Cold Atmospheric Pressure Plasma Solutions for Sustainable Food Packaging
Abstract
:1. Introduction
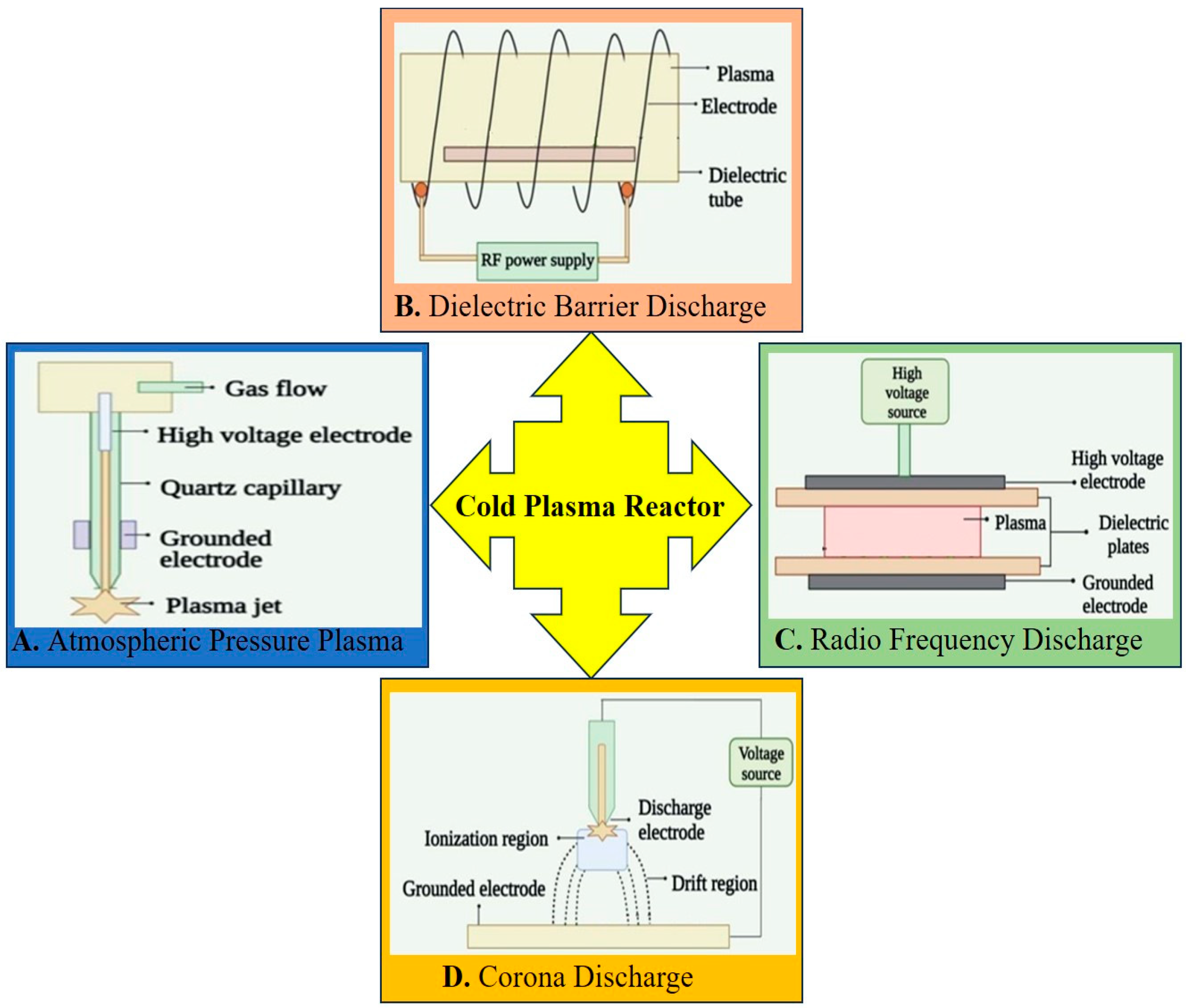
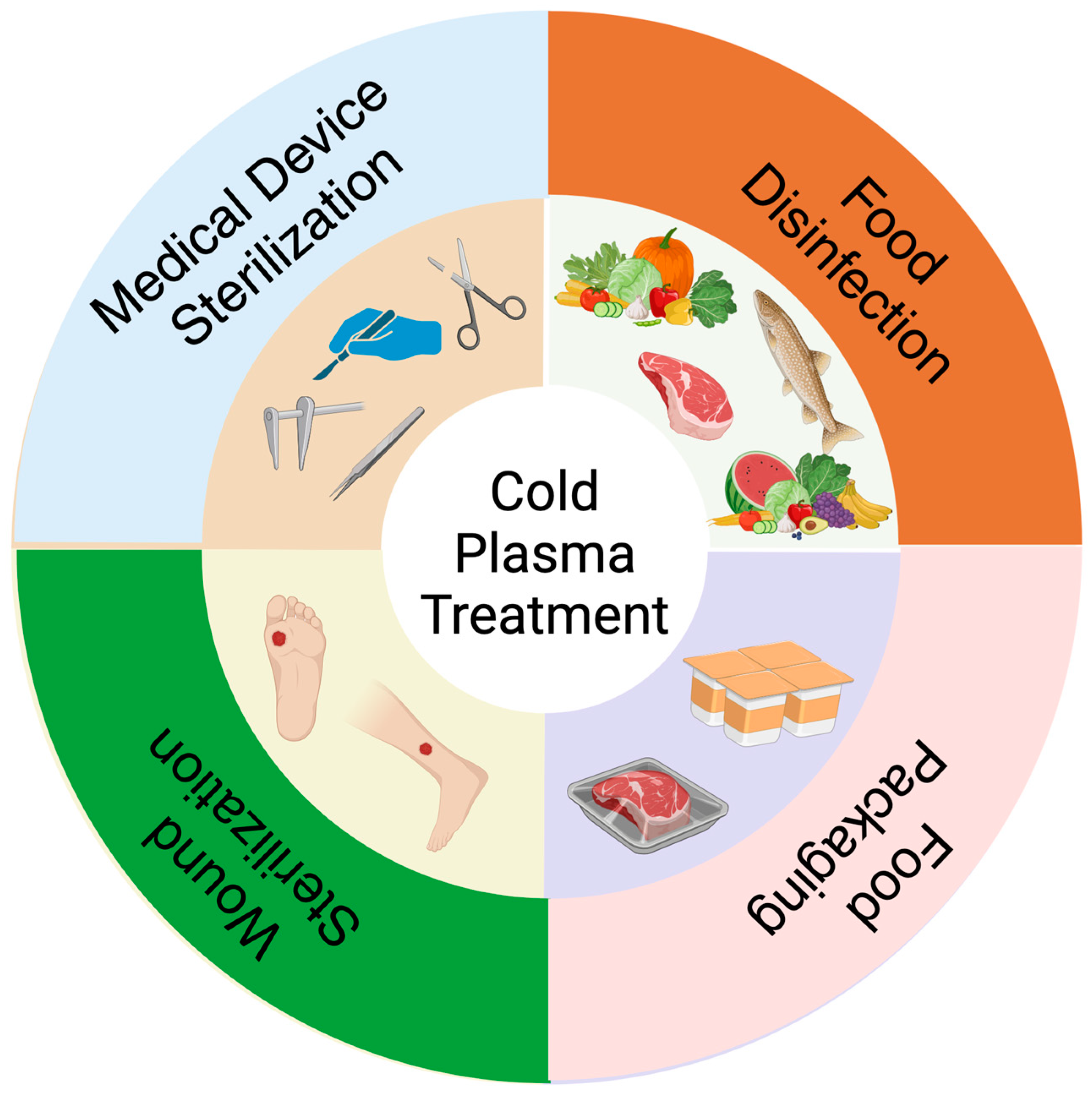
2. Current Advancements in Cold Plasma for Sterilization
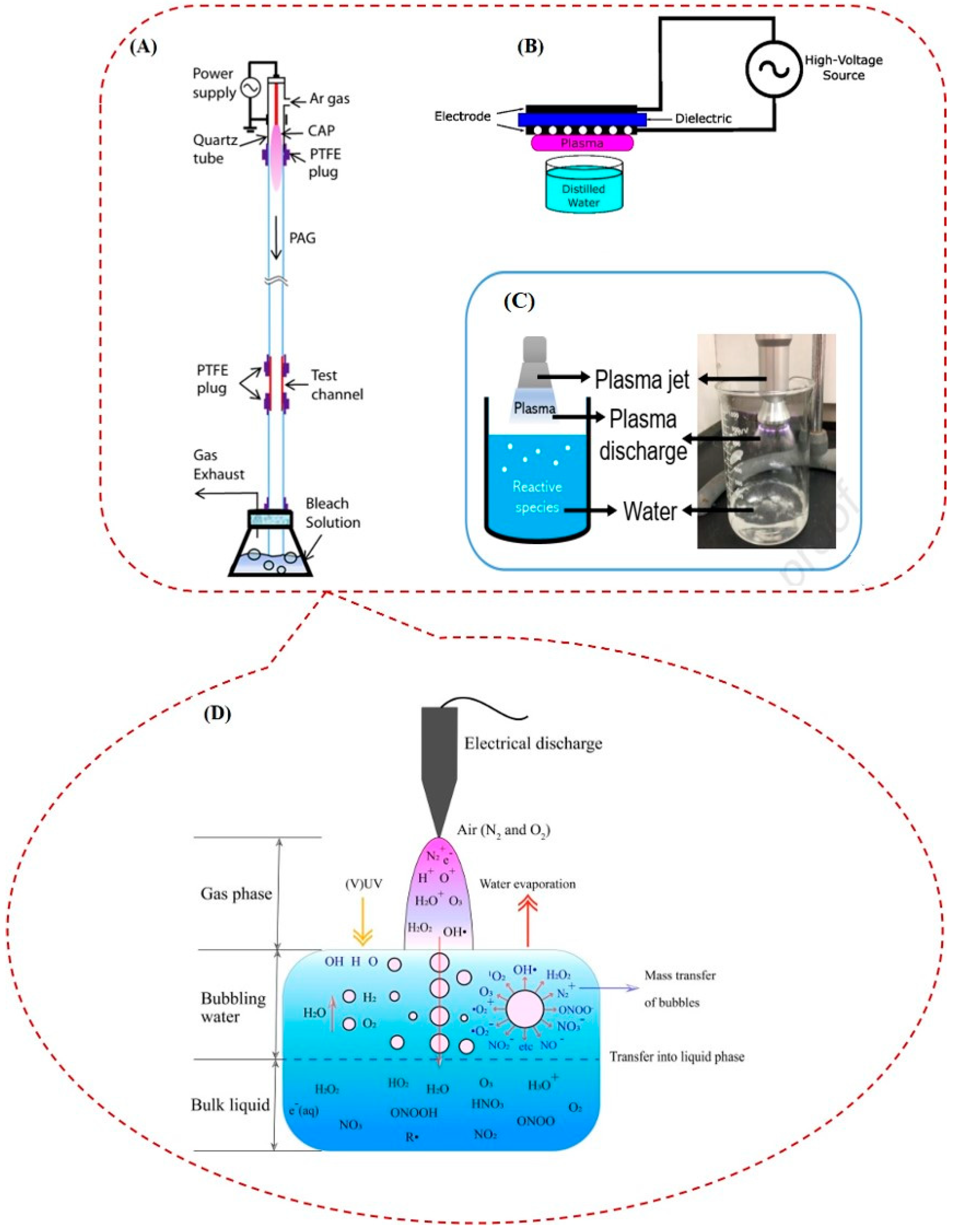
2.1. Cold Atmospheric Plasma and Plasma-Activated Water: Two Different Ways of Using Plasma for Sterilization or Deactivation of Microorganisms
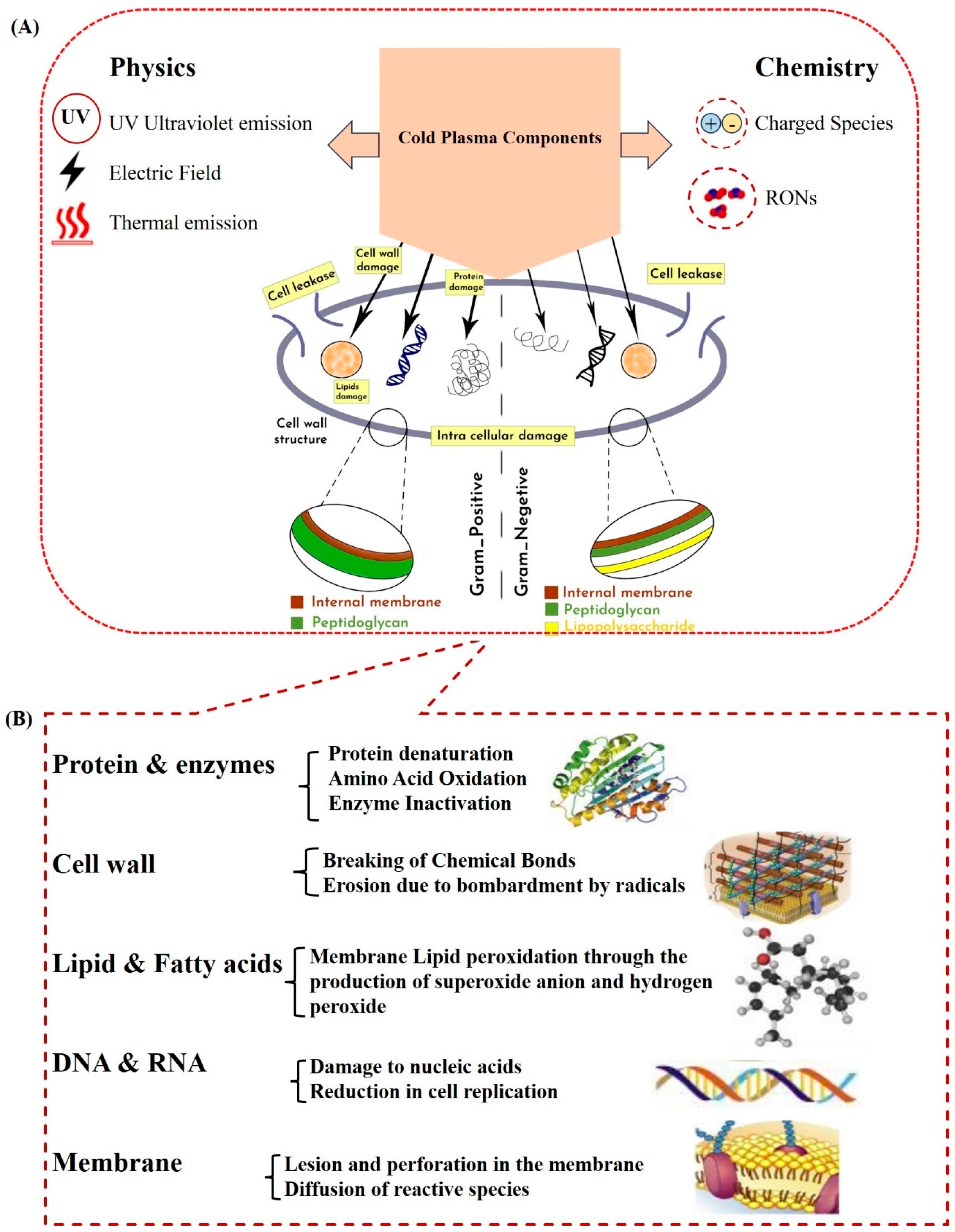
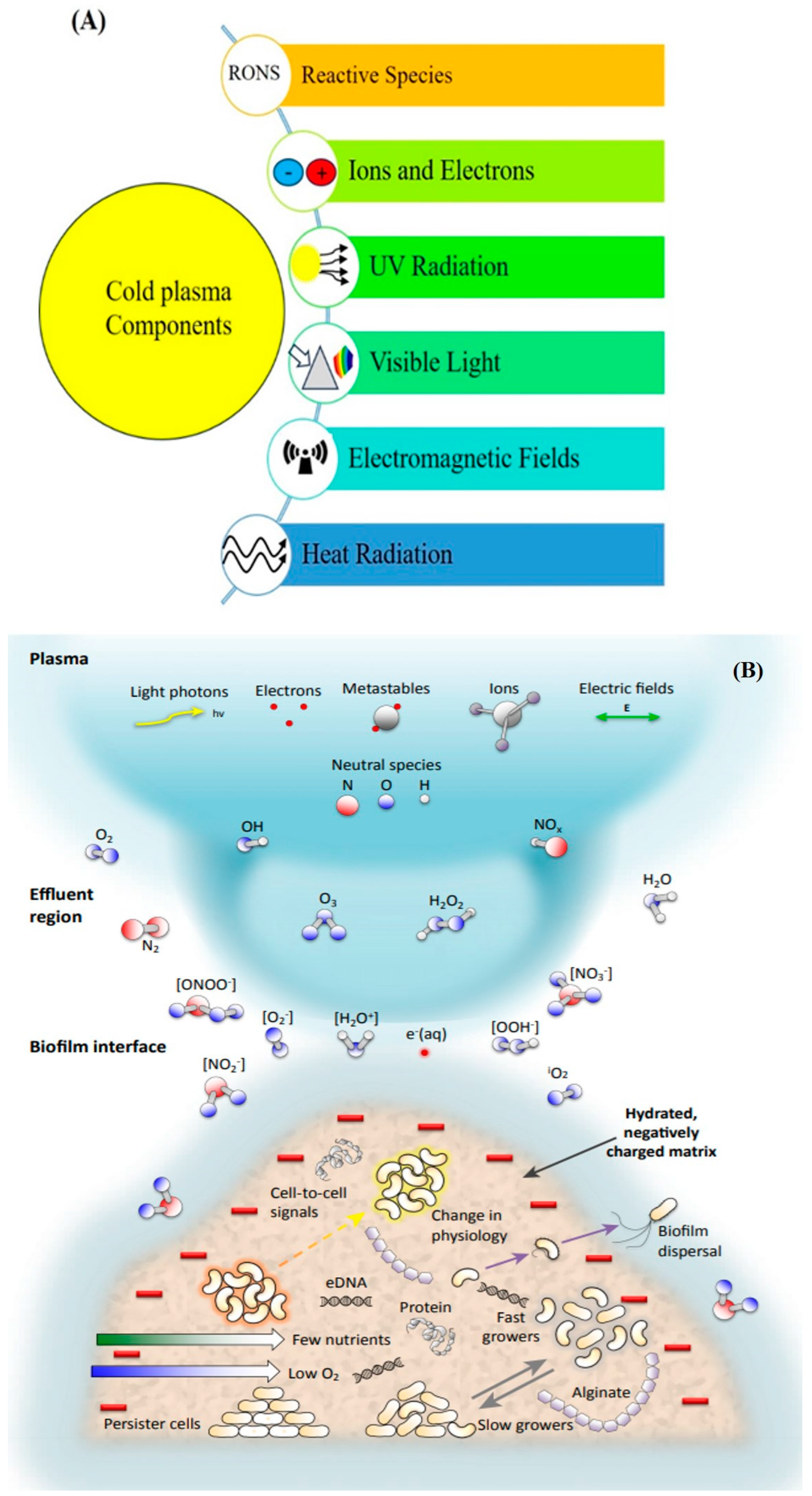
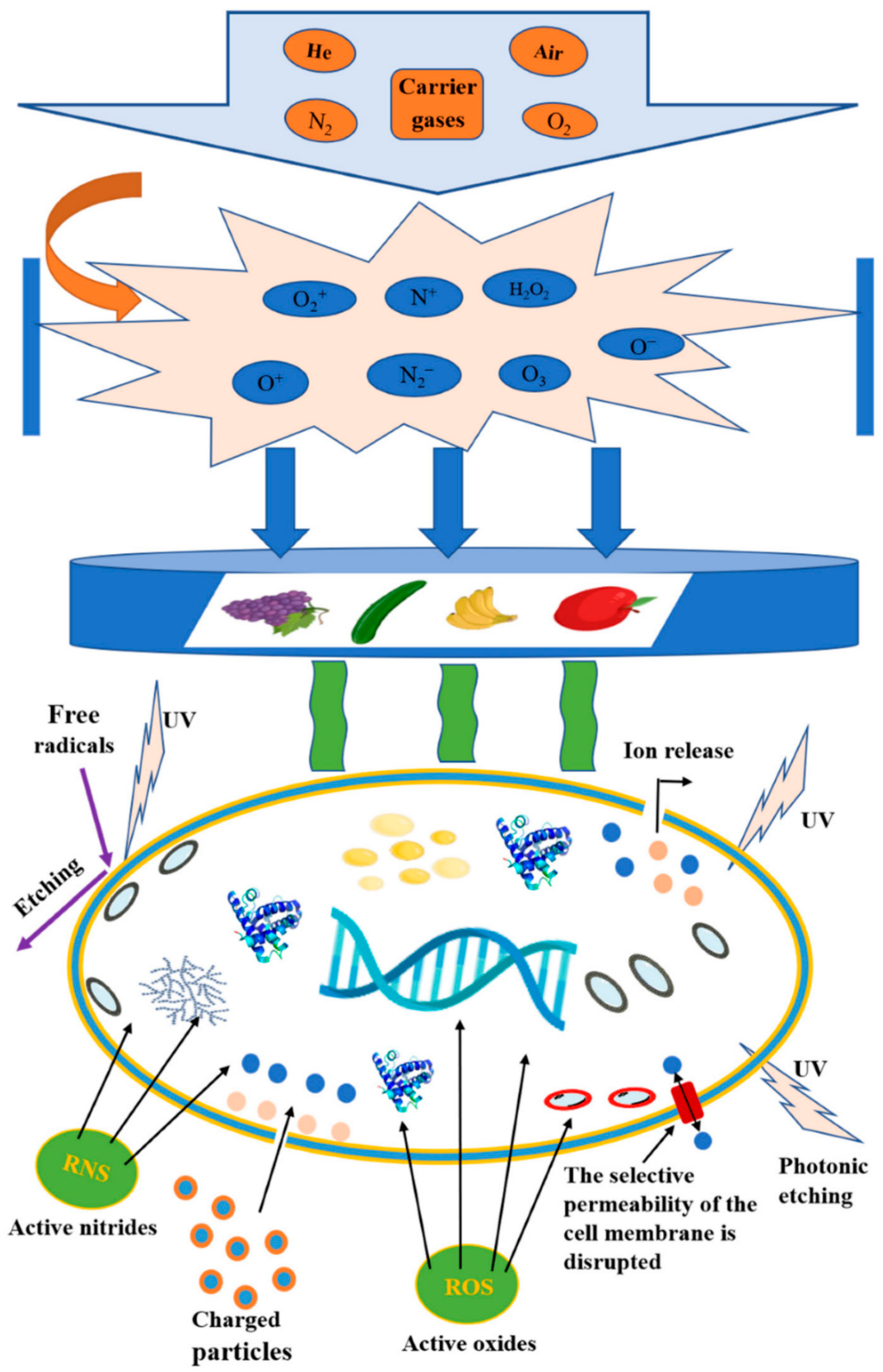
2.2. Cold Plasma and Inactivation of Microorganisms (Mechanisms and Factors Affecting) In Vitro and In Vivo
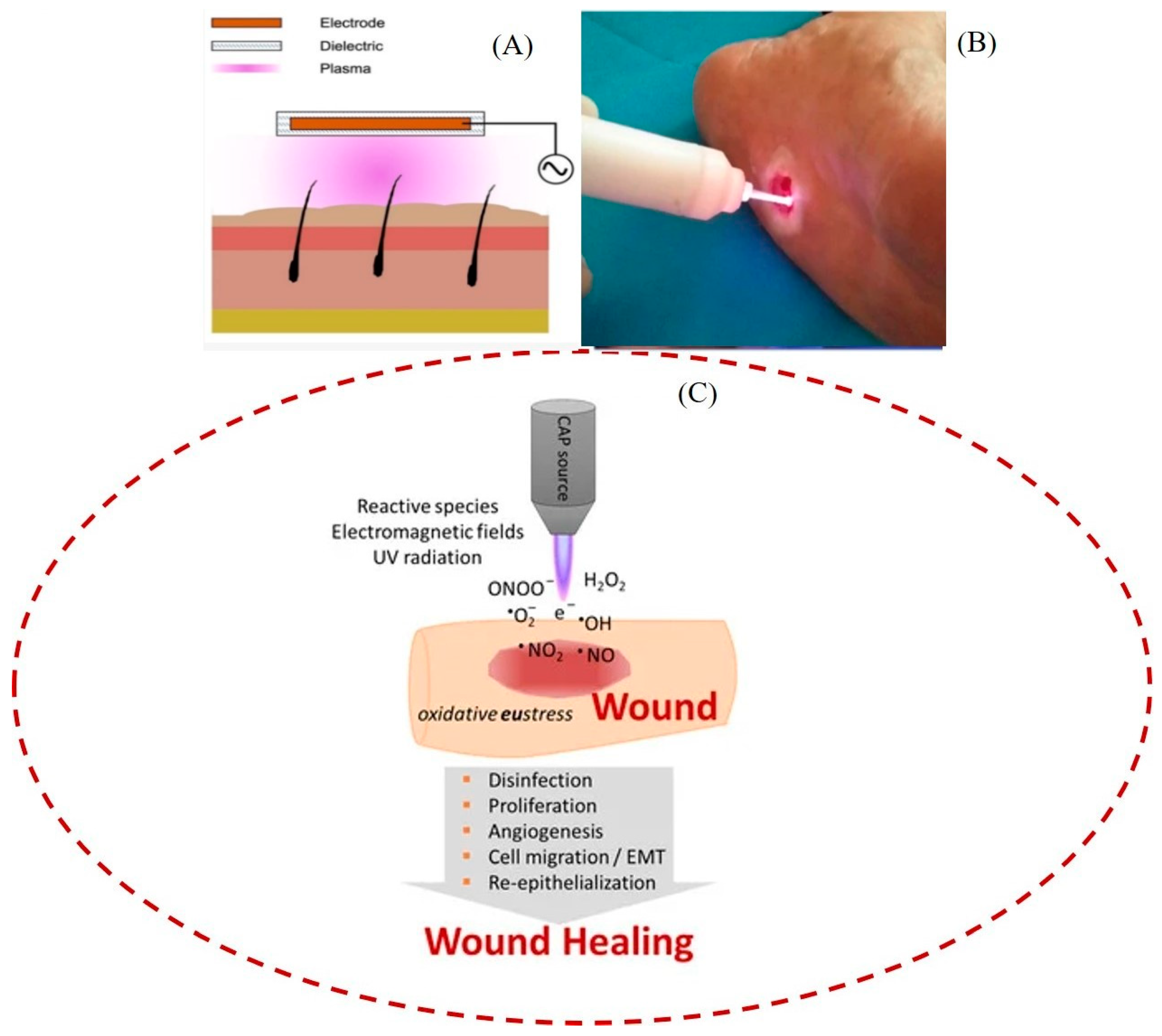
2.3. Medical Device Sterilization
3. Plasma and Food Industry
3.1. Cold Plasma Treatment of Food Packaging Material Surface
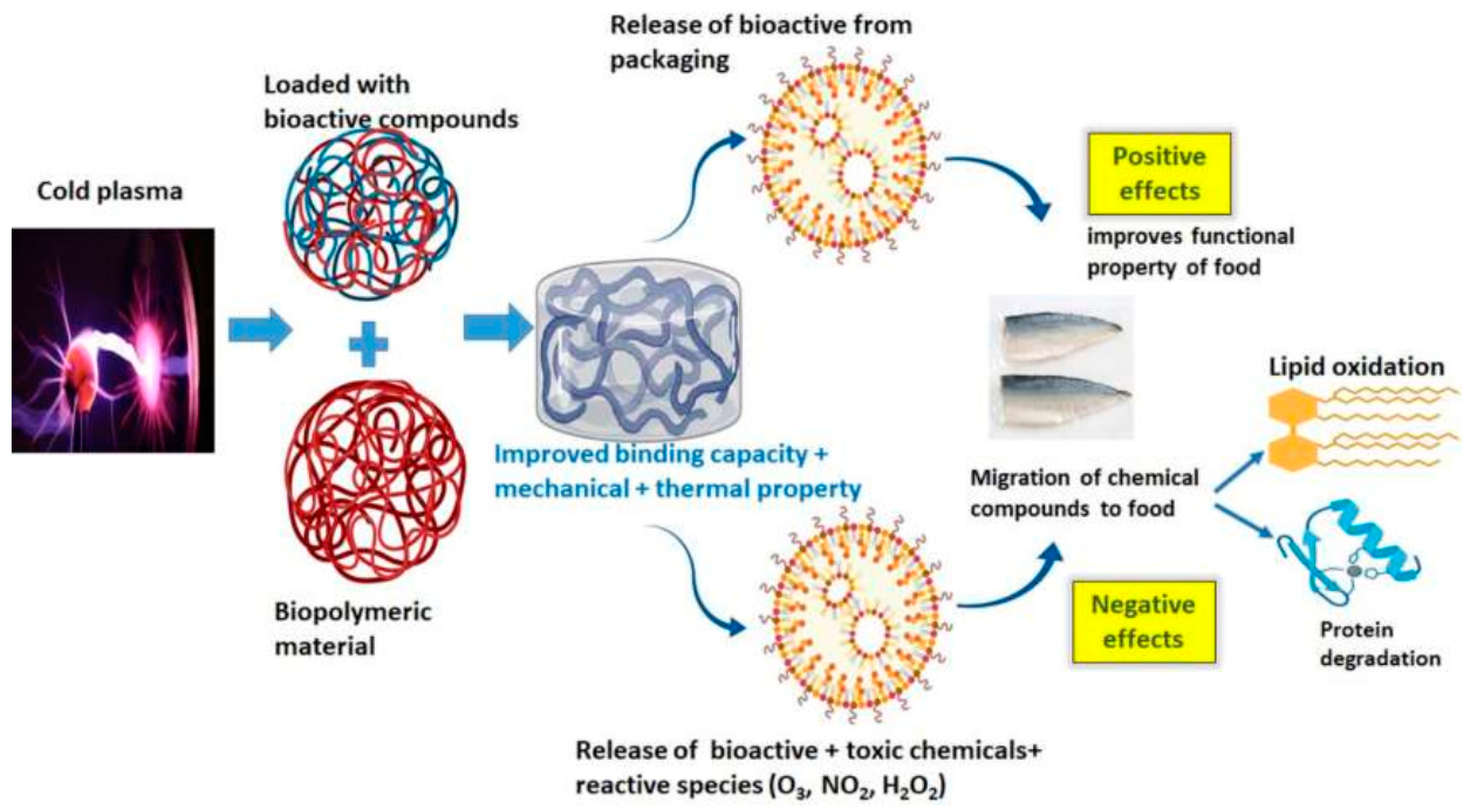
3.2. Disadvantages of Cold Plasma Technology on Food Products
3.3. Safety of Cold Plasma for Food Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shelar, A.; Singh, A.V.; Dietrich, P.; Maharjan, R.S.; Thissen, A.; Didwal, P.N.; Shinde, M.; Laux, P.; Luch, A.; Mathe, V. Emerging cold plasma treatment and machine learning prospects for seed priming: A step towards sustainable food production. RSC Adv. 2022, 12, 10467–10488. [Google Scholar] [CrossRef] [PubMed]
- Koulik, P.; Begounov, S.; Goloviatinskii, S. Atmospheric plasma sterilization and deodorization of dielectric surfaces. Plasma Chem. Plasma Process. 1999, 19, 311–326. [Google Scholar] [CrossRef]
- Boekema, B.; Stoop, M.; Vlig, M.; van Liempt, J.; Sobota, A.; Ulrich, M.; Middelkoop, E. Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing. Appl. Microbiol. Biotechnol. 2021, 105, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B. Cold atmospheric pressure plasma in wound healing and cancer treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Kotov, D.; Krivenchuk, D.; Osipov, A.; Patseev, S.; Aksiuchyts, A.; Ponomarenko, G.; Kulchitsky, V. Cold Atmospheric Plasma Treatment of Medical Devices. Biomed. J. Sci. Tech. Res. 2019, 22, 16745–16749. [Google Scholar]
- Lee, K.H.; Woo, K.S.; Yong, H.I.; Jo, C.; Lee, S.K.; Lee, B.W.; Oh, S.-K.; Lee, Y.-Y.; Lee, B.; Kim, H.-J. Assessment of microbial safety and quality changes of brown and white cooked rice treated with atmospheric pressure plasma. Food Sci. Biotechnol. 2018, 27, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Beyrer, M.; Smeu, I.; Martinet, D.; Howling, A.; Pina-Pérez, M.C.; Ellert, C. Cold atmospheric plasma inactivation of microbial spores compared on reference surfaces and powder particles. Food Bioprocess Technol. 2020, 13, 827–837. [Google Scholar] [CrossRef]
- Kumar, H.; Ahuja, A.; Kadam, A.A.; Rastogi, V.K.; Negi, Y.S. Antioxidant film based on chitosan and tulsi essential oil for food packaging. Food Bioprocess Technol. 2023, 16, 342–355. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.; Milosavljević, V.; O’donnell, C.; Bourke, P.; Keener, K.; Cullen, P. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Sheng, H.; Nakamura, K.; Kanno, T.; Sasaki, K.; Niwano, Y. (Eds.) Microbicidal activity of artificially generated hydroxyl radicals. In Proceedings of the Interface Oral Health Science 2014: Innovative Research on Biosis-Abiosis Intelligent Interface, Sendai, Japan, 20–21 January 2014; pp. 203–215. [Google Scholar]
- Whittaker, A.; Graham, E.; Baxter, R.; Jones, A.; Richardson, P.; Meek, G.; Campbell, G.; Aitken, A.; Baxter, H. Plasma cleaning of dental instruments. J. Hosp. Infect. 2004, 56, 37–41. [Google Scholar] [CrossRef]
- Abuzairi, T.; Ramadhanty, S.; Puspohadiningrum, D.F.; Ratnasari, A.; Poespawati, N.R.; Purnamaningsih, R.W. (Eds.) Investigation on physicochemical properties of plasma-activated water for the application of medical device sterilization. In Proceedings of the AIP Conference Proceedings, Bali, Indonesia, 25–26 July 2017; AIP Publishing: College Park, MD, USA, 2017. [Google Scholar]
