Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases
Abstract
:1. Introduction
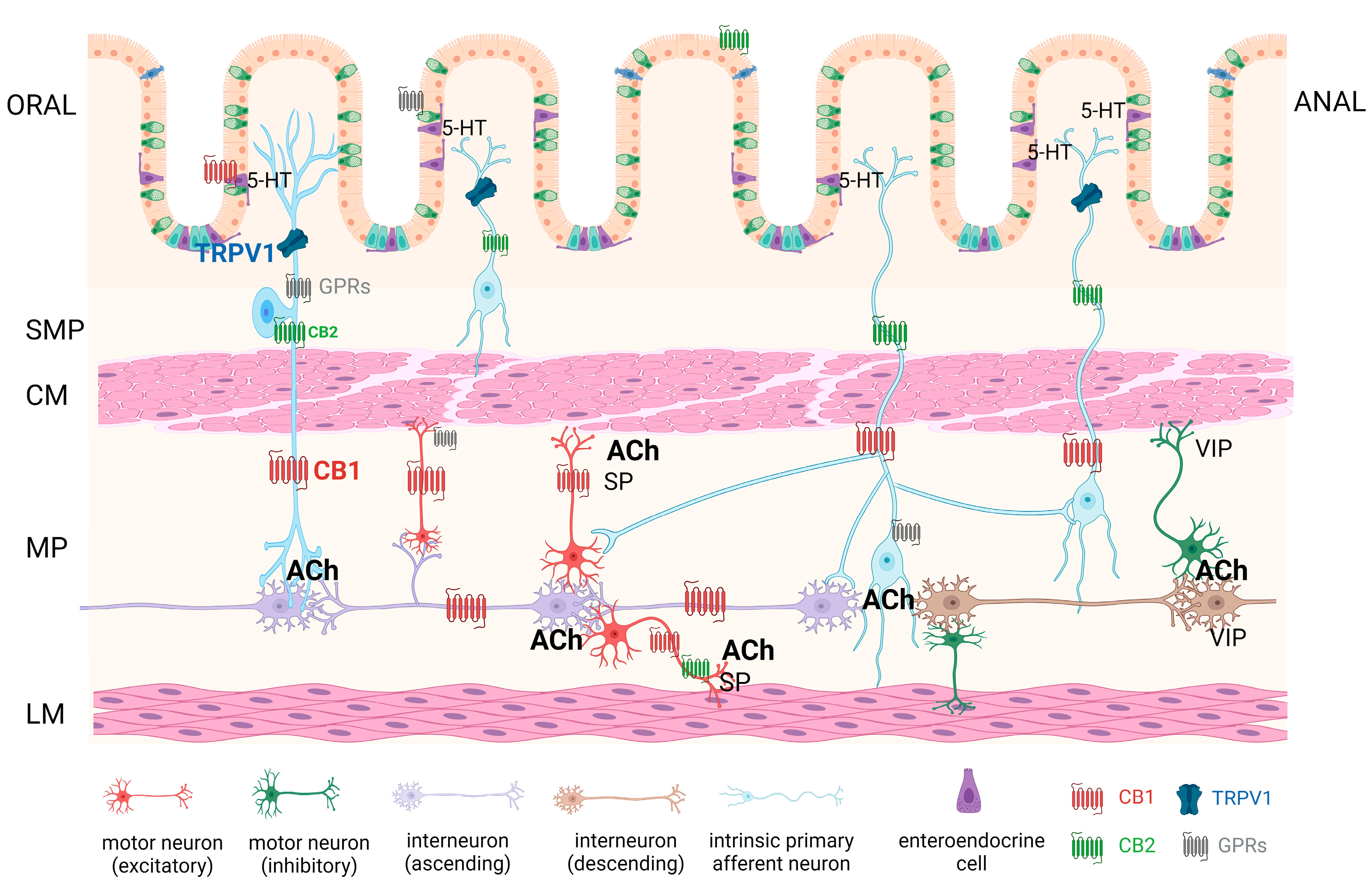
2. Endocannabinoid System (ECS)
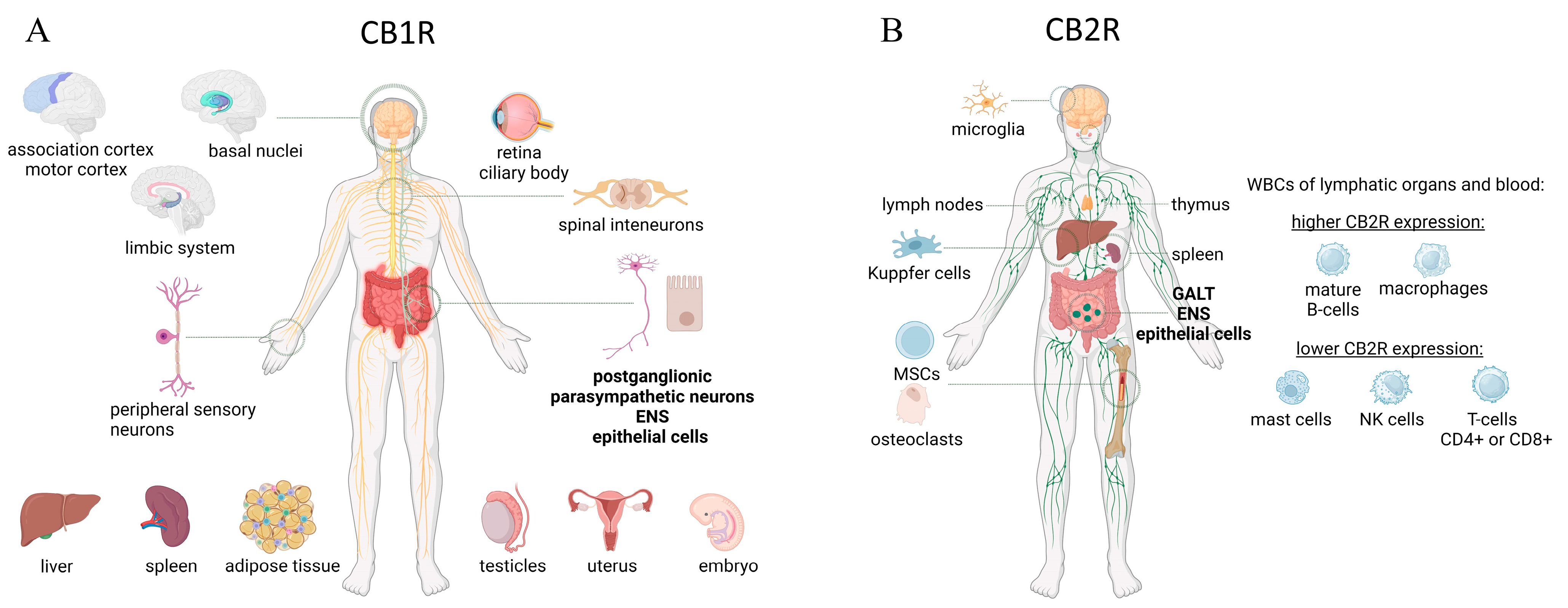
2.1. ECS—Cannabinoid Receptors
2.1.1. ECS—CB1 Receptors
2.1.2. ECS—CB2 Receptors
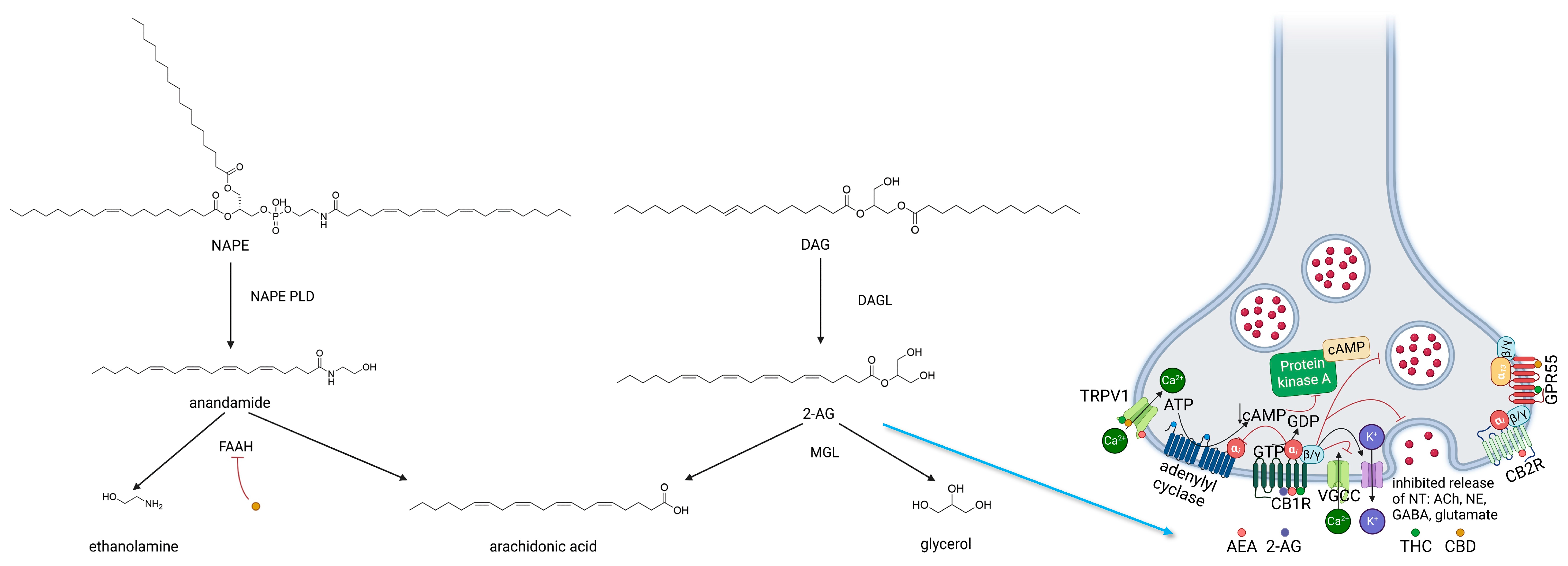
2.1.3. ECS—Enzymes Regulating Biosynthesis and Biodegradation of Cannabinoids
2.2. Endocannabinoids
2.2.1. Anandamide
2.2.2. Endocannabinoids—2-AG
2.2.3. Other Endocannabinoids
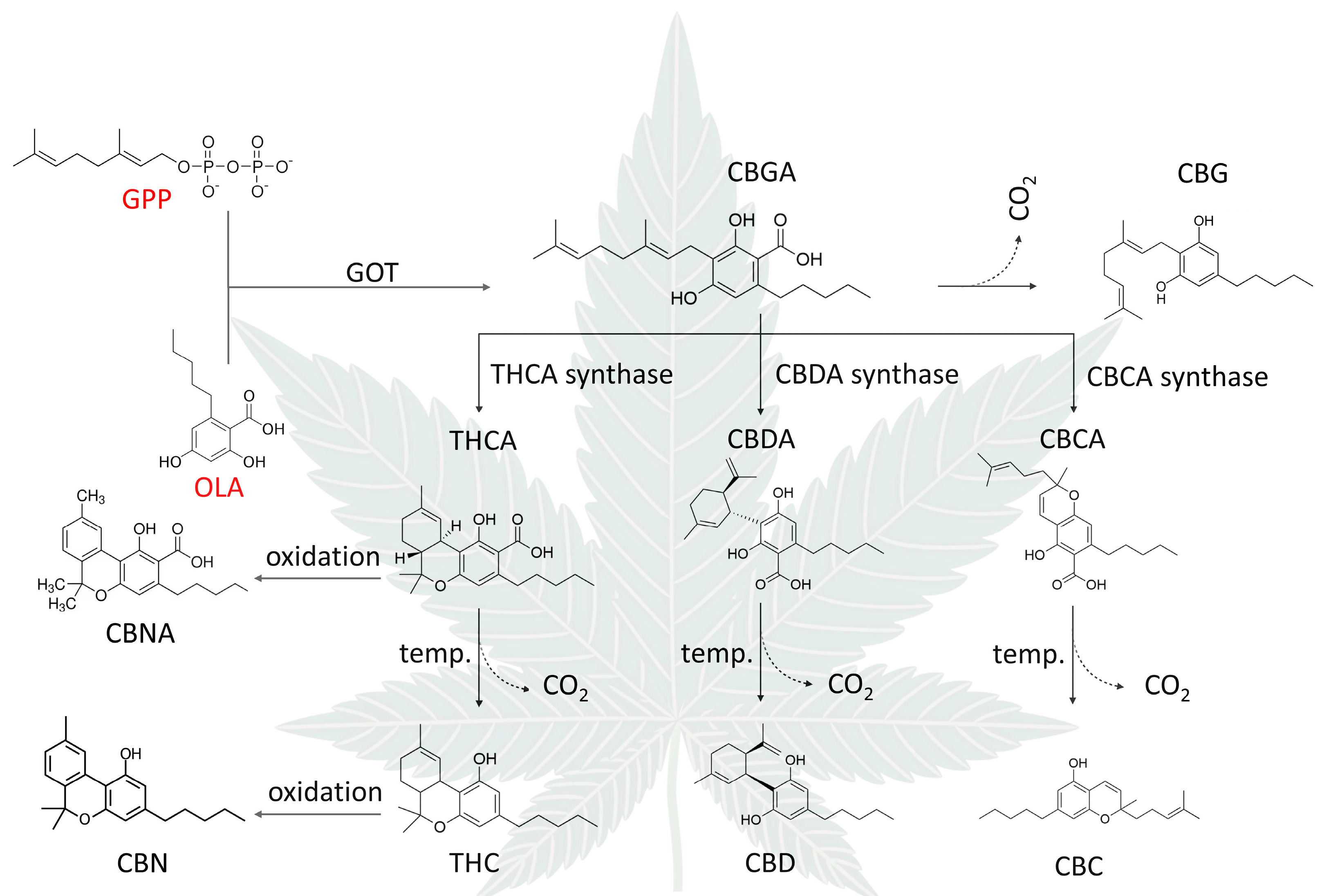
3. Phytocannabinoids
3.1. Phytocannabinoids—THC
3.2. Phytocannabinoids—CBD
3.3. Other Phytocannabinoids
3.4. Phytocannabinoid Properties
4. Effects of Cannabinoids on Gastrointestinal Motility
4.1. Endocannabinoids
4.1.1. Effects of AEA and 2-AG on Gastrointestinal Motility
4.1.2. Effects of Endocannabinoid-like Compounds on Gastrointestinal Motility
4.1.3. Summary of Endocannabinoid Influence on Gastrointestinal Motility
4.2. Effects of Phytocannabinoids on Gastrointestinal Motility
4.2.1. THC
4.2.2. Effect of CBD on Gastrointestinal Motility
4.2.3. Effects of Other Phytocannabinoids on Gastrointestinal Motility
4.2.4. Summary of Phytocannabinoid Influence on Gastrointestinal Motility
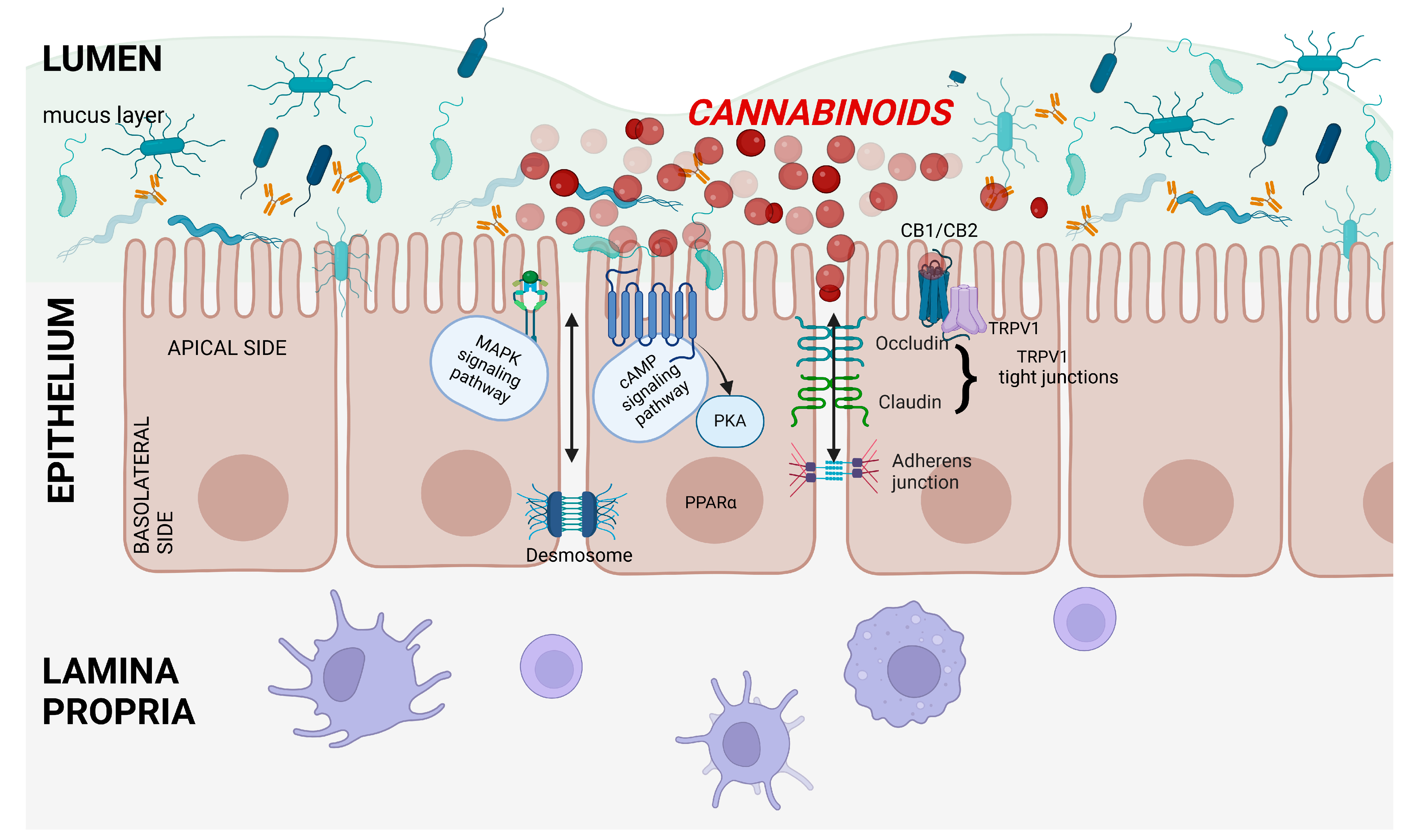
5. Effects of Cannabinoids on Intestinal Barrier Permeability
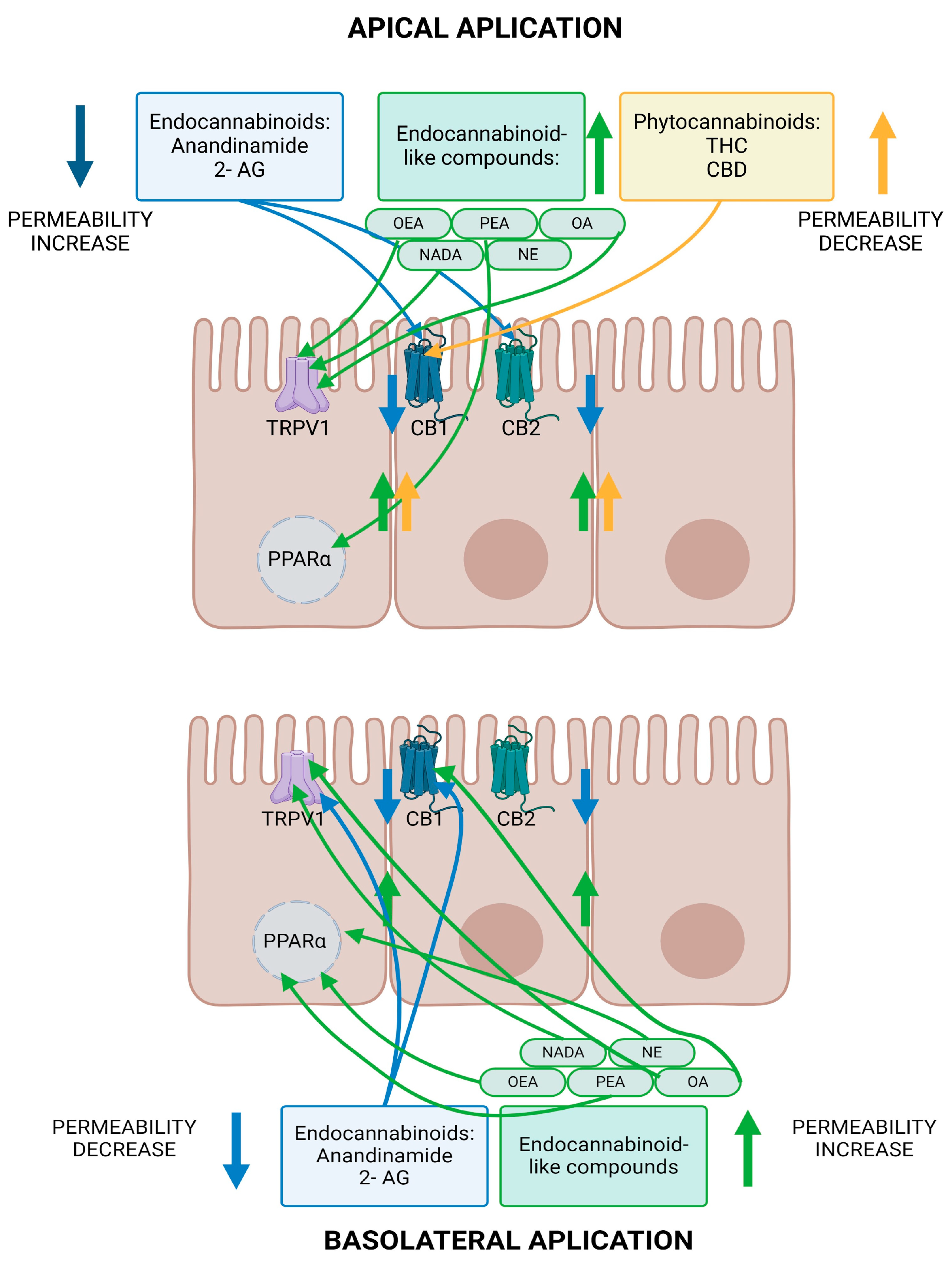
5.1. Effects of Endocannabinoids on Intestinal Barrier Permeability
5.1.1. Anandamide and 2-AG
5.1.2. Effects of Endocannabinoid-like Compounds on Intestinal Barrier Permeability
5.1.3. Summary of Endocannabinoid Influence on Intestinal Barrier Permeability
5.2. Effects of Phytocannabinoids on Intestinal Barrier Permeability
Summary of Phytocannabinoid Influence on Intestinal Permeability
6. Therapeutic Potential of Cannabinoids in Gut Diseases
6.1. Endocannabinoids
6.1.1. Endocannabinoids and Diarrhea
6.1.2. Endocannabinoids and Intestinal Inflammation
6.2. Therapeutic Potential of Phytocannabinoids
6.2.1. Therapeutic Potential of THC and CBD
Infectious Diseases
Therapeutic Potential of THC and CBD in Gastrointestinal Tract Motility
Therapeutic Potential of THC and CBD in Inflammatory Diseases
6.2.2. Therapeutic Potential of Other Phytocannabinoids
6.2.3. Summary of Phytocannabinoid Clinical Trials
| Phytocannabinoid | Disease Status and Number of Subjects | Cannabinoid Dose | Study Type | Effect | Year | Reference |
|---|---|---|---|---|---|---|
| THC | Healthy: 30 | 7.5 mg; 5 mg b.i.d. | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2006 | [109] |
| THC | Healthy: 52 | 7.5 mg | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2007 | [110] |
| CBD, PEA | Healthy: 30 | CBD: 600 mg PEA: 600 mg | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2019 | [92] |
| THC | IBS-C: 35 IBS-D: 35 IBS-A: 5 | 2.5 mg, 5 mg | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2011 | [118] |
| THC | IBS-D: 36 | 2.5 mg; 5 mg b.i.d. | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2012 | [119] |
| CBD | IBS: 32 | 50 mg (ad libitum) | double-blinded, randomized, placebo-controlled, cross-over trial |
| 2022 | [115] |
| THC (in cannabis flowers) | Crohn’s Disease: 21 | 11.5 mg per cigarette | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2013 | [114] |
| CBD | Crohn’s disease: 21 | 10 mg b.i.d. | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2013 | [116] |
| THC, CBD | Crohn’s disease: 30 Ulcerative colitis: 19 | 2 mg/mL THC and 8 mg/mL CBD in oil, up to 1 mL b.i.d. (Crohn’s disease) 11.5 mg THC per cigarette (ulcerative colitis) | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2021 | [100] |
| CBD | Ulcerative Colitis: 60 | 250 mg b.i.d. | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2015 | [117] |
| CBD | Functional dyspepsia with normal gastric emptying: 48 | 10 mg/kg b.i.d. | double-blinded, randomized, placebo-controlled, parallel-group study |
| 2022 | [108] |
6.2.4. Phytocannabinoid Adverse Effects
7. Conclusions
8. Literature Search Methodology
Author Contributions
Funding
Conflicts of Interest
References
