Genome-Wide Identification and Characterization of Lignin Synthesis Genes in Maize
Abstract
1. Introduction
2. Results
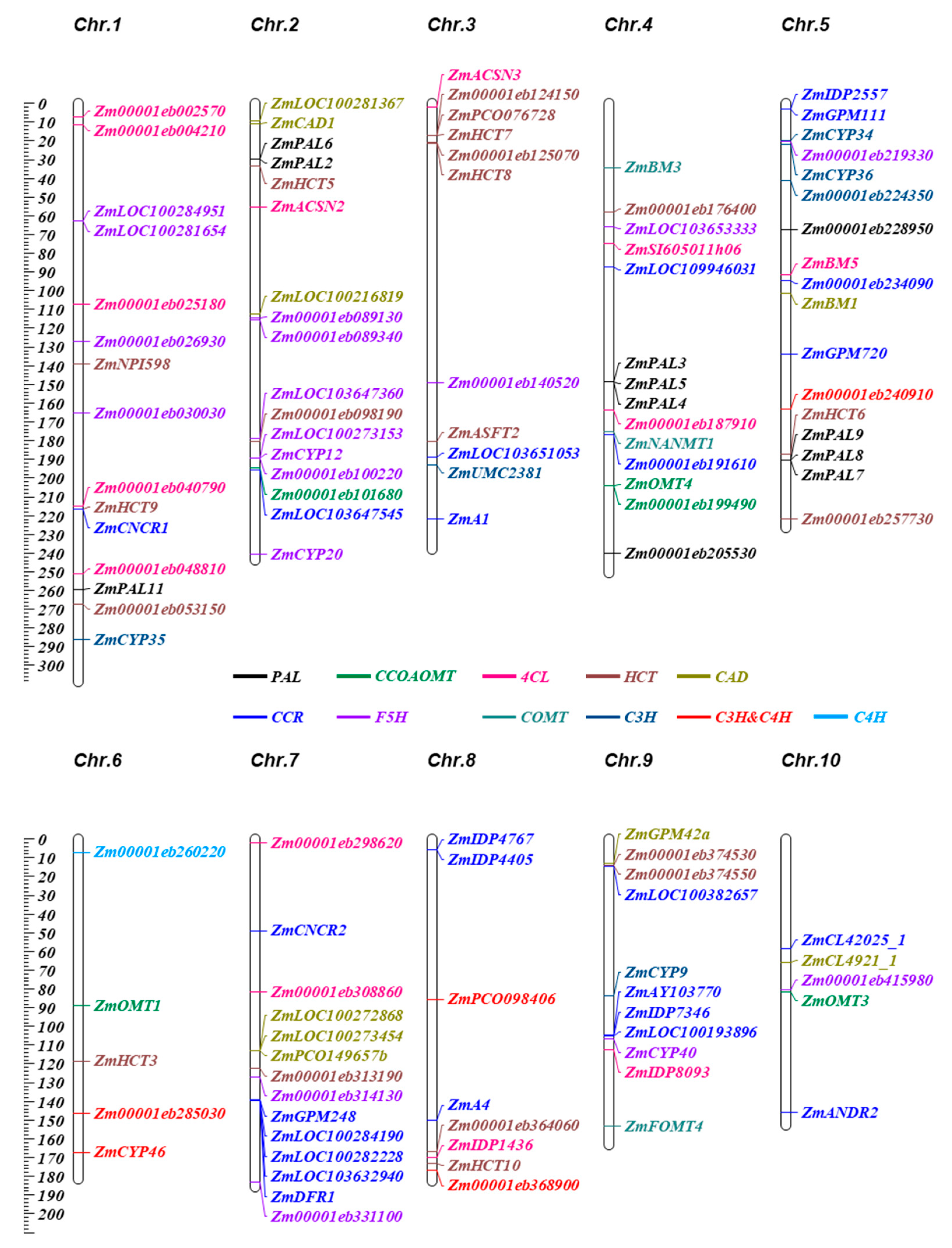
2.1. Identification of Gene Families Involved in the Lignin Synthetic Pathway in Maize
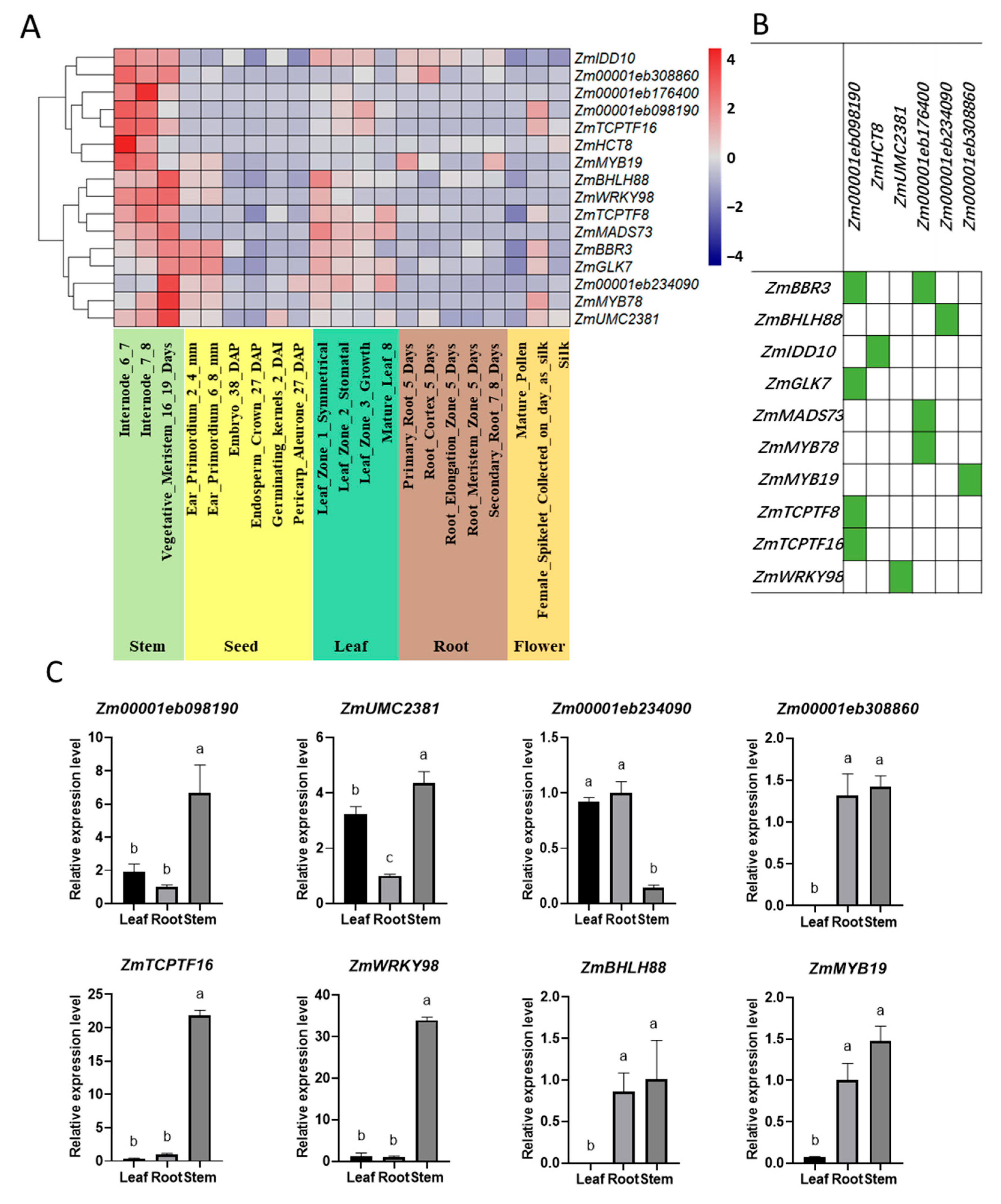
2.2. Gene Expression Pattern Analysis of the Identified Synthetic Genes
2.3. Prediction of Transcriptional Regulatory Network in Lignin Synthetic Pathway
2.4. Gene Expression Analysis of Lignin Synthesis Genes and TFs in Different Tissues
2.5. Candidate Lignin Synthesis Gene Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of Gene Family Members and Sequence Analysis
4.3. Potential Transcriptional Regulation Analysis of Lignin Biosynthesis Genes
4.4. Expression Pattern Analysis of Lignin Synthesis Gene and Visualization
4.5. Different Varieties Stained for Lignin in Stem Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Méchin, V.; Argillier, O.; Menanteau, V.; Barrière, Y.; Mila, I.; Pollet, B.; Lapierre, C. Relationship of cell wall composition to in vitro cell wall digestibility of maize inbred line stems. J. Sci. Food Agric. 2000, 80, 574–580. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, A.; He, L.; Kong, H. Advances in Study of Lignin Biosynthesis and its Genetic Manipulation. Mol. Plant Breed. 2006, 14, 431–437. [Google Scholar]
- Li, Y. A Study on the Anatomical Structure, Lignin Content, and Expression of Key Genes Involved in Lignin Synthesis of Roots and Stems of Lodging Resistant Rapeseed. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2010. [Google Scholar]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Murata, K.; Yamazaki, M.; Onosato, K.; Miyao, A. Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol. 2003, 133, 73–83. [Google Scholar] [CrossRef]
- Islam, M.S.; Peng, S.; Visperas, R.M.; Ereful, N.; Bhuiya, M.S.U.; Julfiquar, A.W. Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res. 2007, 101, 240–248. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, Y. Rice Brittleness Mutants: A Way to Open the ‘Black Box’ of Monocot Cell Wall Biosynthesis. J. Integr. Plant Biol. 2011, 53, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Dong, Z.; Wang, C.; Wei, X.; Long, Y.; Wan, X. Genetic structure and molecular mechanism underlying the stalk lodging traits in maize (Zea mays L.). Comput. Struct. Biotechnol. J. 2023, 21, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhong, G.; Gu, Y.; Lu, T.; Li, W.; Yang, S.; Zhu, C.; Zhu, C.; Li, S.; Wang, Y.; et al. QTL mapping and candidate gene analysis of cell wall related components in rice stem cells. Bull. Bot. 2023, 58, 882–892. [Google Scholar]