- Angerer, J.; Bader, M.; Krämer, A. Ambient and biochemical effect monitoring of workers exposed to ethylene oxide. Int. Arch. Occup. Environ. Health 1998, 71, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.A. Compatibility of medical devices and materials with low-temperature hydrogen gas plasma. Med. Device Diagn. Ind. 1997, 19, 57–62. [Google Scholar]
- Sadeghi-Nejad, B.; Shiravi, F.; Ghanbari, S.; Alinejadi, M.; Zarrin, M. Antifungal activity of Satureja khuzestanica (Jamzad) leaves extracts. Jundishapur J. Microbiol. 2010, 3, 36–40. [Google Scholar]
- Dijksteel, G.S.; Ulrich, M.M.; Vlig, M.; Sobota, A.; Middelkoop, E.; Boekema, B.K. Safety and bactericidal efficacy of cold atmospheric plasma generated by a flexible surface Dielectric Barrier Discharge device against Pseudomonas aeruginosa in vitro and in vivo. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, K.; Sato, T.; Nakajima, T.; Nagasawa, T.; Nakatani, T.; Fujimura, S. Sterilization in liquids by air plasma under intermittent discharge. Mech. Eng. J. 2020, 7, 19-00431. [Google Scholar] [CrossRef]
- Hervé, R.; Kong, M.G.; Bhatt, S.; Chen, H.-L.; Comoy, E.; Deslys, J.; Secker, T.; Keevil, C. Evaluation of cold atmospheric plasma for the decontamination of flexible endoscopes. J. Hosp. Infect. 2023, 136, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sainz-García, A.; Toledano, P.; Muro-Fraguas, I.; Álvarez-Erviti, L.; Múgica-Vidal, R.; López, M.; Sainz-García, E.; Rojo-Bezares, B.; Sáenz, Y.; Alba-Elías, F. Mask disinfection using atmospheric pressure cold plasma. Int. J. Infect. Dis. 2022, 123, 145–156. [Google Scholar] [CrossRef]
- Kolomiets, R.; Nikitchuk, T.; Morozov, D.; Hrek, O. Application of cold atmospheric plasma for the sterilization of objects of complex form. J. Zhytomyr State Technol. Univ. Ser. Eng. 2018, 1, 69–73. [Google Scholar] [CrossRef]
- Kostov, K.; Rocha, V.; Koga-Ito, C.; Matos, B.; Algatti, M.; Honda, R.Y.; Kayama, M.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, Y.S.; You, Y.S.; Huh, J.Y.; Kim, K.; Hong, Y.C.; Kim, C.-H. Antimicrobial effects of microwave plasma-activated water with skin protective effect for novel disinfectants in pandemic era. Sci. Rep. 2022, 12, 5968. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of bacterial inhibition and tolerance around cold atmospheric plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Bai, J.; Min, S.C. Inactivation of indigenous microorganisms and Salmonella in Korean rice cakes by in-package cold plasma treatment. Int. J. Environ. Res. Public Health 2021, 18, 3360. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The state of research on antimicrobial activity of cold plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge cold atmospheric plasma: Bacterial inactivation mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Winter, S.; Meyer-Lindenberg, A.; Wolf, G.; Reese, S.; Nolff, M.C. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. J. Microbiol. Methods 2020, 169, 105728. [Google Scholar] [CrossRef]
- Mohseni, P.; Ghorbani, A.; Fariborzi, N. Exploring the potential of cold plasma therapy in treating bacterial infections in veterinary medicine: Opportunities and challenges. Front. Vet. Sci. 2023, 10, 1240596. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold plasmas for biofilm control: Opportunities and challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Flynn, P.B.; Busetti, A.; Wielogorska, E.; Chevallier, O.P.; Elliott, C.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Non-thermal plasma exposure rapidly attenuates bacterial AHL-dependent quorum sensing and virulence. Sci. Rep. 2016, 6, 26320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K.K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.-W.; Yoo, M.; Chang, H.-J.; Lee, N. Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution. Foods 2023, 12, 2461. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Patel, D.; Butani, S.; Nema, S.K. Investigation of physicochemical properties of plasma activated water and its bactericidal efficacy. Plasma Chem. Plasma Process. 