- Di Marzo, V.; Izzo, A.A. Endocannabinoid overactivity and intestinal inflammation. Gut 2006, 55, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Corcoran, L.; Roche, M.; Finn, D.P. The Role of the Brain’s Endocannabinoid System in Pain and Its Modulation by Stress. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 125, pp. 203–255. [Google Scholar]
- Zielonka, D.M.; Kiraga, Ł.; Kozłowski, R.M. Medical potential of Cannabis: An overview. Handb. Nat. Fibres 2020, 2, 419–448. [Google Scholar]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Abood, M.E. CB1 & CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Chiarlone, A.; Bellocchio, L.; Blázquez, C.; Resel, E.; Soria-Gómez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Finn, D.P. Chapter Thirteen—Cannabinoids and Pain: Sites and Mechanisms of Action. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Cannabinoid Pharmacology; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 437–475. [Google Scholar]
- Pacher, P.; Bátkai, S.; Kunos, G. Cardiovascular Pharmacology of Cannabinoids. In Cannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 599–625. ISBN 978-3-540-26573-3. [Google Scholar]
- Massa, F.; Storr, M.; Lutz, B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J. Mol. Med. 2005, 83, 944–954. [Google Scholar] [CrossRef]
- McCallum, R.W.; Bashashati, M. Cannabis for Gastroparesis: Hype or Hope? Am. J. Gastroenterol. 2019, 114, 865–866. [Google Scholar] [CrossRef]
- Domenici, M.R.; Azad, S.C.; Marsicano, G.; Schierloh, A.; Wotjak, C.T.; Dodt, H.-U.; Zieglgänsberger, W.; Lutz, B.; Rammes, G. Cannabinoid Receptor Type 1 Located on Presynaptic Terminals of Principal Neurons in the Forebrain Controls Glutamatergic Synaptic Transmission. J. Neurosci. 2006, 26, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N. Cannabinoid receptor with an “identity crisis” gets a second look. Nat. Med. 2015, 21, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Xu, A.-H.; Yang, Y.; Zhang, J.-X.; Yu, A.-W. Activation of Cannabinoid Receptor 2 Enhances Osteogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells. BioMed Res. Int. 2015, 2015, e874982. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Cannabinoids and gastrointestinal motility: Pharmacology, clinical effects, and potential therapeutics in humans. Neurogastroenterol. Motil. 2018, 30, e13370. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.L.; Duncan, M.; Sharkey, K.A. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Br. J. Pharmacol. 2008, 153, 263. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Hu, S.S.; Rimmerman, N.; Juknat, A.; Vogel, Z.; Walker, J.M.; Bradshaw, H.B. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef] [PubMed]
- Biringer, R.G. The rise and fall of anandamide: Processes that control synthesis, degradation, and storage. Mol. Cell. Biochem. 2021, 476, 2753–2775. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Tribute to Professor Raphael Mechoulam, The Founder of Cannabinoid and Endocannabinoid Research. Molecules 2022, 27, 323. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- di Tomaso, E.; Beltramo, M.; Piomelli, D. Brain cannabinoids in chocolate. Nature 1996, 382, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Justinová, Z.; Yasar, S.; Redhi, G.H.; Goldberg, S.R. The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys. J. Neurosci. 2011, 31, 7043–7048. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological actions of cannabinoids. In Cannabinoids; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 1–51. [Google Scholar] [CrossRef]
- Pertwee, R.G. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005, 7, E625–E654. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytocannabinoids Biosynthesis in Angiosperms, Fungi, and Liverworts and Their Versatile Role. Plants 2021, 10, 1307. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Nigro, E.; Formato, M.; Crescente, G.; Daniele, A. Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds? Molecules 2021, 26, 2668. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30 (Suppl. 1), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar] [PubMed]