- Yin, N.; Li, B.; Liu, X.; Liang, Y.; Lian, J.; Xue, Y.; Qu, C.; Lu, K.; Wei, L.; Wang, R.; et al. Two types of cinnamoyl-CoA reductase function divergently in accumulation of lignins, flavonoids and glucosinolates and enhance lodging resistance in Brassica napus. Crop J. 2022, 10, 647–660. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, B.; Li, B.; Hu, J.; Li, X.; Gou, L. Study on the Relationship between Stalk Strength Formation and Lignin Accumulation in Maize. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1389–1395. [Google Scholar]
- Zhan, X.; Kong, F.; Liu, Q.; Lan, T.; Liu, Y.; Xu, J.; Ou, Q.; Chen, L.; Kessel, G.; Kempenaar, C.; et al. Maize basal internode development significantly affects stalk lodging resistance. Field Crops Res. 2022, 286, 108611. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Kim, J.I.; Tobimatsu, Y.; Ciesielski, P.N.; Anderson, N.A.; Ximenes, E.; Maeda, J.; Ralph, J.; Donohoe, B.S.; Ladisch, M. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature 2014, 509, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Jannoey, P.; Pongprasert, W.; Lumyong, S.; Roytrakul, S.; Nomura, M. Comparative proteomic analysis of two rice cultivars (Oryza sativa L.) contrasting in brown planthopper (BPH) stress resistance. Plant Omics 2015, 8, 96–105. [Google Scholar]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of Cell Wall Composition on Maize Resistance to Pests and Diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Wang, R.; Zhang, J.; Owens, G. Cadmium adsorption by willow root: The role of cell walls and their subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Dalcorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2014, 5, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. PhytoKeys 2013, 4, 273. [Google Scholar] [CrossRef]
- Espiñeira, J.M.; Uzal, E.N.; Ros, L.V.G.; Carrión, J.S.; Merino, F.; Barceló, A.R.; Pomar, F. Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol. 2011, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chabannes, M.; Ruel, K.; Yoshinaga, A.; Chabbert, B.; Jauneau, A.; Joseleau, J.P.; Boudet, A.M. In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels. Plant J. 2001, 28, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Ennos, A.R.; Turner, S.R. Cloning and characterization of irregular xylem4 (irx4): A severely lignin-deficient mutant of Arabidopsis. Plant J. 2001, 26, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, C.; Li, P.; Wu, C.; Cao, M.; Yang, L. Research progress on synthesis and regulation of plant lignin. J. Shanxi Agric. Sci. 2016, 44, 1406–1411. [Google Scholar]
- Chen, Y.; Tan, X.; Clapham, D. Lignin biosynthesis and genetic regulation. Acta Agric. Univ. Jiangxie 2003, 4, 613–617. [Google Scholar]
