The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant
Abstract
1. Introduction
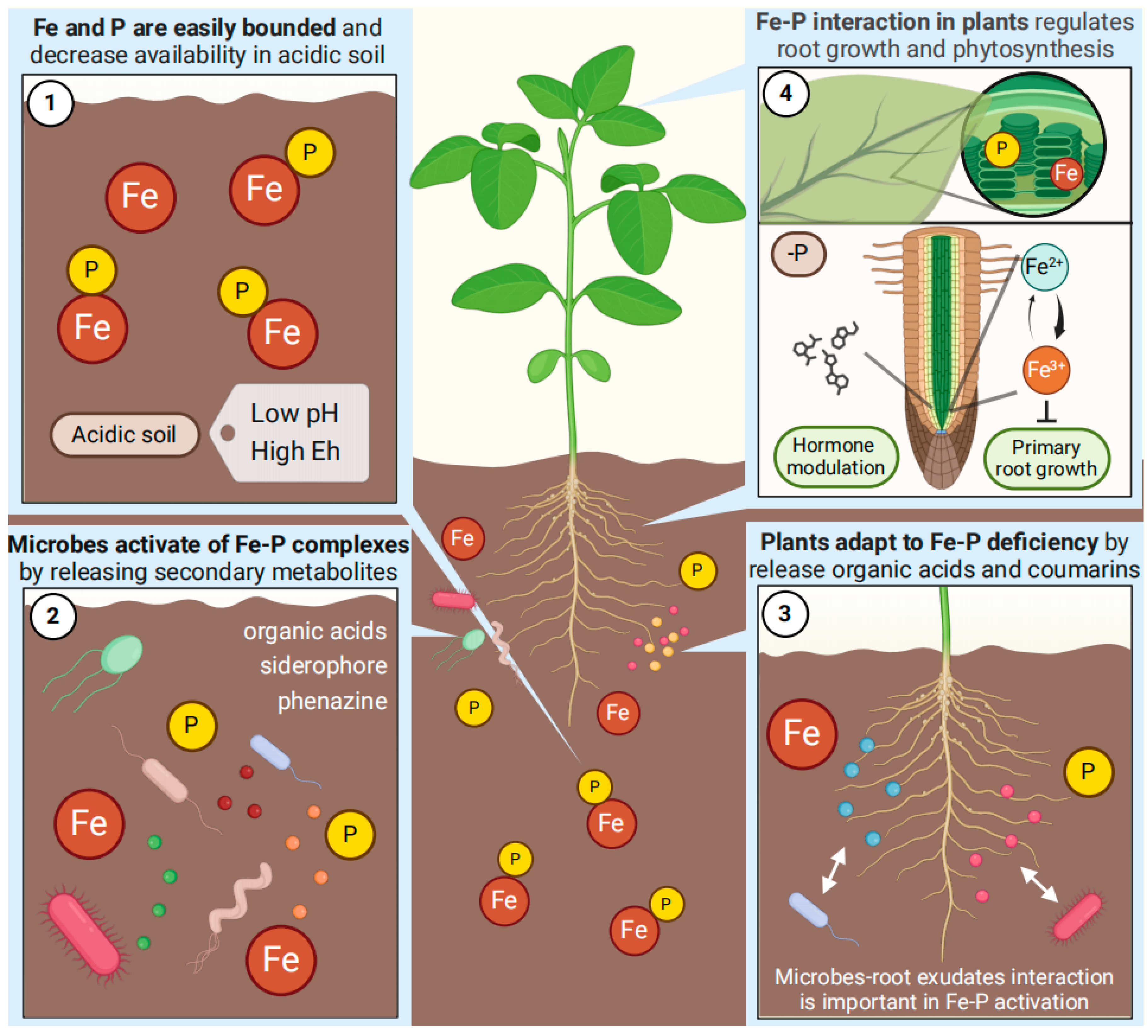
2. P-Fe Interactions in Soil
2.1. The Influences of Soil Properties on P-Fe Interactions
2.2. The P-Fe Interaction in Soil Affected by Microorganisms
| Mechanism | Function | Bacteria or Substance | References |
|---|---|---|---|
| Release organic acids | In acidic environment, secrete oxalic acid in response to insoluble P, such as goethite (P-Fe oxides) | Aspergillus niger | [10,36] |
| Low-P soil promotes P-solubilizing bacteria accumulation and organic acids secretion | P-solubilizing bacteria | [33] | |
| Release citric acid to adapt to acidic environment | Aspergillus niger | [35] | |
| Dissolve P from goethite via secreting low-molecular-weight organic acids, enhance P content of Solanum lycopersicum | Rhizophagus irregularis | [39] | |
| Siderophore secretion | Solubilization of P from Fe-phosphates at acid pH | desferrioxamine-B and desferriferrichrome | [54] |
| Solubilize P from Fe-phytate complexes | Siderophore-secreting bacteria | [55] | |
| Activate insoluble Fe-P complexes in rhizosphere, enhance P and Fe in Camellia oleifera | Streptomyces sp. CoT10 | [56] | |
| Redox ability | Release P from Fe-rich sludge via reducing Fe3+ to Fe2+ | Fe-reducing bacteria | [57] |
| Release phenazine-1-carboxamide to promote Fe reduction and dissolve Fe (hydr)oxides | Pseudomonas chlororaphis | [59] | |
| Phenazines reductively dissolve ferrihydrite and hematite the reaction rate is increases as the pH decreases | Pyocyanin, phenazine-1-carboxylate, phenazine-1-carboxamide and 1-hydroxyphenazine | [60] | |
| Release phenazine to reduce Fe3+ to Fe2+, solubilize P from phosphate hydrous ferric oxides and marine sediments | Pseudomonas aeruginosa | [61] | |
| Increase Fe-P in sediment and oxidizing Fe2+ to Fe3+ | Fe-oxidizing bacteria | [65] |
3. Interactions of P-Fe in Plants
3.1. Fe uptake Process Influenced by P
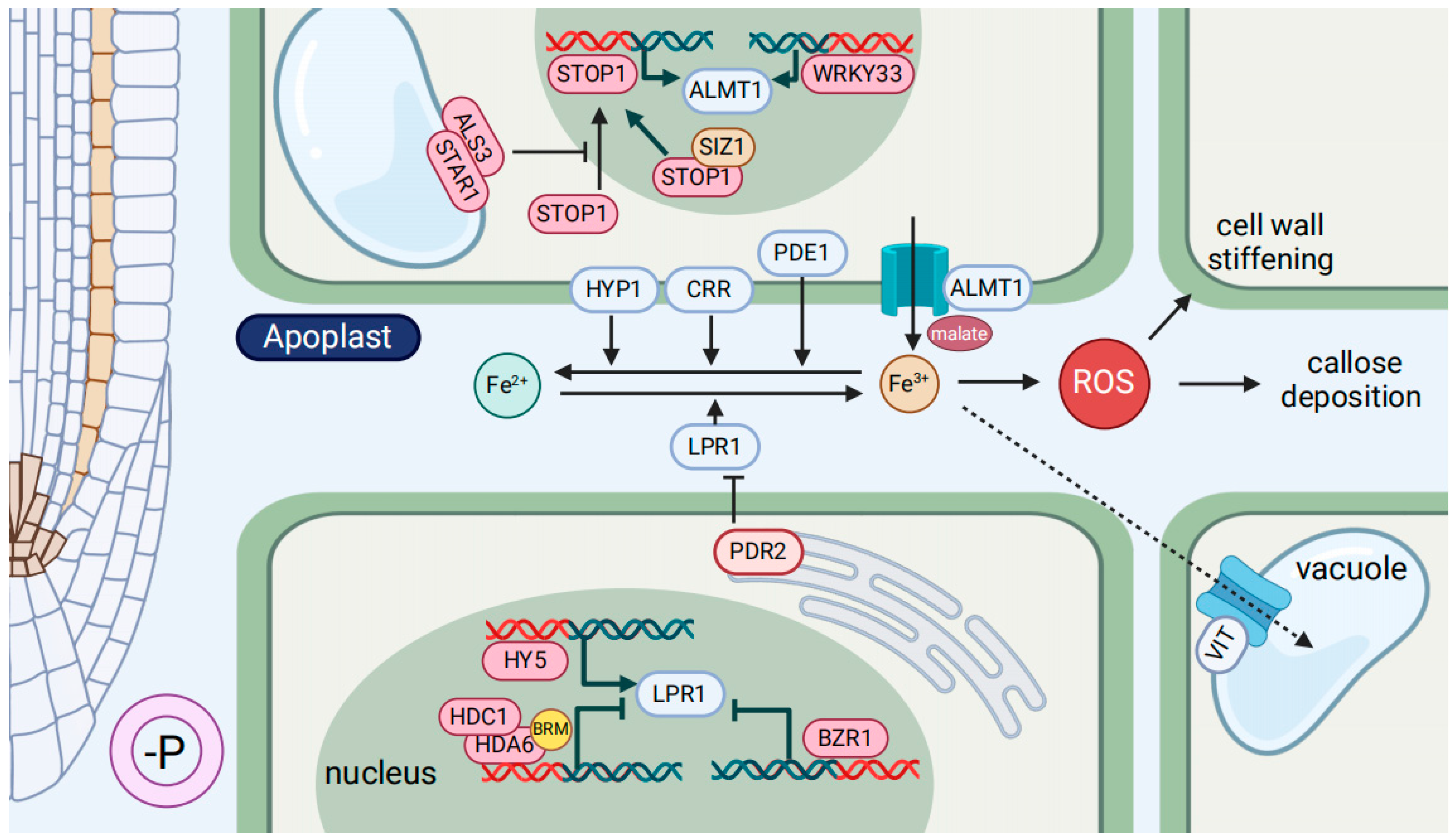
3.2. The Fe-Mediated Root Development Response to Low-P
3.3. The Exudates Metabolism Response to Fe-P Interaction
3.4. The Fe-Influenced Hormone Metabolism Response to Low-P
3.5. The Photosynthesis Affected by Fe-P Interaction
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.Y.; Lin, M.F.; Huang, G.H.; Mao, J.P.; Gao, Z.; Wang, X.L. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Thielemann, L.; Dippold, M.A.; Guggenberger, G.; Kuzyakov, Y.; Banfield, C.C.; Ge, T.; Guenther, S.; Bork, P.; Horn, M.A.; et al. Can the reductive dissolution of ferric iron in paddy soils compensate phosphorus limitation of rice plants and microorganisms? Soil Biol. Biochem. 2022, 168, 108653. [Google Scholar] [CrossRef]
- Xue, C.W.; Li, W.F.; Shen, R.F.; Lan, P. Impacts of iron on phosphate starvation-induced root hair growth in Arabidopsis. Plant Cell Environ. 2023, 46, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, S.; Santoro, V.; Schiavon, M.; Celi, L.; Martin, M.; Said-Pullicino, D. The influence of phosphorus availability on rice root traits driving iron plaque formation and dissolution, and implications for phosphorus uptake. Plant Soil 2024, 494, 603–616. [Google Scholar] [CrossRef]
- Clúa, J.; Montpetit, J.; Jimenez-Sandoval, P.; Naumann, C.; Santiago, J.; Poirier, Y. A CYBDOM protein impacts iron homeostasis and primary root growth under phosphate deficiency in Arabidopsis. Nat. Commun. 2024, 15, 423. [Google Scholar] [CrossRef]
- Li, S.J.; Lin, Z.G.; Liu, M.; Jiang, F.Z.; Chen, J.; Yang, X.J.; Wang, S.X. Effect of ferric chloride on phosphorus immobilization and speciation in Dianchi Lake sediments. Ecotoxicol. Environ. Saf. 2020, 197, 110637. [Google Scholar] [CrossRef]
- Su, M.; Meng, L.Z.; Zhao, L.; Tang, Y.K.; Qiu, J.J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]
- Memon, M. Role of Fe-Oxides for Predicting Phosphorus Sorption in Calcareous Soils; KIT Scientific Publishing: Karlsruhe, Germany, 2008. [Google Scholar]