- Dhir, A. Chapter 14—Cannabidiol in Refractory Epilepsy. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 419–438. [Google Scholar]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Mang, C.F.; Erbelding, D.; Kilbinger, H. Differential effects of anandamide on acetylcholine release in the guinea-pig ileum mediated via vanilloid and non-CB1 cannabinoid receptors. Br. J. Pharmacol. 2001, 134, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.R.; Lichtman, A.; Dewey, W.L.; Akbarali, H.I. Evidence for the putative cannabinoid receptor (GPR55)-mediated inhibitory effects on intestinal contractility in mice. Pharmacology 2012, 90, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Sugiura, T.; Waku, K.; Kamikawa, Y. Contractile response to a cannabimimetic eicosanoid, 2-arachidonoylglycerol, of longitudinal smooth muscle from the guinea-pig distal colon in vitro. Eur. J. Pharmacol. 2002, 444, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, S.; Serio, R.; Mule’, F. Cannabinoid CB(1) receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur. J. Pharmacol. 2008, 582, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mulè, F.; Amato, A.; Baldassano, S.; Serio, R. Evidence for a modulatory role of cannabinoids on the excitatory NANC neurotransmission in mouse colon. Pharmacol. Res. 2007, 56, 132–139. [Google Scholar] [CrossRef]
- Izzo, A.A.; Mascolo, N.; Borrelli, F.; Capasso, F. Excitatory transmission to the circular muscle of the guinea-pig ileum: Evidence for the involvement of cannabinoid CB1 receptors. Br. J. Pharmacol. 1998, 124, 1363–1368. [Google Scholar] [CrossRef]
- Smid, S.D.; Bjorklund, C.K.; Svensson, K.M.; Heigis, S.; Revesz, A. The endocannabinoids anandamide and 2-arachidonoylglycerol inhibit cholinergic contractility in the human colon. Eur. J. Pharmacol. 2007, 575, 168–176. [Google Scholar] [CrossRef]
- Sibaev, A.; Yuece, B.; Allescher, H.D.; Saur, D.; Storr, M.; Kurjak, M. The endocannabinoid anandamide regulates the peristaltic reflex by reducing neuro-neuronal and neuro-muscular neurotransmission in ascending myenteric reflex pathways in rats. Pharmacol. Rep. 2014, 66, 256–263. [Google Scholar] [CrossRef]
- Troy-Fioramonti, S.; Demizieux, L.; Gresti, J.; Muller, T.; Vergès, B.; Degrace, P. Acute activation of cannabinoid receptors by anandamide reduces gastrointestinal motility and improves postprandial glycemia in mice. Diabetes 2015, 64, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Dékány, A.; Benko, R.; Szombati, V.; Bartho, L. The contractile effect of anandamide in the guinea-pig small intestine is mediated by prostanoids but not TRPV1 receptors or capsaicin-sensitive nerves. Basic Clin. Pharmacol. Toxicol. 2013, 112, 341–345. [Google Scholar] [CrossRef]
- Izzo, A.A.; Fezza, F.; Capasso, R.; Bisogno, T.; Pinto, L.; Iuvone, T.; Esposito, G.; Mascolo, N.; Di Marzo, V.; Capasso, F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001, 134, 563–570. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.A.; Ligresti, A.; Costagliola, A.; Pinto, L.; Cascio, M.G.; Maffia, P.; Cecio, A.; Capasso, F.; Di Marzo, V. The endocannabinoid system and the molecular basis of paralytic ileus in mice. FASEB J. 2002, 16, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Piscitelli, F.; Capasso, R.; Aviello, G.; Romano, B.; Borrelli, F.; Petrosino, S.; Di Marzo, V. Peripheral endocannabinoid dysregulation in obesity: Relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br. J. Pharmacol. 2009, 158, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Begg, M.; Molleman, A.; Parsons, M. Modulation of the release of endogenous gamma-aminobutyric acid by cannabinoids in the guinea pig ileum. Eur. J. Pharmacol. 2002, 434, 87–94. [Google Scholar] [CrossRef]
- Begg, M.; Dale, N.; Llaudet, E.; Molleman, A.; Parsons, M.E. Modulation of the release of endogenous adenosine by cannabinoids in the myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum. Br. J. Pharmacol. 2002, 137, 1298–1304. [Google Scholar] [CrossRef]