2021, 41, 871–902. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Jacobs, P.T. Sterilization process utilizing low-temperature plasma. In Disinfection, Sterilization and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, X.; Bi, Z.; Zong, Z.; Niu, J.; Song, Y.; Liu, D.; Yang, S. Inactivation of Escherichia coli cells in aqueous solution by atmospheric-pressure N2, He, Air, and O2 microplasmas. Appl. Environ. Microbiol. 2015, 81, 5257–5265. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, R.; Zhou, R.; Chen, M.; Li, J.; Sun, Y.; Chen, Q.; Yang, S.; Liu, Q.H. Treatment of Ribonucleoside Solution With Atmospheric-Pressure Plasma. Plasma Process. Polym. 2016, 13, 429–437. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gajula, V.P.; Mohapatra, S.; Singh, G.; Kar, S. Role of cold atmospheric plasma in microbial inactivation and the factors affecting its efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Helmke, A.; Gerling, T.; Weltmann, K.-D. Plasma sources for biomedical applications. In Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Cham, Switzerland, 2018; pp. 23–41. [Google Scholar]
- Prasad, P.; Kar, S.; Sahu, J.K. Atmospheric Pressure Non-Thermal Plasma in Food Processing. In Food Processing; CRC Press: Boca Raton, FL, USA, 2021; pp. 223–251. [Google Scholar]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K. Introduction to plasma medicine. In Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Cham, Switzerland, 2018; pp. 3–21. [Google Scholar]
- Korachi, M.; Turan, Z.; Şentürk, K.; Şahin, F.; Aslan, N. An investigation into the biocidal effect of high voltage AC/DC atmospheric corona discharges on bacteria, yeasts, fungi and algae. J. Electrost. 2009, 67, 678–685. [Google Scholar] [CrossRef]
- Cooper, M.; Fridman, G.; Staack, D.; Gutsol, A.F.; Vasilets, V.N.; Anandan, S.; Cho, Y.I.; Fridman, A.; Tsapin, A. Decontamination of surfaces from extremophile organisms using nonthermal atmospheric-pressure plasmas. IEEE Trans. Plasma Sci. 2009, 37, 866–871. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.; Rosenberg, M. Plasma interaction with microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Cheng, C.; Hu, S.; Lan, Y.; Chu, P.K. In vitro antimicrobial effects and mechanism of atmospheric-pressure He/O2 plasma jet on Staphylococcus aureus biofilm. J. Phys. D Appl. Phys. 2017, 50, 105201. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Xu, Z.; Hu, S.; Li, Y.; Xue, X.; Cai, Q.; Zhou, X.; Shen, J.; Lan, Y. Antimicrobial mechanism and the effect of atmospheric pressure N2 plasma jet on the regeneration capacity of Staphylococcus aureus biofilm. Biofouling 2018, 34, 935–949. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Algwari, Q.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS ONE 2012, 7, e44289. [Google Scholar] [CrossRef]
- Martines, E. Interaction of cold atmospheric plasmas with cell membranes in plasma medicine studies. Jpn. J. Appl. Phys. 2019, 59, SA0803. [Google Scholar] [CrossRef]
- O’connor, N.; Cahill, O.; Daniels, S.; Galvin, S.; Humphreys, H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J. Hosp. Infect. 2014, 88, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh Colagar, A.; Memariani, H.; Sohbatzadeh, F.; Valinataj Omran, A. Nonthermal atmospheric argon plasma jet effects on Escherichia coli biomacromolecules. Appl. Biochem. Biotechnol. 2013, 171, 1617–1629. [Google Scholar] [CrossRef]
- Liu, X.; Hong, F.; Guo, Y.; Zhang, J.; Shi, J. Sterilization of Staphylococcus aureus by an atmospheric non-thermal plasma jet. Plasma Sci. Technol. 2013, 15, 439. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Sharma, A.; Pruden, A.; Stan, O.; Collins, G.J. Bacterial inactivation using an RF-powered atmospheric pressure plasma. IEEE Trans. Plasma Sci. 2006, 34, 1290–1296. [Google Scholar] [CrossRef]
- Guimin, X.; Guanjun, Z.; Xingmin, S.; Yue, M.; Ning, W.; Yuan, L. Bacteria inactivation using DBD plasma jet in atmospheric pressure argon. Plasma Sci. Technol. 2009, 11, 83. [Google Scholar] [CrossRef]