- Celi, L.; Prati, M.; Magnacca, G.; Santoro, V.; Martin, M. Role of crystalline iron oxides on stabilization of inositol phosphates in soil. Geoderma 2020, 374, 114442. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Pérez, C.; Mariño, S.; Arce, F.; Gondar, D.; López, R. Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J. Colloid Interface Sci. 2010, 347, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Devau, N.; Hinsinger, P.; Le Cadre, E.; Colomb, B.; Gérard, F. Fertilization and pH effects on processes and mechanisms controlling dissolved inorganic phosphorus in soils. Geochim. Cosmochim. Acta 2011, 75, 2980–2996. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; Hodgkinson, R.A.; Rollett, A.; Dyer, C.; Dils, R.; Collins, A.L.; Bilsborrow, P.E.; Bailey, G.; Sylvester-Bradley, R. Reducing soil phosphorus fertility brings potential long-term environmental gains: A UK analysis. Environ. Res. Lett. 2017, 12, 063001. [Google Scholar] [CrossRef]
- Islam, M.; Siddique, K.H.; Padhye, L.P.; Pang, J.; Solaiman, Z.M.; Hou, D.; Srinivasarao, C.; Zhang, T.; Chandana, P.; Venu, N.; et al. A critical review of soil phosphorus dynamics and biogeochemical processes for unlocking soil phosphorus reserves. Adv. Agron. 2024, 185, 153–249. [Google Scholar]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. Agrotecnol. 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Henderson, R.; Kabengi, N.; Mantripragada, N.; Cabrera, M.; Hassan, S.; Thompson, A. Anoxia-induced release of colloid-and nanoparticle-bound phosphorus in grassland soils. Environ. Sci. Technol. 2012, 46, 11727–11734. [Google Scholar] [CrossRef]
- Chiriţă, P.; Schlegel, M.L. Reaction of FeS with Fe (III)-bearing acidic solutions. Chem. Geol. 2012, 334, 131–138. [Google Scholar] [CrossRef]
- Limmer, M.A.; Evans, A.E.; Seyfferth, A.L. A new method to capture the spatial and temporal heterogeneity of aquatic plant iron root plaque in situ. Environ. Sci. Technol. 2021, 55, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, X.; Wang, Y.; Sun, Q.; Chen, M.S.; Zhang, C.S.; Ding, S.M.; Dai, Z.H. Root-mediated acidification, phosphatase activity and the phosphorus-cycling microbial community enhance phosphorus mobilization in the rhizosphere of wetland plants. Water Res. 2024, 255, 121548. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Y.; Wu, F.F.; Song, K.; Lin, Y.; Bai, Y.C.; Zhu, Y.R.; Giesy, J.P. Competitive interaction between soil-derived humic acid and phosphate on goethite. Appl. Geochem. 2013, 36, 125–131. [Google Scholar] [CrossRef]
- Sundman, A.; Karlsson, T.; Sjöberg, S.; Persson, P. Impact of iron-organic matter complexes on aqueous phosphate concentrations. Chem. Geol. 2016, 426, 109–117. [Google Scholar] [CrossRef]
- Gerke, J. Humic (organic matter)-Al(Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Gerke, J. Review Article: The effect of humic substances on phosphate and iron acquisition by higher plants: Qualitative and quantitative aspects. J. Plant Nutr. Soil Sci. 2021, 184, 329–338. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in plant growth and development: Roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Li, F.Y.; Koopal, L.; Tan, W.F. Roles of different types of oxalate surface complexes in dissolution process of ferrihydrite aggregates. Sci. Rep. 2018, 8, 2060. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, T.S.; Dai, L.T.; Wang, F.W.; Tao, J.J.; Meng, S.T.; Hu, Y.X.; Wang, S.M.; Hu, S.J. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, X.W.; Shao, X.Q.; Wu, Y.L.; Zhang, X.Y.; Wang, S.M.; Pan, J.J.; Hu, S.J.; Li, Z. Lead immobilization assisted by fungal decomposition of organophosphate under various pH values. Sci. Rep. 2019, 9, 13353. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Andrino, A.; Boy, J.; Mikutta, R.; Sauheitl, L.; Guggenberger, G. Carbon investment required for the mobilization of inorganic and organic phosphorus bound to goethite by an arbuscular mycorrhiza (Solanum lycopersicum x Rhizophagus irregularis). Front. Environ. Sci. 2019, 7, 26. [Google Scholar] [CrossRef]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of organic acids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxide. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Perotto, S. Mineral transformations by mycorrhizal fungi. Geomicrobiol. J. 2010, 27, 609–623. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter-the unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Fan, X.X.; Chang, W.; Feng, F.J.; Song, F.Q. Responses of photosynthesis-related parameters and chloroplast ultrastructure to atrazine in alfalfa (Medicago sativa L.) inoculated with arbuscular mycorrhizal fungi. Ecotoxicol. Environ. Saf. 2018, 166, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Debnath, T.; Das, U.; Prity, S.A.; Haque, A.; Rahman, M.M.; Parvez, M.S. Arbuscular mycorrhizal fungi alleviate Fe-deficiency symptoms in sunflower by increasing iron uptake and its availability along with antioxidant defense. Plant Physiol. Biochem. 2020, 150, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis-model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Hamdali, H.; Moursalou, K.; Tchangbedji, G.; Ouhdouch, Y.; Hafidi, M. Isolation and characterization of rock phosphate solubilizing actinobacteria from a Togolese phosphate mine. Afr. J. Biotechnol. 2012, 11, 312. [Google Scholar] [CrossRef]
- Han, L.C.; Wang, X.; Li, B.G.; Shen, G.F.; Tao, S.; Fu, B.; Han, Y.M.; Li, W.; Long, S.X.; Peng, S.Y.; et al. Enhanced Fe-bound phosphate availability by the combined use of Mg-modified biochar and phosphate-solubilizing bacteria. J. Environ. Chem. Eng. 2022, 10, 107232. [Google Scholar] [CrossRef]
- Gu, S.H.; Wei, Z.; Shao, Z.Y.; Friman, V.P.; Cao, K.H.; Yang, T.J.; Kramer, J.; Wang, X.F.; Li, M.; Mei, X.L.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Q.; Wang, T.Q.; Chen, Y.; Wang, M.; Lu, Q.F.; Wang, K.G.; Dou, Z.C.; Chi, Z.G.; Qiu, W.; Dai, J.; et al. Microbiome convergence enables siderophore-secreting-rhizobacteria to improve iron nutrition and yield of peanut intercropped with maize. Nat. Commun. 2024, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Elena, M.; de Villegas, D. Microbial Siderophores; Varma, A., Chincholkar, S.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–231. [Google Scholar]
- Jones, D.L.; Oburger, E. Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. [Google Scholar]
- Vindeirinho, J.M.; Soares, H.M.V.M.; Soares, E.V. Modulation of siderophore production by Pseudomonas fluorescens through the manipulation of the culture medium composition. Appl. Biochem. Biotechnol. 2021, 193, 607–618. [Google Scholar] [CrossRef]
- Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-solubilizing microorganisms: A key to sustainable agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Reid, R.K.; Reid, C.P.P.; Szaniszlo, P.J. Effects of synthetic and microbially produced chelates on the diffusion of iron and phosphorus to a simulated root in soil. Biol. Fert. Soils 1985, 1, 45–52. [Google Scholar] [CrossRef]
- Wang, S.; Walker, R.; Schicklberger, M.; Nico, P.S.; Fox, P.M.; Karaoz, U.; Chakraborty, R.; Brodie, E.L. Microbial phosphorus mobilization strategies across a natural nutrient limitation gradient and evidence for linkage with iron solubilization traits. Front. Microbiol. 2021, 12, 572212. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.P.; Xu, T.; Chen, J.W.; Yang, H.Y.; Liu, X.M.; Zhuo, R.; Peng, Y.H.; Tang, W.; Wang, R.; Chen, L.S.; et al. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Li, R.H.; Wang, W.J.; Zhao, R.X.; Zhang, J.Y.; Sun, L.; Li, X.Y.; Li, B. New insights into the microbial-driven metal reductive dissolution for enhanced phosphorus release from iron-rich sludge. J. Clean. Prod. 2023, 392, 136290. [Google Scholar] [CrossRef]