- Grider, J.R.; Mahavadi, S.; Li, Y.; Qiao, L.-Y.; Kuemmerle, J.F.; Murthy, K.S.; Martin, B.R. Modulation of motor and sensory pathways of the peristaltic reflex by cannabinoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G539–G549. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Matias, I.; Lutz, B.; Borrelli, F.; Capasso, F.; Marsicano, G.; Mascolo, N.; Petrosino, S.; Monory, K.; Valenti, M.; et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 2005, 129, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Izzo, A.A.; Fezza, F.; Pinto, A.; Capasso, F.; Mascolo, N.; Di Marzo, V. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 2001, 134, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Keenan, C.M.; Lutz, B.; Piomelli, D.; Sharkey, K.A. The identification of peroxisome proliferator-activated receptor alpha-independent effects of oleoylethanolamide on intestinal transit in mice. Neurogastroenterol. Motil. 2009, 21, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Matias, I.; Capasso, R.; Petrosino, S.; Borrelli, F.; Orlando, P.; Romano, B.; Capasso, F.; Di Marzo, V.; Izzo, A.A. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J. Mol. Med. 2008, 86, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, A.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; De Silva, M.; Pietra, M.; Fracassi, F.; Chiocchetti, R. Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract. Histochem. Cell Biol. 2020, 153, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.F.; Jackson, D.M.; Chesher, G.B. Interaction of delta9-tetrahydrocannabinol and cannabidiol on intestinal motility in mice. J. Pharm. Pharmacol. 1974, 26, 136–137. [Google Scholar] [CrossRef]
- Chesher, G.B.; Dahl, C.J.; Everingham, M.; Jackson, D.M.; Marchant-Williams, H.; Starmer, G.A. The effect of cannabinoids on intestinal motility and their antinociceptive effect in mice. Br. J. Pharmacol. 1973, 49, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Rosell, S.; Agurell, S. Effects of 7-hydroxy-delta-6-tetrahydrocannabinol and some related cannabinoids on the guinea pig isolated ileum. Acta Physiol. Scand. 1975, 94, 142–144. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Stevenson, L.A.; Elrick, D.B.; Mechoulam, R.; Corbett, A.D. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br. J. Pharmacol. 1992, 105, 980–984. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Fernando, S.R.; Nash, J.E.; Coutts, A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996, 118, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.M.; Malor, R.; Chesher, G.B.; Starmer, G.A.; Welburn, P.J.; Bailey, R. The interaction between prostaglandin E1 and delta 9-tetrahydrocannabinol on intestinal motility and on the abdominal constriction response in the mouse. Psychopharmacologia 1976, 47, 187–193. [Google Scholar] [CrossRef]
- Makwana, R.; Molleman, A.; Parsons, M.E. Pharmacological characterization of cannabinoid receptor activity in the rat-isolated ileum myenteric plexus-longitudinal muscle preparation. Br. J. Pharmacol. 2010, 159, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Borrelli, F.; Aviello, G.; Romano, B.; Scalisi, C.; Capasso, F.; Izzo, A.A. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br. J. Pharmacol. 2008, 154, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Naylor, R.J.; Whittle, B.A.; Javid, F.A. The effects of cannabidiolic acid and cannabidiol on contractility of the gastrointestinal tract of Suncus murinus. Arch. Pharm. Res. 2011, 34, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Chłopecka, M.; Kiraga, Ł.; Crowley, K.; Jank, M.; Latek, U.; Mendel, M.; Karlik, W. Diclofenac and dexamethasone modulate the effect of cannabidiol on the rat colon motility ex vivo. J. Vet. Res. 2023, 67, 289–295. [Google Scholar] [CrossRef]
- Wei, D.; Wang, H.; Yang, J.; Dai, Z.; Yang, R.; Meng, S.; Li, Y.; Lin, X. Effects of O-1602 and CBD on TNBS-induced colonic disturbances. Neurogastroenterol. Motil. 2020, 32, e13756. [Google Scholar] [CrossRef]
- Pinto, L.; Izzo, A.A.; Cascio, M.G.; Bisogno, T.; Hospodar-Scott, K.; Brown, D.R.; Mascolo, N.; Di Marzo, V.; Capasso, F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002, 123, 227–234. [Google Scholar] [CrossRef]
- Okumura, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Funayama, T.; Kumei, S.; Ohhira, M. Oxytocin acts centrally in the brain to improve leaky gut through the vagus nerve and a cannabinoid signaling in rats. Physiol. Behav. 2022, 254, 113914. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.; Park, S.; Heo, G.; Chung, H.Y.; Im, E. Cannabinoid receptor type 1 in the aging gut regulates the mucosal permeability via miR-191-5p. Front. Endocrinol. 2023, 14, 1241097. [Google Scholar] [CrossRef]
