 975.1 * | 1651.0 ± 405.7 ** |
| acta2 | 63.2 ± 20.2 | 53.1 ± 13.6 | 86.6 ± 65.0 | 330.2 ± 112.2 ** | 718.8 ± 247.9 ** | 142.7 ± 137.9 | 360.5 ± 66.0 ** |
| Genes | Control | Non-Reperfused MI | Reperfused MI |
|---|---|---|---|
| col1a1 | 1.10 ± 0.53 | 27.75 ± 17.70 * | 77.62 ± 52.38 * |
| col3a1 | 1.18 ± 0.70 | 24.83 ± 16.25 * | 70.88 ± 43.19 * |
| tgfb1 | 1.04 ± 0.32 | 4.29 ± 2.12 ** | 5.43 ± 2.18 ** |
| ccn2 | 1.34 ± 1.18 | 6.04 ± 4.27 | 14.43 ± 14.15 |
| acta2 | 1.04 ± 0.34 | 2.79 ± 0.77 * | 3.41 ± 1.12 ** |
| Genes | Spearman Rank-Order Correlation | p-Value |
|---|---|---|
| col1a1 | 0.89 | 0.00011 |
| col3a1 | 0.89 | 0.00011 |
| tgfb1 | 0.90 | 7.7 × 10−5 |
| ccn2 | 0.91 | 3.7 × 10−5 |
| acta2 | 0.90 | 5.4 × 10−5 |
| Patient | Clinical Data | Autopsy Results |
|---|---|---|
| #1 | 78-year-old male Time elapsed since infarction: 5 years Cause of death: pneumonia | Infarct scar area: multiple foci in the lower wall of the left ventricle |
| #2 | 72-year-old female Time elapsed since infarction: 17 years Cause of death: aortic valve prosthesis dysfunction | Infarct scar area: 2.5 cm left ventricle |
| #3 | 62-year-old male Time elapsed since infarction: 1 year Cause of death: cerebral edema (diffuse large B-cell lymphoma) | Infarct scar area: 0.5 cm interventricular septum |
| #4 | 69-year-old male Time elapsed since infarction: 3 years Cause of death: multilobar pneumonia | Infarct scar area: left ventricle |
| #5 | 76-year-old male Time elapsed since infarction: 10 years Cause of death: myocardial infarction | Infarct scar area: ill-defined area in interventricular septum and posterior wall |
| #6 | 72-year-old male Time elapsed since infarction: 10 years Cause of death: refractory bradycardia and arrest after intervention | Infarct scar area: multiple foci in left ventricle |
| #7 | 84-year-old female Time elapsed since infarction: 1 year Cause of death: cardiogenic shock | Infarct scar area: 5 cm in the anterior wall of the left ventricle and interventricular septum |
| #8 | 76-year-old female Time elapsed since infarction: 1 year Cause of death: cardiac tamponade after bypass | Infarct scar area: multiple foci in the anterior side of the left ventricle |
| Patient | Clinical Data |
|---|---|
| #1 | 89-year-old male Cause of death: gastric adenocarcinoma |
| #2 | 62-year-old female Cause of death: hemorrhagic peritonitis |
| #3 | 93-year-old female Cause of death: pulmonary embolism |
| #4 | 66-year-old female Cause of death: acute pancreatitis with necrosis |
| Marker | Specie | Concentration | Antigen Retrieval | Reference |
|---|---|---|---|---|
| Col2a1 | Human | 1:100 | Enzymatic | Abcam St. Louis, MO, USA (ab185430) |
| Col8a1 | Human | 1:50 | pH High | LSBio (Shirley, MA, USA) (aa583-743) |
| Col8a2 | Human | 1:100 | pH High | Thermofisher (Waltham, MA, USA) (PA5-51280) |
| Col11a1 | Human | 1:100 | pH High | Thermofisher (Waltham, MA, USA) (PA5-68410) |
| Col12a1 | Human | 1:50 | pH High | Thermofisher (Waltham, MA, USA) (PA5-52655) |
| Molecule | Area (μm2) | Diameter (μm) | Major Axis (μm) | Minor Axis (μm) |
|---|---|---|---|---|
| Col2a1 | 40–180 | 1–7 | 19–40 | 1.0–8 |
| Col8a1 | 15–50 | 2–8 | 10–21 | 1.5–5 |
| Col8a2 | 15–50 | 2–8 | 10–21 | 1.5–5 |
| Col11a1 | 15–50 | 2–8 | 10–21 | 1.5–5 |
| Col12a1 | 15–50 | 2–8 | 10–21 | 1.5–5 |
| Gene | Specie | Reference |
|---|---|---|
| col2a1 | Human | Hs01060337_g1 |
| col8a1 | Human | Hs05426099_s1 |
| col8a2 | Human | Hs07287101_m1 |
| col11a1 | Human | Hs01097671_g1 |
| col12a1 | Human | Hs01054146_m1 |
| col2a1 | Mouse | Mm01309565_m1 |
| col8a1 | Mouse | Mm01344185_m1 |
| col8a2 | Mouse | Mm02344867_g1 |
| col11a1 | Mouse | Mm00483387_m1 |
| col12a1 | Mouse | Mm01148576_m1 |
| col1a1 | Mouse | Mm00801666_g1 |
| col3a1 | Mouse | Mm00802300_m1 |
| acta2 | Mouse | Mm01546133_m1 |
| ccn2 | Mouse | Mm01192933_g1 |
| tgfb1 | Mouse | Mm00441727_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.; Fábrega-García, M.M.; Molina-García, T.; Gavara, J.; de Dios, E.; Pérez-Solé, N.; Marcos-Garcés, V.; Padilla-Esquivel, J.J.; Diaz, A.; Martinez-Dolz, L.; et al. Novel Fibrillar and Non-Fibrillar Collagens Involved in Fibrotic Scar Formation after Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 6625. https://doi.org/10.3390/ijms25126625
Ortega M, Fábrega-García MM, Molina-García T, Gavara J, de Dios E, Pérez-Solé N, Marcos-Garcés V, Padilla-Esquivel JJ, Diaz A, Martinez-Dolz L, et al. Novel Fibrillar and Non-Fibrillar Collagens Involved in Fibrotic Scar Formation after Myocardial Infarction. International Journal of Molecular Sciences. 2024; 25(12):6625. https://doi.org/10.3390/ijms25126625
Chicago/Turabian StyleOrtega, María, Maria Mar Fábrega-García, Tamara Molina-García, Jose Gavara, Elena de Dios, Nerea Pérez-Solé, Víctor Marcos-Garcés, Jaime José Padilla-Esquivel, Ana Diaz, Luis Martinez-Dolz, and et al. 2024. "Novel Fibrillar and Non-Fibrillar Collagens Involved in Fibrotic Scar Formation after Myocardial Infarction" International Journal of Molecular Sciences 25, no. 12: 6625. https://doi.org/10.3390/ijms25126625
APA StyleOrtega, M., Fábrega-García, M. M., Molina-García, T., Gavara, J., de Dios, E., Pérez-Solé, N., Marcos-Garcés, V., Padilla-Esquivel, J. J., Diaz, A., Martinez-Dolz, L., Jimenez-Navarro, M., Rios-Navarro, C., Bodí, V., & Ruiz-Saurí, A. (2024). Novel Fibrillar and Non-Fibrillar Collagens Involved in Fibrotic Scar Formation after Myocardial Infarction. International Journal of Molecular Sciences, 25(12), 6625. https://doi.org/10.3390/ijms25126625