- Dokter, J.; Brusselaers, N.; Hendriks, W.; Boxma, H. Bacteriological cultures on admission of the burn patient: To do or not to do, that’s the question. Burns 2016, 42, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial activity of plasma. In Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Cham, Switzerland, 2018; pp. 113–125. [Google Scholar]
- Zimmermann, J.; Shimizu, T.; Schmidt, H.; Li, Y.; Morfill, G.; Isbary, G. Test for bacterial resistance build-up against plasma treatment. New J. Phys. 2012, 14, 073037. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Goerlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT 01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Chuangsuwanich, A.; Assadamongkol, T.; Boonyawan, D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: A randomized controlled trial. Int. J. Low. Extrem. Wounds 2016, 15, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Nicol, M.J.; Bilén, S.G.; Kirimanjeswara, G.S.; Knecht, S.D. Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control. Plasma 2024, 7, 233–257. [Google Scholar] [CrossRef]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Mohajeri Tehrani, M.; Amini, M.R. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci. Rep. 2020, 10, 10440. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.L.; Mehlhorn, T.A. A review of cold atmospheric pressure plasmas for trauma and acute care. Front. Phys. 2021, 9, 774. [Google Scholar] [CrossRef]
- Akter, M.; Yadav, D.K.; Ki, S.H.; Choi, E.H.; Han, I. Inactivation of infectious bacteria using nonthermal biocompatible plasma cabinet sterilizer. Int. J. Mol. Sci. 2020, 21, 8321. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Du, J.; Reid, B.; Deng, X.; Liu, Z.; Zong, Z.; Wang, H.; Yao, B.; Yang, C. Electric fields guide migration of epidermal stem cells and promote skin wound healing. Wound Repair Regen. 2012, 20, 840–851. [Google Scholar] [CrossRef]
- Peters, E.J.; Lagrand, R.; Smits, P.; Pemen, A.; Sobota, A.; Zeper, B.; Sabelis, L.W. Cold Atmospheric Pressure Plasma as a Novel Treatment Modality in Diabetic Foot Ulcers—A Pilot Study. Diabetes 2018, 67 (Suppl. 1), 44-LB. [Google Scholar] [CrossRef]
- Fushimi, R.; Takashina, M.; Yoshikawa, H.; Kobayashi, H.; Okubo, T.; Nakata, S.; Kaku, M. Comparison of adenosine triphosphate, microbiological load, and residual protein as indicators for assessing the cleanliness of flexible gastrointestinal endoscopes. Am. J. Infect. Control 2013, 41, 161–164. [Google Scholar] [CrossRef]
- Ofstead, C.L.; Wetzler, H.P.; Doyle, E.M.; Rocco, C.K.; Visrodia, K.H.; Baron, T.H.; Tosh, P.K. Persistent contamination on colonoscopes and gastroscopes detected by biologic cultures and rapid indicators despite reprocessing performed in accordance with guidelines. Am. J. Infect. Control 2015, 43, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Hervé, R.C.; Keevil, C.W. Persistent residual contamination in endoscope channels; a fluorescence epimicroscopy study. Endoscopy 2016, 48, 609–616. [Google Scholar] [CrossRef]
- Muscarella, L.F. Use of ethylene-oxide gas sterilisation to terminate multidrug-resistant bacterial outbreaks linked to duodenoscopes. BMJ Open Gastroenterol. 2019, 6, e000282. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, I.; Silas, D.; Chi, K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest. Endosc. 2016, 84, 259–262. [Google Scholar] [CrossRef]
- Bhatt, S.; Mehta, P.; Chen, C.; Schneider, C.L.; White, L.N.; Chen, H.-L.; Kong, M.G. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest. Endosc. 2019, 89, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K.K. Atmospheric pressure plasmas: Infection control and bacterial responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Walsh, J.L.; Kong, M.G. Room-temperature atmospheric argon plasma jet sustained with submicrosecond high-voltage pulses. Appl. Phys. Lett. 2007, 91, 221502. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of plasma-activated water (PAW) as a novel disinfectant: Effectiveness on Escherichia coli and Listeria innocua, physicochemical properties, and storage stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef] [PubMed]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed]