- Alhamoruni, A.; Lee, A.C.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J. Pharmacol. Exp. Ther. 2010, 335, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Alhamoruni, A.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br. J. Pharmacol. 2012, 165, 2598–2610. [Google Scholar] [CrossRef] [PubMed]
- Karwad, M.A.; Couch, D.G.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. The role of CB(1) in intestinal permeability and inflammation. FASEB J. 2017, 31, 3267–3277. [Google Scholar] [CrossRef]
- Wiley, M.B.; DiPatrizio, N.V. Diet-Induced Gut Barrier Dysfunction Is Exacerbated in Mice Lacking Cannabinoid 1 Receptors in the Intestinal Epithelium. Int. J. Mol. Sci. 2022, 23, 10549. [Google Scholar] [CrossRef]
- Karwad, M.A.; Macpherson, T.; Wang, B.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARα. FASEB J. 2017, 31, 469–481. [Google Scholar] [CrossRef]
- Karwad, M.A.; Couch, D.G.; Wright, K.L.; Tufarelli, C.; Larvin, M.; Lund, J.; O’Sullivan, S.E. Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα. Biochem. Pharmacol. 2019, 168, 465–472. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.F.; Wilkinson, D.J.; England, T.J.; O’Sullivan, S.E. The Pharmacological Effects of Plant-Derived versus Synthetic Cannabidiol in Human Cell Lines. Med. Cannabis Cannabinoids 2021, 4, 86–96. [Google Scholar] [CrossRef]
- Kumar, V.; Mansfield, J.; Fan, R.; MacLean, A.; Li, J.; Mohan, M. miR-130a and miR-212 Disrupt the Intestinal Epithelial Barrier through Modulation of PPARγ and Occludin Expression in Chronic Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Immunol. 2018, 200, 2677–2689. [Google Scholar] [CrossRef]
- Gigli, S.; Seguella, L.; Pesce, M.; Bruzzese, E.; D’Alessandro, A.; Cuomo, R.; Steardo, L.; Sarnelli, G.; Esposito, G. Cannabidiol restores intestinal barrier dysfunction and inhibits the apoptotic process induced by Clostridium difficile toxin A in Caco-2 cells. United Eur. Gastroenterol. J. 2017, 5, 1108–1115. [Google Scholar] [CrossRef]
- Harvey, B.S.; Nicotra, L.L.; Vu, M.; Smid, S.D. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine 2013, 63, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Governa, P.; Borgonetti, V.; Tinazzi, M.; Peron, G.; Catanzaro, D.; Berretta, M.; Biagi, M.; Manetti, F.; Dall’Acqua, S.; et al. Cannabidiol Isolated From Cannabis sativa L. Protects Intestinal Barrier From In Vitro Inflammation and Oxidative Stress. Front. Pharmacol. 2021, 12, 641210. [Google Scholar] [CrossRef]
- Silvestri, C.; Pagano, E.; Lacroix, S.; Venneri, T.; Cristiano, C.; Calignano, A.; Parisi, O.A.; Izzo, A.A.; Di Marzo, V.; Borrelli, F. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front. Pharmacol. 2020, 11, 585096. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Iannotti, F.A.; Piscitelli, F.; Romano, B.; Lucariello, G.; Venneri, T.; Di Marzo, V.; Izzo, A.A.; Borrelli, F. Efficacy of combined therapy with fish oil and phytocannabinoids in murine intestinal inflammation. Phytother. Res. 2021, 35, 517–529. [Google Scholar] [CrossRef]
- Tartakover Matalon, S.; Azar, S.; Meiri, D.; Hadar, R.; Nemirovski, A.; Abu Jabal, N.; Konikoff, F.M.; Drucker, L.; Tam, J.; Naftali, T. Endocannabinoid Levels in Ulcerative Colitis Patients Correlate With Clinical Parameters and Are Affected by Cannabis Consumption. Front. Endocrinol. 2021, 12, 685289. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Carlson, P.; McKinzie, S.; Grudell, A.; Busciglio, I.; Burton, D.; Baxter, K.; Ryks, M.; Zinsmeister, A.R. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G13–G19. [Google Scholar] [CrossRef]
- Fichna, J.; Sałaga, M.; Stuart, J.; Saur, D.; Sobczak, M.; Zatorski, H.; Timmermans, J.-P.; Bradshaw, H.B.; Ahn, K.; Storr, M.A. Selective inhibition of FAAH produces antidiarrheal and antinociceptive effect mediated by endocannabinoids and cannabinoid-like fatty acid amides. Neurogastroenterol. Motil. 2014, 26, 470–481. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, F.; Costagliola, A.; Bisogno, T.; Marsicano, G.; Ligresti, A.; Matias, I.; Capasso, R.; Pinto, L.; Borrelli, F.; et al. An endogenous cannabinoid tone attenuates cholera toxin-induced fluid accumulation in mice. Gastroenterology 2003, 125, 765–774. [Google Scholar] [CrossRef]