- Dar, D.; Thomashow, L.S.; Weller, D.M.; Newman, D.K. Global landscape of phenazine biosynthesis and biodegradation reveals species-specific colonization patterns in agricultural soils and crop microbiomes. eLife 2020, 9, e59726. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Kappler, A.; Newman, D.K. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 2004, 70, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Newman, D.K. Redox reactions of phenazine antibiotics with ferric(hydr)oxides and molecular oxygen. Environ. Sci. Technol. 2008, 42, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- McRose, D.L.; Newman, D.K. Redox-active antibiotics enhance phosphorus bioavailability. Science 2021, 371, 1033–1037. [Google Scholar] [CrossRef]
- Jensen, V.; Löns, D.; Zaoui, C.; Bredenbruch, F.; Meissner, A.; Dieterich, G.; Münch, R.; Häussler, S. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and-independent pathways. J. Bacteriol. 2006, 188, 8601–8606. [Google Scholar] [CrossRef]
- Smolders, E.; Baetens, E.; Verbeeck, M.; Nawara, S.; Diels, J.; Verdievel, M.; Peeters, B.; De Cooman, W.; Baken, S. Internal loading and redox cycling of sediment iron explain reactive phosphorus concentrations in lowland rivers. Environ. Sci. Technol. 2017, 51, 2584–2592. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Xiao, K.; Rensing, C.; Zhou, S.G. Molecular evidence for the adaptive evolution of Geobacter sulfurreducens to perform dissimilatory iron reduction in natural environments. Mol. Microbiol. 2020, 113, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Gao, M.M.; Yang, Y.J.; Lu, S.P.; Wang, G.L.; Qian, X.Q. Interactions of Vallisneria natans and iron-oxidizing bacteria enhance iron-bound phosphorus formation in eutrophic lake sediments. Microorganisms 2022, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Zeng, Q.; Sheng, Y.Z.; Chen, C.M.; Yu, G.G.; Kappler, A. Coupled iron cycling and organic matter transformation across redox interfaces. Nat. Rev. Earth Environ. 2023, 4, 659–673. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhou, J.C.; Rengel, Z.; George, T.S.; Feng, G. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: Processes and ecological functions. Plant Soil 2022, 481, 1–22. [Google Scholar] [CrossRef]
- Chen, L.; Liao, H. Engineering crop nutrient efficiency for sustainable agriculture. J. Integr. Plant Biol. 2017, 59, 710–735. [Google Scholar] [CrossRef] [PubMed]
- Bouain, N.; Korte, A.; Satbhai, S.B.; Nam, H.I.; Rhee, S.Y.; Busch, W.; Rouached, H. Systems genomics approaches provide new insights into Arabidopsis thaliana root growth regulation under combinatorial mineral nutrient limitation. PLoS Genet. 2019, 15, e1008392. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Lucena, C.; Romera, F.J. Ethylene and nitric oxide involvement in the regulation of Fe and P deficiency responses in dicotyledonous plants. Int. J. Mol. Sci. 2021, 22, 4904. [Google Scholar] [CrossRef]
- Puga, M.I.; Poza-Carrión, C.; Martinez-Hevia, I.; Perez-Liens, L.; Paz-Ares, J. Recent advances in research on phosphate starvation signaling in plants. J. Plant Res. 2024, 137, 315–330. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V. Strategies of plants for acquisition of iron. Plant Soil 1994, 165, 261–274. [Google Scholar] [CrossRef]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Vert, G.g.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.d.r.; Guerinot, M.L.; Briat, J.F.o.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021, 26, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Paffrath, V.; Tandron Moya, Y.A.; Weber, G.; von Wirén, N.; Giehl, R.F.H. A major role of coumarin-dependent ferric iron reduction in strategy I-type iron acquisition in Arabidopsis. Plant Cell 2024, 36, 642–664. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Urabe, A.; Sasaki, S.; Tsugawa, R.; Nishio, S.; Mukaiyama, H.; Murata, Y.; Masuda, H.; Aung, M.S.; Mera, A.; et al. Development of a mugineic acid family phytosiderophore analog as an iron fertilizer. Nat. Commun. 2021, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T. Iron delivery to the growing leaves associated with leaf chlorosis in mugineic acid family phytosiderophores-generating graminaceous crops. Soil Sci. Plant Nutr. 2021, 67, 415–426. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Lan, P. Genome-wide analysis of overlapping genes regulated by iron deficiency and phosphate starvation reveals new interactions in Arabidopsis roots. BMC Res. Notes 2015, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Hoehenwarter, W.; Mönchgesang, S.; Neumann, S.; Majovsky, P.; Abel, S.; Müller, J. Comparative expression profiling reveals a role of the root apoplast in local phosphate response. BMC Plant Biol. 2016, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.C.; Läuchli, A. Phosphorus efficiency and phosphate-iron interaction in maize. Agron. J. 1985, 77, 399–403. [Google Scholar] [CrossRef]