- Burlica, R.; Grim, R.; Shih, K.Y.; Balkwill, D.; Locke, B. Bacteria inactivation using low power pulsed gliding arc discharges with water spray. Plasma Process. Polym. 2010, 7, 640–649. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.S.; Islam, R.; Rana, R.; Sayem, A.; Sad, M.A.A.; Matin, A.; Raposo, A.; Zandonadi, R.P.; Han, H. Plasma-activated water for food safety and quality: A review of recent developments. Int. J. Environ. Res. Public Health 2022, 19, 6630. [Google Scholar] [CrossRef] [PubMed]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contribution to the chemistry of plasma-activated water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Knorr, D.; Augustin, M. Food processing needs, advantages and misconceptions. Trends Food Sci. Technol. 2021, 108, 103–110. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A cold plasma technology for ensuring the microbiological safety and quality of foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Zhuang, H.; Rothrock, M.J., Jr.; Hiett, K.L.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M. In-package air cold plasma treatment of chicken breast meat: Treatment time effect. J. Food Qual. 2019, 2019, 1837351. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. New Bioactive Edible Packing Systems: Synbiotic Edible Films/Coatings as Carries of Probiotics and Prebiotics. Food Bioprocess Technol. 2023, 16, 1413–1428. [Google Scholar] [CrossRef]
- Chaichi, M.; Badii, F.; Mohammadi, A.; Hashemi, M. Novel Bioactive Composite Films Based on Pectin-Nanocellulose-Synergistic Triple Essential Oils: Development and Characterization. Food Bioprocess Technol. 2023, 16, 1794–1805. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sharma, S.; Mir, S.A.; Dar, B. Nanobiocomposite films: A “greener alternate” for food packaging. Food Bioprocess Technol. 2021, 14, 1013–1027. [Google Scholar] [CrossRef]
- Behera, K.; Kumari, M.; Chang, Y.-H.; Chiu, F.-C. Chitosan/boron nitride nanobiocomposite films with improved properties for active food packaging applications. Int. J. Biol. Macromol. 2021, 186, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.-Q.; Kang, X.; Zeng, K. Preparation of water-soluble dialdehyde cellulose enhanced chitosan coating and its application on the preservation of mandarin fruit. Int. J. Biol. Macromol. 2022, 203, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, S.; Ahari, H.; Givianrad, M.H.; Hosseini, H. Improving the Barrier Properties of Food Packaging by Al2O3@TiO2 & Al2O3@SiO2 Nanoparticles. Food Bioprocess Technol. 2021, 14, 1287–1300. [Google Scholar]
- Ott, L.C.; Appleton, H.J.; Shi, H.; Keener, K.; Mellata, M. High voltage atmospheric cold plasma treatment inactivates Aspergillus flavus spores and deoxynivalenol toxin. Food Microbiol. 2021, 95, 103669. [Google Scholar] [CrossRef] [PubMed]
- Rashvand, M.; Matera, A.; Altieri, G.; Genovese, F.; Nikzadfar, M.; Feyissa, A.H.; Di Renzo, G.C. Effect of dielectric barrier discharge cold plasma on the bio-nanocomposite film and its potential to preserve the quality of strawberry under modified atmosphere packaging. Food Bioprocess Technol. 2023, 17, 1247–1264. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Ghorbani, M.; Shahbazi, N.; Izadi, F.; Pilevar, Z.; Mortazavian, A.M. The effects of novel thermal and nonthermal technologies on the properties of edible food packaging. Food Eng. Rev. 2020, 12, 333–345. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Zhou, N.; Schiappacasse, C.; Cheng, Y.; Chen, D.; Addy, M.; Zhang, Y.; Anderson, E.; Fan, L. Packed food and packaging materials disinfected by cold plasma. In Advances in Cold Plasma Applications for Food Safety and Preservation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–286. [Google Scholar]