- D’Argenio, G.; Petrosino, S.; Gianfrani, C.; Valenti, M.; Scaglione, G.; Grandone, I.; Nigam, S.; Sorrentini, I.; Mazzarella, G.; Di Marzo, V. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J. Mol. Med. 2007, 85, 523–530. [Google Scholar] [CrossRef]
- de Filippis, D.; Iuvone, T.; d’amico, A.; Esposito, G.; Steardo, L.; Herman, A.G.; Pelckmans, P.A.; de Winter, B.Y.; de Man, J.G. Effect of cannabidiol on sepsis-induced motility disturbances in mice: Involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol. Motil. 2008, 20, 919–927. [Google Scholar] [CrossRef]
- Beaumont, H.; Jensen, J.; Carlsson, A.; Ruth, M.; Lehmann, A.; Boeckxstaens, G. Effect of Δ9-tetrahydrocannabinol, a cannabinoid receptor agonist, on the triggering of transient lower oesophageal sphincter relaxations in dogs and humans. Br. J. Pharmacol. 2009, 156, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; BouSaba, J.; Taylor, A.; Dilmaghani, S.; Busciglio, I.; Carlson, P.; Torres, M.; Ryks, M.; Burton, D.; Harmsen, W.S.; et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin. Gastroenterol. Hepatol. 2023, 21, 3405–3414.e4. [Google Scholar] [CrossRef] [PubMed]
- Atieh, J.; Maselli, D.; Breen-Lyles, M.; Torres, M.; Katzka, D.; Ryks, M.; Busciglio, I.; Burton, D.; Carlson, P.; Harmsen, W.S.; et al. Cannabidiol for Functional Dyspepsia With Normal Gastric Emptying: A Randomized Controlled Trial. Am. J. Gastroenterol. 2022, 117, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, T.; Camilleri, M.; Ferber, I.; Burton, D.; Baxter, K.; Zinsmeister, A. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: A randomized, placebo-controlled study. Neurogastroenterol. Motil. 2006, 18, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, T.; Camilleri, M.; Busciglio, I.; Burton, D.; Baxter, K.; Zinsmeister, A.R. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: A randomized, placebo-controlled study. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G137–G145. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.S.; Kichloo, A.; Shaka, H.; Singh, J.; Edigin, E.; Solanki, D.; Eseaton, P.O.; Wani, F. Gastroparesis with Cannabis Use: A Retrospective Study from the Nationwide Inpatient Sample. Postgrad. Med. 2021, 133, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.H.; Schwaitzberg, S.D. The successful use of dronabinol for failure to thrive secondary to intestinal dysmotility. Int. J. Surg. Case Rep. 2015, 11, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Zemrani, B.; Lambe, C.; Goulet, O. Cannabinoids Improve Gastrointestinal Symptoms in a Parenteral Nutrition-Dependent Patient With Chronic Intestinal Pseudo-Obstruction. JPEN J. Parenter. Enteral Nutr. 2021, 45, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Schleider, L.B.-L.; Dotan, I.; Lansky, E.P.; Benjaminov, F.S.; Konikoff, F.M. Cannabis induces a clinical response in patients with Crohn’s disease: A prospective placebo-controlled study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1271. [Google Scholar] [CrossRef]
- van Orten-Luiten, A.-C.B.; de Roos, N.M.; Majait, S.; Witteman, B.J.M.; Witkamp, R.F. Effects of Cannabidiol Chewing Gum on Perceived Pain and Well-Being of Irritable Bowel Syndrome Patients: A Placebo-Controlled Crossover Exploratory Intervention Study with Symptom-Driven Dosing. Cannabis Cannabinoid Res. 2022, 7, 436–444. [Google Scholar] [CrossRef]
- Naftali, T.; Mechoulam, R.; Gabay, G.; Stein, A.; Bronshtein, M.; Mari, A.; Konikoff, F.M. 983 Cannabidiol Treatment Does Not Effect Active Crohn’s Disease. Gastroenterology 2013, 5, S-180. [Google Scholar] [CrossRef]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.L.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A. Sa1264 A randomised, double-blind, placebo-controlled, parallel group, multi-centred pilot study to assess the symptomatic treatment of ulcerative colitis with Cannabidiol. Gastroenterology 2015, 148, S-275. [Google Scholar] [CrossRef]