- Giordano, A.; Liu, Z.; Panter, S.N.; Dimech, A.M.; Shang, Y.; Wijesinghe, H.; Fulgueras, K.; Ran, Y.; Mouradov, A.; Rochfort, S.; et al. Reduced lignin content and altered lignin composition in the warm season forage grass Paspalum dilatatum by down-regulation of a Cinnamoyl CoA reductase gene. Transgenic Res. 2014, 23, 503–517. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Gu, F.; Ke, S.; Sun, D.; Dong, S.; Liu, W.; Huang, M.; Xiao, W.; Yang, G.; et al. 4-Coumarate-CoA Ligase-Like Gene OsAAE3 Negatively Mediates the Rice Blast Resistance, Floret Development and Lignin Biosynthesis. Front. Plant Sci. 2017, 7, 2041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Zeng, J.K.; Li, X.; Zhang, J.; Ge, H.; Yin, X.R.; Chen, K.S. Regulation of loquat fruit low temperature response and lignification involves interaction of heat shock factors and genes associated with lignin biosynthesis. Plant Cell Environ. 2016, 39, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, Z.; Liu, Y.; Li, Y.; Su, K.; Bai, Z.; Guo, S.; Hu, Z.; Zhang, Z.; Bao, Y.; et al. Mutation of 4-coumarate: Coenzyme A ligase 1 gene affects lignin biosynthesis and increases the cell wall digestibility in maize brown midrib5 mutants. Biotechnol. Biofuels 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Funnell-Harris, D.L.; Pedersen, J.F. Brown midrib mutations and their importance to the utilization of maize, sorghum, and pearl millet lignocellulosic tissues. Plant Sci. 2010, 178, 229–238. [Google Scholar] [CrossRef]
- Halpin, C.; Holt, K.; Chojecki, J.; Oliver, D.; Foxon, G.A. Brown-midrib maize (bm1)—A mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J. 1998, 14, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Afifi, O.A.; Tobimatsu, Y.; Lam, P.Y.; Martin, A.F.; Miyamoto, T.; Osakabe, Y.; Osakabe, K.; Umezawa, T. Genome-edited rice deficient in two 4-COUMARATE:COENZYME A LIGASE genes displays diverse lignin alterations. Plant Physiol. 2022, 190, 2155–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Chen, T.; Wang, W.; He, Q. Transcriptome sequencing analysis of the mechanism of stem lodging resistance in Qianmai 1175. J. Triticeae Crops 2023, 43, 958–967. [Google Scholar]
- Legay, S.; Lacombe, E.; Goicoechea, M.; Brière, C.; Séguin, A.; Mackay, J.; Grima-Pettenati, J. Molecular characterization of EgMYB1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Sci. 2007, 173, 542–549. [Google Scholar] [CrossRef]
- Xiang, S. Molecular Mechanism of BnMYB52a Involved in the Regulation of Lignin Biosynthesis in Brassica napus L. Master’s Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar]
- Wang, H. Function and Gene Editing of NAC Transcription Factors Related to Lignin Synthesis in Chrysanthemum lavandulifolium. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Zhong, R.; Richardson, E.A.; Ye, Z.H. Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 2007, 225, 1603–1611. [Google Scholar] [CrossRef]