- Prysiazhnyi, V.; Zaporojchenko, V.; Kersten, H.; Černák, M. Influence of humidity on atmospheric pressure air plasma treatment of aluminium surfaces. Appl. Surf. Sci. 2012, 258, 5467–5471. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, C.-m.; Zou, F.-l.; Sun, Y.; Shang, N.; Wu, W. Impacts of cold plasma technology on sensory, nutritional and safety quality of food: A Review. Foods 2022, 11, 2818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, P.; Jia, C.; Chen, M.; Li, B. Effects of air dielectric barrier discharge plasma treatment time on surface properties of PBO fiber. Appl. Surf. Sci. 2011, 258, 513–520. [Google Scholar] [CrossRef]
- Liu, C.; Brown, N.M.; Meenan, B.J. Uniformity analysis of dielectric barrier discharge (DBD) processed polyethylene terephthalate (PET) surface. Appl. Surf. Sci. 2006, 252, 2297–2310. [Google Scholar] [CrossRef]
- Muranyi, P.; Wunderlich, J.; Heise, M. Sterilization efficiency of a cascaded dielectric barrier discharge. J. Appl. Microbiol. 2007, 103, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Muranyi, P.; Wunderlich, J.; Heise, M. Influence of relative gas humidity on the inactivation efficiency of a low temperature gas plasma. J. Appl. Microbiol. 2008, 104, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Gao, J.; Guo, Y. Plasma sterilization using the RF glow discharge. Appl. Surf. Sci. 2009, 255, 8960–8964. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.; Rajauria, G. Application of cold plasma on food matrices: A review on current and future prospects. J. Food Process. Preserv. 2021, 45, e15070. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Fernandez-Gutierrez, S.A.; Pedrow, P.D.; Pitts, M.J.; Powers, J. Cold atmospheric-pressure plasmas applied to active packaging of apples. IEEE Trans. Plasma Sci. 2010, 38, 957–965. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lecce, L.; De Vietro, N.; Favia, P.; Del Nobile, M.A. Plasma deposition processes from acrylic/methane on natural fibres to control the kinetic release of lysozyme from PVOH monolayer film. J. Food Eng. 2011, 104, 373–379. [Google Scholar] [CrossRef]
- Karam, L.; Casetta, M.; Chihib, N.-E.; Bentiss, F.; Maschke, U.; Jama, C. Optimization of cold nitrogen plasma surface modification process for setting up antimicrobial low density polyethylene films. J. Taiwan Inst. Chem. Eng. 2016, 64, 299–305. [Google Scholar] [CrossRef]
- Clarke, D.; Tyuftin, A.A.; Cruz-Romero, M.C.; Bolton, D.; Fanning, S.; Pankaj, S.K.; Bueno-Ferrer, C.; Cullen, P.J.; Kerry, J.P. Surface attachment of active antimicrobial coatings onto conventional plastic-based laminates and performance assessment of these materials on the storage life of vacuum packaged beef sub-primals. Food Microbiol. 2017, 62, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.; Thomas, S. Cold plasma applications in food packaging. In Cold Plasma in Food and Agriculture; Nikki Levy: New York, NY, USA; Academic Press: Cambridge, MA, USA, 2016; pp. 293–307. [Google Scholar]
- Ozdemir, M.; Yurteri, C.U.; Sadikoglu, H. Physical polymer surface modification methods and applications in food packaging polymers. Crit. Rev. Food Sci. Nutr. 1999, 39, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Chu, Y.H.; Jo, C. Prospective applications of cold plasma for processing poultry products: Benefits, effects on quality attributes, and limitations. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect plasma treatment of fresh pork: Decontamination efficiency and effects on quality attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Prendeville, J.; Jaiswal, A.K.; Jaiswal, S. Cold Plasma Technology in Food Packaging. Coatings 2022, 12, 1896. [Google Scholar] [CrossRef]