- Wong, B.S.; Camilleri, M.; Busciglio, I.; Carlson, P.; Szarka, L.A.; Burton, D.; Zinsmeister, A.R. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology 2011, 141, 1638–1647.e1–7. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Camilleri, M.; Eckert, D.; Carlson, P.; Ryks, M.; Burton, D.; Zinsmeister, A.R. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol. Motil. 2012, 24, 358.e169. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef] [PubMed]
- Attout, H.; Amichi, S.; Josse, F.; Appavoupoule, V.; Randriajohany, A.; Thirapathi, Y. Cannabis Hyperemesis Syndrome: A Still Under-Recognized Syndrome. Eur. J. Case Rep. Intern. Med. 2020, 7, 001588. [Google Scholar] [CrossRef] [PubMed]
- Leu, N.; Routsolias, J.C. Cannabinoid Hyperemesis Syndrome: A Review of the Presentation and Treatment. J. Emerg. Nurs. 2021, 47, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Senderovich, H.; Meaney, C.; Vashishtha, S. Cannabis induced gastrointestinal tract symptoms in the adult population: A systematic review. Med. Princ Pract. 2024, 33, 90–101. [Google Scholar] [CrossRef]
- Samuel, S.; Michael, M.; Tadros, M. Should gastroenterologists prescribe cannabis? The highs, the lows and the unknowns. World J. Clin. Cases 2023, 11, 4210. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.D.R.; Pacheco, J.C.; Rossi, G.N.; de-Paulo, B.O.; Zuardi, A.W.; Guimarães, F.S.; Hallak, J.E.; Crippa, J.A.; Dos Santos, R.G. Adverse effects of oral cannabidiol: An updated systematic review of randomized controlled trials (2020–2022). Pharmaceutics 2022, 14, 2598. [Google Scholar] [CrossRef] [PubMed]

| Phytocan-Nabinoid | CB1 Agoni-Zation | CB1 Antago-Nization | CB2 Agonization | CB2 Antago-Nization | Additional Mechanisms | References |
|---|---|---|---|---|---|---|
| THC | + | − | + | − |
| [42,44] |
| CBD | − | Very weak | − | Very weak |
| [4,45,46] |
| CBG | weak | − | partial | − |
| [41,47] |
| CBN | partial | partial |
| [42,47] | ||
| CBC | − | − | − | − |
| [4] |
| CBDV | − | − | − | − |
| [47,48] |
| THCV | − | + | partial | − | [43] | |
| Cannabinoid acids | − | − | − | − |
| [43] |
| Substance | Species | Experiment Type | GIT Segment | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| Anandamide | Mouse | Ex vivo | Ileal longitudinal muscle strips | Reduction in spontaneous contractility | CB1 | [53] |
| Anandamide | Mouse | Ex vivo | Colon | Reduction in electrically evoked contractions | CB1 | [54] |
| Anandamide | Mouse | In vivo | Stomach and small intestine |
| n/d | [58] |
| Anandamide | Guinea pig | Ex vivo | Small intestine circular muscle preparations | Reduction in cholinergic and NANC contractility | CB1 | [55] |
| Anandamide, 2-AG | Human | Ex vivo | Colonic longitudinal and circular muscle preparations | Reduction in contractile response to ACh | Non-CB1, non- CB2 pathway | [56] |
| Anandamide | Rat | Ex vivo | Ileal preparations | Reduction in contractile response to ACh | CB1 | [57] |
| Anandamide | Guinea pig | Ex vivo | Proximal small intestine myenteric plexus longitudinal muscle strips | Increase in basal ACh release and muscle tone | TRPV1 | [50] |
| Anandamide | Guinea pig | Ex vivo | Proximal small intestine myenteric plexus longitudinal muscle strips | Inhibition of electrically evoked ACh release and contractions | n/d | [50] |
| Anandamide, 2-AG | Guinea pig | Ex vivo | Distal colon longitudinal muscle strips | Increase in contraction | Possible involvement of lipoxygenase metabolites | [52] |
| Anandamide | Guinea pig | Ex vivo | Ileum | Increase in contraction | Possible involvement of cyclooxygenase metabolites | [59] |
| Anandamide | Mouse | In vivo | Small intestine | Possible causative role in paralytic ileus | CB1 | [62] |
| Anandamide | Mouse | In vivo | Small intestine | Decrease in anandamide levels linked to increased GIT transit | n/d | [63] |
| Anandamide | Guinea Pig | Ex vivo | Small intestine myenteric plexus longitudinal muscle strips |
|
| [64] |
| Anandamide | Guinea Pig | Ex vivo | Small intestine myenteric plexus longitudinal muscle strips | Inhibition of electrically induced adenosine release | CB1 | [65] |
| Anandamide | Rat | Ex vivo | Colon |
| CB1 | [66] |
| PEA | Mouse | In vivo | Small intestine | Inhibition of GI transit | n/d | [67] |
| OEA | Mouse | In vivo | Small intestine |
| n/d | [67,69] |
| OEA | Mouse | In vivo | Stomach | Inhibition of gastric emptying | n/d | [70] |