- Romera, F.J.; Alcántara, E.; de la Guardia, M.D. Effects of bicarbonate, phosphate and high pH on the reducing capacity of Fe-deficient sunflower and cucumber plants. J. Plant Nutr. 1992, 15, 1519–1530. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J. The severity of iron chlorosis in sensitive plants is related to soil phosphorus levels. J. Sci. Food Agric. 2014, 94, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Wang, X.N.; Sun, W.; Wang, X.F.; Tong, X.S.; Ji, X.L.; An, J.P.; Zhao, Q.; You, C.X.; Hao, Y.J. Phosphate regulates malate/citrate-mediated iron uptake and transport in apple. Plant Sci. 2020, 297, 110526. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Schmidt, W. One way. Or another? Iron uptake in plants. New Phytol. 2017, 214, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Q.; Huang, F.L.; Narsai, R.; Wu, J.J.; Giraud, E.; He, F.; Cheng, L.J.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.G.; Ren, M.L.; Zhang, P.; Li, Z.N.; Chen, S.; Ge, C.L.; Wang, Y.L. Iron and callose homeostatic regulation in rice roots under low phosphorus. BMC Plant Biol. 2018, 18, 326. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Venuti, S.; Zamboni, A.; Varanini, Z.; Tomasi, N.; Pinton, R. Transcriptional and physiological analyses of Fe deficiency response in maize reveal the presence of Strategy I components and Fe/P interactions. BMC Genom. 2017, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef] [PubMed]
- Svistoonoff, S.; Creff, A.; Reymond, M.; Sigoillot-Claude, C.; Ricaud, L.; Blanchet, A.; Nussaume, L.; Desnos, T. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet. 2007, 39, 792–796. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Lucero, R.D.; Sakhonwasee, S.; Adamson, A.W.; Creff, A.; Nussaume, L.; Desnos, T.; Abel, S. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc. Natl. Acad. Sci. USA 2009, 106, 14174–14179. [Google Scholar] [CrossRef]
- Péret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhu, X.F.; Xue, D.W.; Zheng, S.J.; Jin, C.W. Beyond iron-storage pool: Functions of plant apoplastic iron during stress. Trends Plant Sci. 2023, 28, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef]
- Müller, J.; Toev, T.; Heisters, M.; Teller, J.; Moore, K.L.; Hause, G.; Dinesh, D.C.; Bürstenbinder, K.; Abel, S. Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev. Cell 2015, 33, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Balzergue, C.; Dartevelle, T.; Godon, C.; Laugier, E.; Meisrimler, C.; Teulon, J.-M.; Creff, A.; Bissler, M.; Brouchoud, C.; Hagège, A.; et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 2017, 8, 15300. [Google Scholar] [CrossRef] [PubMed]
- Mora-Macías, J.; Ojeda-Rivera, J.O.; Gutiérrez-Alanís, D.; Yong-Villalobos, L.; Oropeza-Aburto, A.; Raya-González, J.; Jiménez-Domínguez, G.; Chávez-Calvillo, G.; Rellán-Álvarez, R.; Herrera-Estrella, L. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E3563–E3572. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Fridman, Y.; Holland, N.; Ackerman-Lavert, M.; Zananiri, R.; Jaillais, Y.; Henn, A.; Savaldi-Goldstein, S. Interdependent nutrient availability and steroid hormone signals facilitate root growth plasticity. Dev. Cell 2018, 46, 59–72.e54. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, Z.; Zheng, Z.; Dong, J.; Song, L.; Sui, L.Q.; Nussaume, L.; Desnos, T.; Liu, D. Genetic dissection of Fe-dependent signaling in root developmental responses to phosphate deficiency. Plant Physiol. 2019, 179, 300–316. [Google Scholar] [CrossRef]