- Kulawik, P.; Alvarez, C.; Cullen, P.J.; Aznar-Roca, R.; Mullen, A.M.; Tiwari, B. The effect of non-thermal plasma on the lipid oxidation and microbiological quality of sushi. Innov. Food Sci. Emerg. Technol. 2018, 45, 412–417. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Khaneghah, A.M.; Barba, F.J.; Misra, N. A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci. Technol. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Gavahian, M.; Hashemi, S.; Khaneghah, A.M.; Tehrani, M.M. Ohmically extracted Zenyan essential oils as natural antioxidant in mayonnaise. Int. Food Res. J. 2013, 20, 3189. [Google Scholar]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.; Ferreira, I.C.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Bora, J.; Khan, T.; Mahnot, N.K. Cold plasma treatment concerning quality and safety of food: A review. Curr. Res. Nutr. Food Sci. J. 2022, 10, 427–446. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.-y.; Ji, H.; Ran, Y.; Li, S.-h.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; De León, J.E.; Mosher, C.; Colonna, W.; Keener, K.M. High-voltage atmospheric cold plasma treatment of different types of starch films. Starch-Stärke 2017, 69, 1700009. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.; O’Neill, L.; Tiwari, B.; Bourke, P.; Cullen, P. Physicochemical characterization of plasma-treated sodium caseinate film. Food Res. Int. 2014, 66, 438–444. [Google Scholar] [CrossRef]
- Bahrami, R.; Zibaei, R.; Hashami, Z.; Hasanvand, S.; Garavand, F.; Rouhi, M.; Jafari, S.M.; Mohammadi, R. Modification and improvement of biodegradable packaging films by cold plasma; a critical review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yong, H.I.; Kim, H.-J.; Choe, W.; Yoo, S.J.; Jang, E.J.; Jo, C. Evaluation of the microbiological safety, quality changes, and genotoxicity of chicken breast treated with flexible thin-layer dielectric barrier discharge plasma. Food Sci. Biotechnol. 2016, 25, 1189–1195. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, R.; Yang, L.; Wang, K.; Zhang, Q.; Su, X.; Yang, B.; Zhang, J.; Fang, J. Non-thermal plasma for inactivated-vaccine preparation. Vaccine 2016, 34, 1126–1132. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Lawrence, K.; Zhang, J. Disinfection of chicken fillets in packages with atmospheric cold plasma: Effects of treatment voltage and time. J. Appl. Microbiol. 2018, 124, 1212–1219. [Google Scholar] [CrossRef]
- Adler, H.J.; Fischer, P.; Heller, A.; Jansen, I.; Kuckling, D.; Komber, H.; Lehmann, D.; Piontek, J.; Pleul, D.; Simon, F. Trends in polymer chemistry 1998. Acta Polym. 1999, 50, 232–239. [Google Scholar] [CrossRef]
- Fan, L.; Hou, F.; Muhammad, A.I.; Ruiling, L.; Watharkar, R.B.; Guo, M.; Ding, T.; Liu, D. Synergistic inactivation and mechanism of thermal and ultrasound treatments against Bacillus subtilis spores. Food Res. Int. 2019, 116, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, W.; Wang, H.; Fan, Y.; Chang, Z. Groundwater contamination source identification based on a hybrid particle swarm optimization-extreme learning machine. J. Hydrol. 2020, 584, 124657. [Google Scholar] [CrossRef]
- Kim, H.-J.; Sung, N.-Y.; Yong, H.I.; Kim, H.; Lim, Y.; Ko, K.H.; Yun, C.-H.; Jo, C. Mutagenicity and immune toxicity of emulsion-type sausage cured with plasma-treated water. Korean J. Food Sci. Anim. Resour. 2016, 36, 494. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.-S.; Baek, K.H.; Jo, C.; Jung, S. No mutagenicity and oral toxicity of winter mushroom powder treated with atmospheric non-thermal plasma. Food Chem. 2021, 338, 127826. [Google Scholar] [CrossRef]
- Han, S.H.; Suh, H.J.; Hong, K.B.; Kim, S.Y.; Min, S.C. Oral toxicity of cold plasma-treated edible films for food coating. J. Food Sci. 2016, 81, T3052–T3057. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavus spores and biofilms: A comparative study. Appl. Environ. Microbiol. 2020, 86, e02619-19. [Google Scholar] [CrossRef]
- Thomas-Popo, E.; Mendonça, A.; Misra, N.; Little, A.; Wan, Z.; Moutiq, R.; Coleman, S.; Keener, K. Inactivation of Shiga-toxin-producing Escherichia coli, Salmonella enterica and natural microflora on tempered wheat grains by atmospheric cold plasma. Food Control 2019, 104, 231–239. [Google Scholar] [CrossRef]