- Wan, X.; Wu, S.; Li, Z.; Dong, Z.; An, X.; Ma, B.; Tian, Y.; Li, J. Maize Genic Male-Sterility Genes and Their Applications in Hybrid Breeding: Progress and Perspectives. Mol. Plant 2019, 12, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, S.; Li, X. Breeding with dominant genic male-sterility genes to boost crop grain yield in the post-heterosis utilization era. Mol. Plant 2021, 14, 531–534. [Google Scholar] [CrossRef]
- Park, H.L.; Bhoo, S.H.; Kwon, M.; Lee, S.-W.; Cho, M.-H. Biochemical and Expression Analyses of the Rice Cinnamoyl-CoA Reductase Gene Family. Front. Plant Sci. 2017, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, M.; Li, D.; Zhang, J.; Jin, Q.; Sheng, L.; Cai, Y.; Lin, Y. Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit. Biol. Open 2017, 6, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.G.; Zhan, Q.W.; Yu, H.B.; Huang, B.H.; Cheng, X.X.; Li, W.Y.; Huang, S.C.; Wang, C.J.; Du, J.L. Genome-wide identification and analysis of maize PAL gene family and its expression profile in response to high-temperature stress. Pak. J. Bot. 2020, 52, 1577–1587. [Google Scholar] [CrossRef]
- Yin, L.; Huang, J. Cloning and Bioinformatic Analysis of ZmCAD4 Gene from Maize. J. Maize Sci. 2023, 31, 29–38. [Google Scholar]
- Liu, S.; Meng, Z.; Zhang, H.; Chu, Y.; Qiu, Y.; Jin, B.; Wang, L. Identification and characterization of thirteen gene families involved in flavonoid biosynthesis in Ginkgo biloba. Ind. Crops Prod. 2022, 188, A115576. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, W.; Zhang, H.; Ma, L.; Li, P.; Ge, L.; Li, G. Genome-wide analysis of the basic Helix-Loop-Helix (bHLH) transcription factor family in maize. BMC Plant Biol. 2018, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, S.; Zhang, D.; Li, Z.; Xie, K.; An, X.; Ma, B.; Hou, Q.; Dong, Z.; Tian, Y.; et al. Genome-wide analysis of maize GPAT gene family and cytological characterization and breeding application of ZmMs33/ZmGPAT6 gene. Theor. Appl. Genet. 2019, 132, 2137–2154. [Google Scholar]
- Lu, L.; Hou, Q.; Wang, L.; Zhang, T.; Zhao, W.; Yan, T.; Zhao, L.; Li, J.; Wan, X. Genome-Wide Identification and Characterization of Polygalacturonase Gene Family in Maize (Zea mays L.). Int. J. Mol. Sci. 2021, 22, 10722. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pu, A.; Liu, Q.; Hou, Q.; Zhang, Y.; An, X.; Long, Y.; Jiang, Y.; Dong, Z.; Wu, S.; et al. The Bibliometric Landscape of Gene Editing Innovation and Regulation in the Worldwide. Cells 2022, 11, 2682. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Jiang, Y.; Yan, T.; Fang, C.; Hou, Q.; Wu, S.; Xie, K.; An, X.; Wan, X. Use of CRISPR/Cas9-Based Gene Editing to Simultaneously Mutate Multiple Homologous Genes Required for Pollen Development and Male Fertility in Maize. Cells 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; An, X.; Li, Z.; Yan, T.; Zhu, T.; Xie, K.; Liu, S.; Hou, Q.; Zhao, L.; Wu, S.; et al. CRISPR/Cas9-based discovery of maize transcription factors regulating male sterility and their functional conservation in plants. Plant Biotechnol. J. 2021, 19, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Fornalé, S.; Shi, X.; Chai, C.; Encina, A.; Irar, S.; Capellades, M.; Fuguet, E.; Torres, J.L.; Rovira, P.; Puigdomènech, P.; et al. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J. 2010, 64, 633–644. [Google Scholar] [CrossRef]
- Li, L.; Hill-Skinner, S.; Liu, S.; Beuchle, D.; Tang, H.M.; Yeh, C.T.; Nettleton, D.; Schnable, P.S. The maize brown midrib4 (bm4) gene encodes a functional folylpolyglutamate synthase. Plant J. 2015, 81, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, S.; Yang, S.; Yang, Z.; Liu, S.; Wang, Y.; Gao, H.; Zhang, S.; Yang, X.; Jiang, C.; et al. Genome assembly and genetic dissection of a prominent drought-resistant maize germplasm. Nat. Genet. 2023, 55, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cano, L.; Gomez-Cano, F.; Dillon, F.M.; Alers-Velazquez, R.; Doseff, A.I.; Grotewold, E.; Gray, J. Discovery of Modules Involved in the Biosynthesis and Regulation of Maize Phenolic Compounds. Plant Sci. 2019, 291, 110364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Sun, Z.; Huang, X.; Yu, N.; Tai, H.; Yang, Q. Comparative transcriptome meta-analysis reveals a set of genes involved in the responses to multiple pathogens in maize. Front. Plant Sci. 2022, 13, 971371. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Cruz-Mendívil, A.; Ibarra-Laclette, E.; García-Pérez, L.M.; Gómez-Peraza, R.L.; Hanako-Rosas, G.; Ruíz-May, E.; Santamaría-Miranda, A.; Singh, R.K.; Campos-Rivero, G.; et al. Cell wall-related genes and lignin accumulation contribute to the root resistance in different maize (Zea mays L.) genotypes to Fusarium verticillioides (Sacc.) Nirenberg infection. Front. Plant Sci. 2023, 14, 1195794. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Qi, S.; Yuan, Y.; Guo, J.; Chen, G.; Ahmad, M.; Jiang, B.; Jin, Y. Effects of the Structure and Molecular Weight of Alkali-Oxygen Lignin Isolated from Rice Straw on the Growth of Maize Seedlings. Biomacromolecules 2023, 24, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Pratyusha, D.S.; Sarada, D.V.L. MYB transcription factors-master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; McCarthy, R.L.; Reeves, C.K.; Jones, E.G.; Ye, Z.-H. Transcriptional Activation of Secondary Wall Biosynthesis by Rice and Maize NAC and MYB Transcription Factors. Plant Cell Physiol. 2011, 52, 1856–1871. [Google Scholar] [CrossRef]
- Barrière, Y.; Courtial, A.; Soler, M.; Grima-Pettenati, J. Toward the identification of genes underlying maize QTLs for lignin content, focusing on colocalizations with lignin biosynthetic genes and their regulatory MYB and NAC transcription factors. Mol. Breed. 2015, 35, 87. [Google Scholar] [CrossRef]