- Xu, Z.R.; Cai, M.L.; Yang, Y.; You, T.T.; Ma, J.F.; Wang, P.; Zhao, F.J. The ferroxidases LPR1 and LPR2 control iron translocation in the xylem of Arabidopsis plants. Mol. Plant 2022, 15, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Wang, Y.; Liu, X.X.; Ye, J.Y.; Zhou, M.; Jing, X.T.; Du, W.X.; Hu, W.J.; He, C.; Zhu, Y.X.; et al. The ferroxidases are critical for Fe(II) oxidation in xylem to ensure a healthy Fe allocation in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 958984. [Google Scholar] [CrossRef]
- Naumann, C.; Heisters, M.; Brandt, W.; Janitza, P.; Alfs, C.; Tang, N.; Toto Nienguesso, A.; Ziegler, J.; Imre, R.; Mechtler, K.; et al. Bacterial-type ferroxidase tunes iron-dependent phosphate sensing during Arabidopsis root development. Curr. Biol. 2022, 32, 2189–2205.e2186. [Google Scholar] [CrossRef]
- Gutiérrez-Alanís, D.; Ojeda-Rivera, J.O.; Yong-Villalobos, L.; Cárdenas-Torres, L.; Herrera-Estrella, L. Adaptation to phosphate scarcity: Tips from Arabidopsis roots. Trends Plant Sci. 2018, 23, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Bu, L.H.; Han, M.L.; Wang, Y.L.; Li, Z.Y.; Liu, H.T.; Chao, D.Y. Long-distance blue light signalling regulates phosphate deficiency-induced primary root growth inhibition. Mol. Plant 2021, 14, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.M.; Wang, Z.Q.; Wang, J.Y.; Li, P.F.; Jin, J.F.; Chen, W.W.; Fan, W.; Kochian, L.V.; Zheng, S.J.; Yang, J.L. Low phosphate represses histone deacetylase complex1 to regulate root system architecture remodeling in Arabidopsis. New Phytol. 2020, 225, 1732–1745. [Google Scholar] [CrossRef]
- Li, T.; Zhang, R.Y.; Satheesh, V.; Wang, P.; Ma, G.J.; Guo, J.F.; An, G.Y.; Lei, M.G. The chromatin remodeler BRAHMA recruits HISTONE DEACETYLASE6 to regulate root growth inhibition in response to phosphate starvation in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Godon, C.; Mercier, C.; Wang, X.Y.; David, P.; Richaud, P.; Nussaume, L.; Liu, D.; Desnos, T. Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. Plant J. 2019, 99, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.S.; Piñeros, M.A.; Li, X.X.; Yang, H.B.; Liu, Y.; Murphy, A.S.; Kochian, L.V.; Liu, D. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol. Plant 2017, 10, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Roux, B.; Have, M.; Le Poder, L.; Duong, N.; David, P.; Leonhardt, N.; Blanchard, L.; Naumann, C.; Abel, S.; et al. Root responses to aluminium and iron stresses require the SIZ1 SUMO ligase to modulate the STOP1 transcription factor. Plant J. 2021, 108, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, D. SIZ1 regulates phosphate deficiency-induced inhibition of primary root growth of Arabidopsis by modulating Fe accumulation and ROS production in its roots. Plant Signal. Behav. 2021, 16, 1946921. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Hou, S.F.; Tu, G.Q.; Lan, W.Z.; Jing, Y.P. Transcription factor WRKY33 mediates the phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in Arabidopsis roots. Int. J. Mol. Sci. 2021, 22, 9275. [Google Scholar] [CrossRef]
- Maniero, R.A.; Picco, C.; Hartmann, A.; Engelberger, F.; Gradogna, A.; Scholz-Starke, J.; Melzer, M.; Künze, G.; Carpaneto, A.; von Wirén, N.; et al. Ferric reduction by a CYBDOM protein counteracts increased iron availability in root meristems induced by phosphorus deficiency. Nat. Commun. 2024, 15, 422. [Google Scholar] [CrossRef]
- Wang, L.Y.; Qian, J.; Li, M.; Zheng, H.; Yang, X.; Zheng, M.; Hsu, Y.F. Arabidopsis PDE1 confers phosphate-deficiency tolerance in primary root growth. Plant Cell Rep. 2023, 43, 8. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Alanís, D.; Yong-Villalobos, L.; Jimenez-Sandoval, P.; Alatorre-Cobos, F.; Oropeza-Aburto, A.; Mora-Macías, J.; Sánchez-Rodríguez, F.; Cruz-Ramírez, A.; Herrera-Estrella, L. Phosphate starvation-dependent iron mobilization induces CLE14 expression to trigger root meristem differentiation through CLV2/PEPR2 signaling. Dev. Cell 2017, 41, 555–570.e553. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M. Arabidopsis Root Hair Development in Adaptation to Iron and Phosphate Supply; Mathematisch-Naturwissenschaftliche Fakultät I; Humboldt-Universität zu Berlin: Berlin, Germany, 2007. [Google Scholar]