- Khosravi, S.; Jafari, S.; Zamani, H.; Nilkar, M. Inactivation of Staphylococcus aureus and Escherichia coli biofilms by air-based atmospheric-pressure DBD plasma. Appl. Biochem. Biotechnol. 2021, 193, 3641–3650. [Google Scholar] [CrossRef] [PubMed]
- Patange, A.D.; Simpson, J.C.; Curtin, J.F.; Burgess, C.M.; Cullen, P.; Tiwari, B.K. Inactivation efficacy of atmospheric air plasma and airborne acoustic ultrasound against bacterial biofilms. Sci. Rep. 2021, 11, 2346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, Z.; Cheng, J.-H.; Sun, D.-W. Modification of cellulose from sugarcane (Saccharum officinarum) bagasse pulp by cold plasma: Dissolution, structure and surface chemistry analysis. Food Chem. 2022, 374, 131675. [Google Scholar] [CrossRef] [PubMed]
| Industry | Application | Advantages | Disadvantages |
|---|---|---|---|
| Healthcare | Surface sterilization of clinical equipment | Effective against various pathogens, non-toxic process | Moderate material degradation, high cost |
| Wound healing application | Accelerates tissue regeneration, reduces infection | Limited clinical studies | |
| Cancer treatment | Selective on cancers, non-toxic process | Limited clinical studies | |
| Dental application | Effective in disinfection, non-invasive | Equipment cost requires skilled staff | |
| Food Packaging | Increasing shelf life | Cold method, maintain nutritional quality | Less penetration in dense materials |
| Surface sterilization | Reduces waste, increases safety | Equipment cost and maintenance | |
| Active packaging | Improves quality of food, enhances preservation, enhances shelf life | Material compatibility and cost | |
| Textiles | Surface modification | Environmentally friendly process, maintain material quality | Cost and maintenance of equipment |
| Microbial inactivation/resistant | Long-term effects, green process | Slight material degradation | |
| Electronics | Surface cleaning | Removes contaminants, improves adhesion | Cost and equipment complex process |
| Etching and patterning | High precision, ecofriendly | Specificity and process rate | |
| Agriculture | Abiotic/biotic Stress removal | Ecofriendly process enhances immunity | Specific equipment or processes required |
| Seed treatment | Improves germination, disease resistance | Variability in outcome | |
| Agri-products sterilization | Decreases contamination, enhances shelf life | Regulatory issues | |
| Environmental | Air purification | Eradicate pollutants, decreases odors/bad smell | Power usage and maintenance |
| Water treatment | Efficient decontamination, Ecofriendly green process | Energy consumption and equipment cost | |
| Soil remediation | Sterilize contaminants, improve quality, eco-friendly | Variable effectiveness | |
| Cosmetics | Skin treatment | Promotes wound healing, reduces oxidative stress, reduces acne, activates skin | Limited clinical studies |
| Anti-aging treatments | Enhance collagen synthesis, reduces senescence | Specified skin product required for market | |
| Pharmaceutical | Drug delivery system | Maintain drug quality, targeted drug delivery | Regulatory challenges |
| Packaging sterilization | Ensures decontamination, cold and green method | Setup cost and maintenance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barjasteh, A.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Cold Atmospheric Pressure Plasma Solutions for Sustainable Food Packaging. Int. J. Mol. Sci. 2024, 25, 6638. https://doi.org/10.3390/ijms25126638
Barjasteh A, Kaushik N, Choi EH, Kaushik NK. Cold Atmospheric Pressure Plasma Solutions for Sustainable Food Packaging. International Journal of Molecular Sciences. 2024; 25(12):6638. https://doi.org/10.3390/ijms25126638
Chicago/Turabian StyleBarjasteh, Azadeh, Neha Kaushik, Eun Ha Choi, and Nagendra Kumar Kaushik. 2024. "Cold Atmospheric Pressure Plasma Solutions for Sustainable Food Packaging" International Journal of Molecular Sciences 25, no. 12: 6638. https://doi.org/10.3390/ijms25126638