- Agarwal, T.; Grotewold, E.; Doseff, A.I.; Gray, J. MYB31/MYB42 Syntelogs Exhibit Divergent Regulation of Phenylpropanoid Genes in Maize, Sorghum and Rice. Sci. Rep. 2016, 6, 28502. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Hazebroek, J.P.; Chamberlin, M.A.; Perkins, M.; Sandhu, A.S.; Gupta, R.; Simcox, K.D.; Li, Y.; Prall, A.; Heetland, L.; et al. Chitinase-like1 Plays a Role in Stalk Tensile Strength in Maize. Plant Physiol. 2019, 181, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nie, S.; Li, G.; Du, J.; Ren, R.; Yang, X.; Liu, B.; Gao, X.; Liu, T.; Zhang, Z.; et al. Identification and Fine Mapping of the Recessive Gene BK-5, Which Affects Cell Wall Biosynthesis and Plant Brittleness in Maize. Int. J. Mol. Sci. 2022, 23, 814. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, L.; Sheng, Z.; Zhao, D.; Tao, J. An R2R3-MYB network modulates stem strength by regulating lignin biosynthesis and secondary cell wall thickening in herbaceous peony. Plant J. 2023, 113, 1237–1258. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, L.; Huang, X.; Zhao, D.; Tao, J. The herbaceous peony transcription factor WRKY41a promotes secondary cell wall thickening to enhance stem strength. Plant Physiol. 2023, 191, 428–445. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Bris, P.L.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, C.; Zhang, K.; Deng, W.; Brown, L.; Wei, H. TGMI: An efficient algorithm for identifying pathway regulators through evaluation of triple-gene mutual interaction. Nucleic Acids Res. 2018, 46, e67. [Google Scholar] [CrossRef]
- Livak, K.J.; Thomas, D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, X.; Yue, L.; Li, H.; Zhu, L.; Dong, Z.; Long, Y. Genome-Wide Identification and Characterization of Lignin Synthesis Genes in Maize. Int. J. Mol. Sci. 2024, 25, 6710. https://doi.org/10.3390/ijms25126710
Wang S, Wang X, Yue L, Li H, Zhu L, Dong Z, Long Y. Genome-Wide Identification and Characterization of Lignin Synthesis Genes in Maize. International Journal of Molecular Sciences. 2024; 25(12):6710. https://doi.org/10.3390/ijms25126710
Chicago/Turabian StyleWang, Shuai, Xiaofang Wang, Liangxu Yue, Huangai Li, Lei Zhu, Zhenying Dong, and Yan Long. 2024. "Genome-Wide Identification and Characterization of Lignin Synthesis Genes in Maize" International Journal of Molecular Sciences 25, no. 12: 6710. https://doi.org/10.3390/ijms25126710
APA StyleWang, S., Wang, X., Yue, L., Li, H., Zhu, L., Dong, Z., & Long, Y. (2024). Genome-Wide Identification and Characterization of Lignin Synthesis Genes in Maize. International Journal of Molecular Sciences, 25(12), 6710. https://doi.org/10.3390/ijms25126710