- Bournier, M.; Tissot, N.; Mari, S.; Boucherez, J.; Lacombe, E.; Briat, J.F.; Gaymard, F. Arabidopsis ferritin 1 (AtFer1) gene regulation by the phosphate starvation response 1 (AtPHR1) transcription factor reveals a direct molecular link between iron and phosphate homeostasis. J. Biol. Chem. 2013, 288, 22670–22680. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant Sci. 2015, 6, 129608. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.N.; Ruan, W.Y.; Zhang, Y.B.; Zhang, Y.X.; Wang, X.Q.; Guo, Z.H.; Wang, L.; Zhou, T.; Paz-Ares, J.; Yi, K.K. A reciprocal inhibitory module for Pi and iron signaling. Mol. Plant 2022, 15, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Darah, P.R.; Kochian, L.V. Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 1996, 180, 57–66. [Google Scholar] [CrossRef]
- Hamada, Y.Z.; Carlson, B.; Dangberg, J. Interaction of malate and lactate with chromium (III) and iron (III) in aqueous solutions. Synth. React. Inorg. Met.-Org. Chem. 2005, 35, 515–522. [Google Scholar] [CrossRef]
- Deiana, S.; Palma, A.; Premoli, A.; Senette, C. Possible role of the polyuronic components in accumulation and mobilization of iron and phosphate at the soil-root interface. Plant Physiol. Biochem. 2007, 45, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Dong, D.F.; Peng, X.X.; Yan, X.L. Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiol. Plant. 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Valentinuzzi, F.; Pii, Y.; Vigani, G.; Lehmann, M.; Cesco, S.; Mimmo, T. Phosphorus and iron deficiencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria × ananassa. J. Exp. Bot. 2015, 66, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.B.; Giehl, R.F.H.; Döll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2013, 164, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Schmidt, S.; Chutia, R.; Müller, J.; Böttcher, C.; Strehmel, N.; Scheel, D.; Abel, S. Non-targeted profiling of semi-polar metabolites in Arabidopsis root exudates uncovers a role for coumarin secretion and lignification during the local response to phosphate limitation. J. Exp. Bot. 2015, 67, 1421–1432. [Google Scholar] [CrossRef]
- DeLoose, M.; Cho, H.; Bouain, N.; Choi, I.; Prom-U-Thai, C.; Shahzad, Z.; Zheng, L.; Rouached, H. PDR9 allelic variation and MYB63 modulate nutrient-dependent coumarin homeostasis in Arabidopsis. Plant J. 2024, 117, 1716–1727. [Google Scholar] [CrossRef]
- Chutia, R.; Abel, S.; Ziegler, J. Iron and phosphate deficiency regulators concertedly control coumarin profiles in Arabidopsis thaliana roots during iron, phosphate, and combined deficiencies. Front. Plant Sci. 2019, 10, 113. [Google Scholar] [CrossRef]
- Chutia, R.; Scharfenberg, S.; Neumann, S.; Abel, S.; Ziegler, J. Modulation of phosphate deficiency-induced metabolic changes by iron availability in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 7609. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wang, Y.; Yeh, K.C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef]
- Rajniak, J.; Giehl, R.F.H.; Chang, E.; Murgia, I.; von Wirén, N.; Sattely, E.S. Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat. Chem. Biol. 2018, 14, 442–450. [Google Scholar] [CrossRef]
- Todorov, L.T.; Kostova, I.P. Coumarin-transition metal complexes with biological activity: Current trends and perspectives. Front. Chem. 2024, 12, 1342772. [Google Scholar] [CrossRef]
- Ladouceur, A.; Tozawa, S.; Alam, S.; Kamei, S.; Kawai, S. Effect of low phosphorus and iron-deficient conditions on phytosiderophore release and mineral nutrition in barley. Soil Sci. Plant Nutr. 2006, 52, 203–210. [Google Scholar] [CrossRef]