| OA | Mouse | In vivo | Small intestine | Inhibition of GI transit | n/d | [67] |
| Substance | Species | Experiment Type | GIT Segment | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| THC | Mouse | In vivo | Small intestine | Inhibition of GI transit | n/d | [72,73,77] |
| THC | Guinea Pig | Ex vivo | Ileum | Inhibition of contractile response to electrical and 5-HT stimulation | n/d | [74] |
| THC | Guinea Pig | Ex vivo | Small intestine myenteric plexus longitudinal muscle strips | Inhibition of contractile response to electrical stimulation | CB1 | [75,76] |
| THC | Rat | Ex vivo | Small intestine myenteric plexus longitudinal muscle strips | Inhibition of contractile response to electrical stimulation | CB1 | [78] |
| CBD | Mouse | In vivo | Small intestine | Normalization of inflammatory hypermotility | CB1 | [79] |
| CBD | Mouse | Ex vivo | Ileum | Inhibition of ACh-induced contractions | CB1 | [79] |
| CBD | Rat | Ex vivo | Colon |
| n/d | [81] |
| CBD | Rat | Ex vivo, in vivo | Upper GIT, colon | Normalization of inflammatory hypermotility | n/d | [82] |
| CBD, CBDA | Asian house shrew | Ex vivo | Proximal and distal intestinal segments |
| n/d | [80] |
| CBC | Mouse | In vivo | Small intestine | Normalization of inflammatory hypermotility | n/d | [61] |
| CBC | Mouse | Ex vivo | Ileum | Inhibition of contractile response to electrical stimulation and ACh | N and L-type Ca2+ channels | [61] |
| CBN | Guinea Pig | Ex vivo | Small intestine myenteric plexus longitudinal muscle strips | Inhibition of contractile response to electrical stimulation | CB1 | [76] |
| CBN | Mouse | In vivo | Colon | Inhibition of colonic propulsion | CB1 | [83] |
| Substance | Species/Cell Culture | Experiment Type | Effect | Mechanism | References |
|---|---|---|---|---|---|
| anandamide | Caco-2 | In vitro |
|
| [86] |
| 2-AG | Caco-2 | In vitro |
|
| [86] |
| Anandamide, 2-AG | Caco-2 | In vitro |
|
| [86] |
| Anandamide | Caco-2 | In vitro |
|
| [88] |
| 2-AG | Caco-2 | In vitro |
|
| [88] |
| OEA | Caco-2 | In vitro |
|
| [90,91] |
| PEA | Caco-2 | In vitro |
|
| [90] |
| NADA, OA | Caco-2 | In vitro |
|
| [91] |
| NE, PEA | Caco-2 | In vitro |
|
| [91] |
| PEA | Caco-2 | In vitro |
| PPARα, PKA, MAPK, and adenylyl cyclase signaling pathways | [92] |
| PEA | Human | Ex vivo |
|
| [92] |
| PEA | Human | In vivo |
|
| [92] |
| Substance | Species/Cell Culture | Experiment Type | GIT Segment | Effect | Mechanism | Reference |
|---|---|---|---|---|---|---|
| THC, CBD | Caco-2 | In vitro | n/a |
|
| [86] |
| THC, CBD | Caco-2 | In vitro | n/a |
|
| [87] |
| CBD | Caco-2 | In vitro | n/a |
|
| [93] |
| THC | Caco-2 | In vitro | n/a |
|
| [94] |
| THC | Rhesus macaque | Ex vivo | Colon |
|
| [94] |
| CBD | Caco-2 | In vitro | n/a |
|
| [95] |
| CBD | Caco-2 | In vitro | n/a |
| CB1, PKA, MAPK, and adenylyl cyclase signaling pathways | [92] |
| CBD | Human | Ex vivo | Colonic mucosa and submucosa |
|
| [92] |
| CBD | Human | In vivo | n/a |
|
| [92] |
| CBD | Mouse | In vivo | n/a |
|
| [98,99] |
| CBG | Mouse | In vivo | n/a |
|
| [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowley, K.; Kiraga, Ł.; Miszczuk, E.; Skiba, S.; Banach, J.; Latek, U.; Mendel, M.; Chłopecka, M. Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases. Int. J. Mol. Sci. 2024, 25, 6682. https://doi.org/10.3390/ijms25126682
Crowley K, Kiraga Ł, Miszczuk E, Skiba S, Banach J, Latek U, Mendel M, Chłopecka M. Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases. International Journal of Molecular Sciences. 2024; 25(12):6682. https://doi.org/10.3390/ijms25126682
Chicago/Turabian StyleCrowley, Kijan, Łukasz Kiraga, Edyta Miszczuk, Sergiusz Skiba, Joanna Banach, Urszula Latek, Marta Mendel, and Magdalena Chłopecka. 2024. "Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases" International Journal of Molecular Sciences 25, no. 12: 6682. https://doi.org/10.3390/ijms25126682
APA StyleCrowley, K., Kiraga, Ł., Miszczuk, E., Skiba, S., Banach, J., Latek, U., Mendel, M., & Chłopecka, M. (2024). Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases. International Journal of Molecular Sciences, 25(12), 6682. https://doi.org/10.3390/ijms25126682