- McRose, D.L.; Li, J.Y.; Newman, D.K. The chemical ecology of coumarins and phenazines affects iron acquisition by pseudomonads. Proc. Natl. Acad. Sci. USA 2023, 120, e2217951120. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Ayala-Rodríguez, J.Á.; López-Bucio, J. Pseudomonas putida configures Arabidopsis root architecture through modulating the sensing systems for phosphate and iron acquisition. Plant Sci. 2024, 342, 112028. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dippold, M.A.; Guggenberger, G.; Kuzyakov, Y.; Guenther, S.; Dorodnikov, M. The wetter the better? Preferences in plant-microbial competition for phosphorus sources in rice cultivation under contrasting irrigation. Soil Biol. Biochem. 2024, 191, 109339. [Google Scholar] [CrossRef]
- Jia, Z.T.; Giehl, R.F.; von Wirén, N. Nutrient-hormone relations: Driving root plasticity in plants. Mol. Plant 2022, 15, 86–103. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; Davière, J.M.; Regnault, T.; Sakvarelidze-Achard, L.; Carrera, E.; Lopez Diaz, I.; Cayrel, A.; Dubeaux, G.; Vert, G.; Achard, P. Tissue-specific regulation of gibberellin signaling fine-tunes Arabidopsis iron-deficiency responses. Dev. Cell 2016, 37, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.F.; Gao, X.H.; Liao, L.L.; Harberd, N.P.; Fu, X.D. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Celletti, S.; Pii, Y.; Valentinuzzi, F.; Tiziani, R.; Fontanella, M.C.; Beone, G.M.; Mimmo, T.; Cesco, S.; Astolfi, S. Physiological responses to Fe deficiency in split-root tomato plants: Possible roles of auxin and ethylene? Agronomy 2020, 10, 1000. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, Y.M.; Sun, C.Y.; Liu, X.Q.; Lv, L.L.; Hu, Z.B.; Yu, D.Y.; Zhang, D. Up-regulating GmETO1 improves phosphorus uptake and use efficiency by promoting root growth in soybean. Plant Cell Environ. 2020, 43, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Luo, N.; Sun, L.C.; Liu, D. HPS4/SABRE regulates plant responses to phosphate starvation through antagonistic interaction with ethylene signalling. J. Exp. Bot. 2012, 63, 4527–4538. [Google Scholar] [CrossRef][Green Version]
- Nolan, T.M.; Vukašinović, N.; Liu, D.R.; Russinova, E.; Yin, Y.H. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021, 93, 29–38. [Google Scholar] [CrossRef]
- Wang, B.L.; Li, Y.; Zhang, W.H. Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. Ann. Bot. 2012, 110, 681–688. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral nutrient signaling controls photosynthesis: Focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2022, 27, 502–509. [Google Scholar] [CrossRef]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-u-thai, C.; Doumas, P.; Rouached, H. The involvement of OsPHO1;1 in the regulation of iron transport through integration of phosphate and zinc deficiency signaling. Front. Plant Sci. 2016, 7, 396. [Google Scholar] [CrossRef]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M.C. Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef]
- Nam, H.I.; Shahzad, Z.; Dorone, Y.; Clowez, S.; Zhao, K.; Bouain, N.; Lay-Pruitt, K.S.; Cho, H.; Rhee, S.Y.; Rouached, H. Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling. Nat. Commun. 2021, 12, 7211. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, C.; Liang, C.; Wang, T.; Tian, J. The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant. Int. J. Mol. Sci. 2024, 25, 6992. https://doi.org/10.3390/ijms25136992
Yang X, Liu C, Liang C, Wang T, Tian J. The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant. International Journal of Molecular Sciences. 2024; 25(13):6992. https://doi.org/10.3390/ijms25136992
Chicago/Turabian StyleYang, Xingqi, Chang Liu, Cuiyue Liang, Tianqi Wang, and Jiang Tian. 2024. "The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant" International Journal of Molecular Sciences 25, no. 13: 6992. https://doi.org/10.3390/ijms25136992
APA StyleYang, X., Liu, C., Liang, C., Wang, T., & Tian, J. (2024). The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant. International Journal of Molecular Sciences, 25(13), 6992. https://doi.org/10.3390/ijms25136992







