Axonal and Glial PIEZO1 and PIEZO2 Immunoreactivity in Human Clitoral Krause’s Corpuscles
Abstract
:1. Introduction
2. Results
2.1. Identification and Immunohistochemical Profile of Clitoral Krause’s Corpuscles
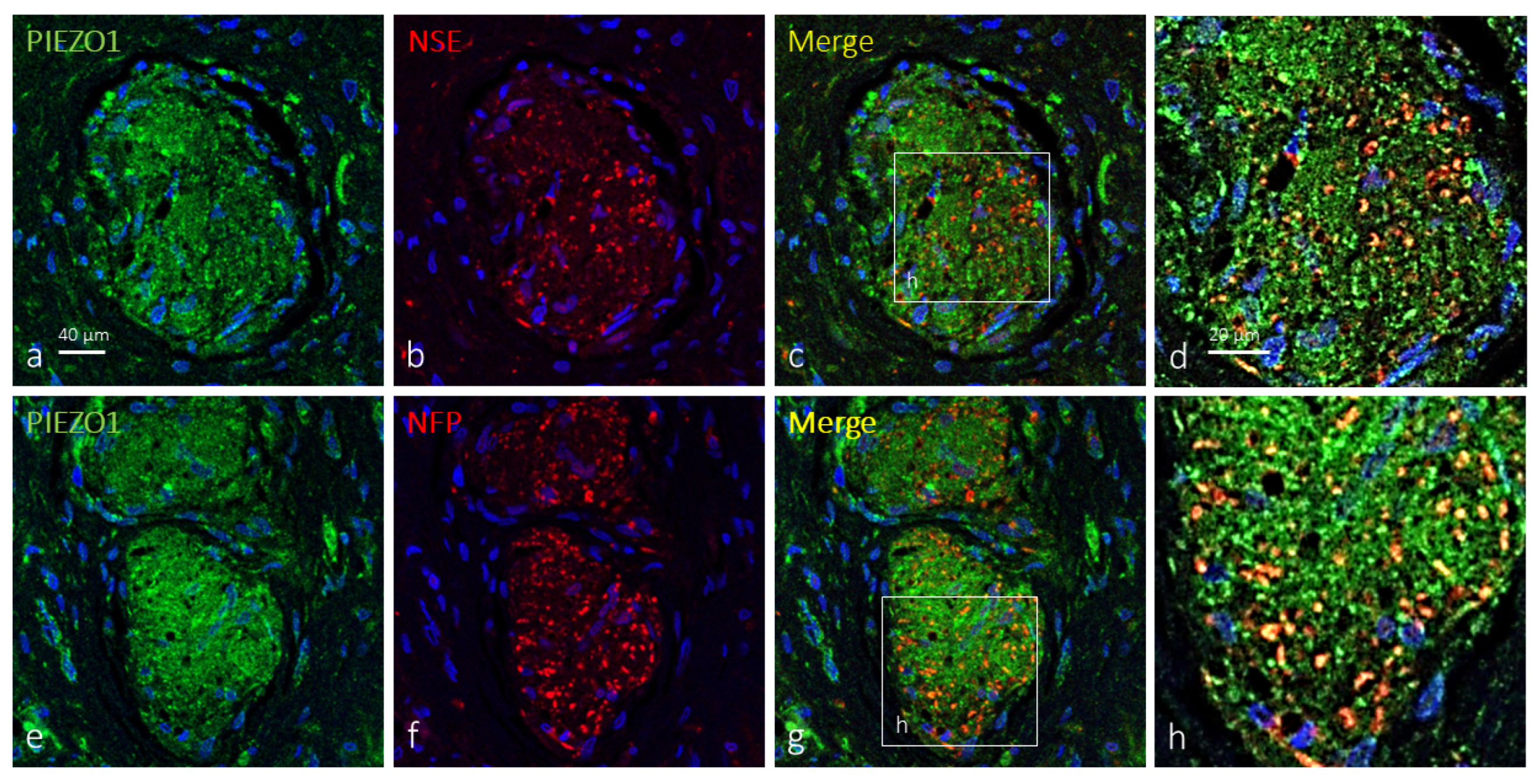
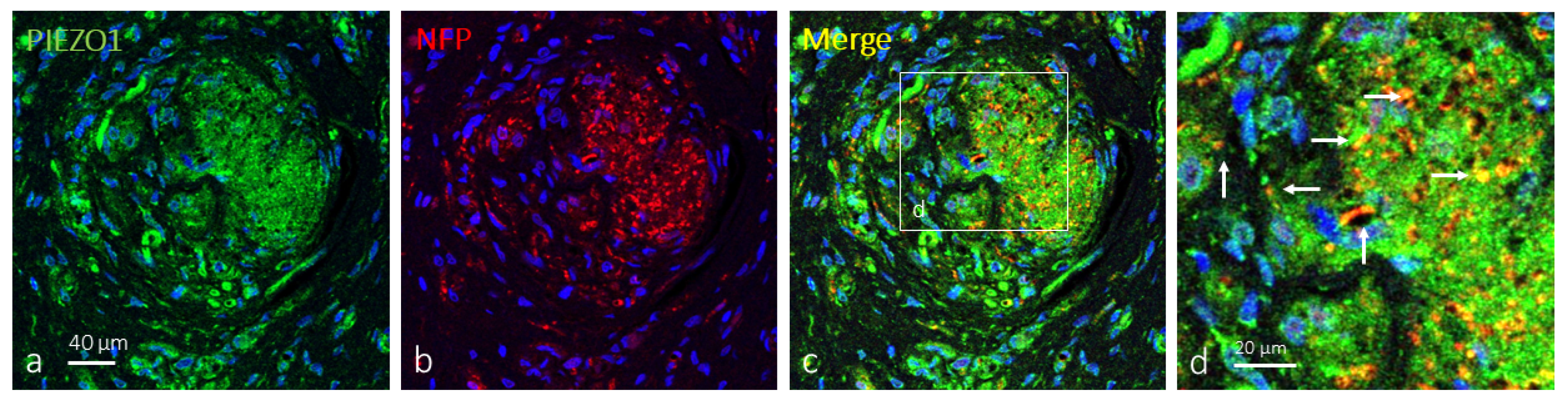
2.2. Clitoral Krause’s Corpuscles Display Axonal and Glial PIEZO1 Immunoreactivity
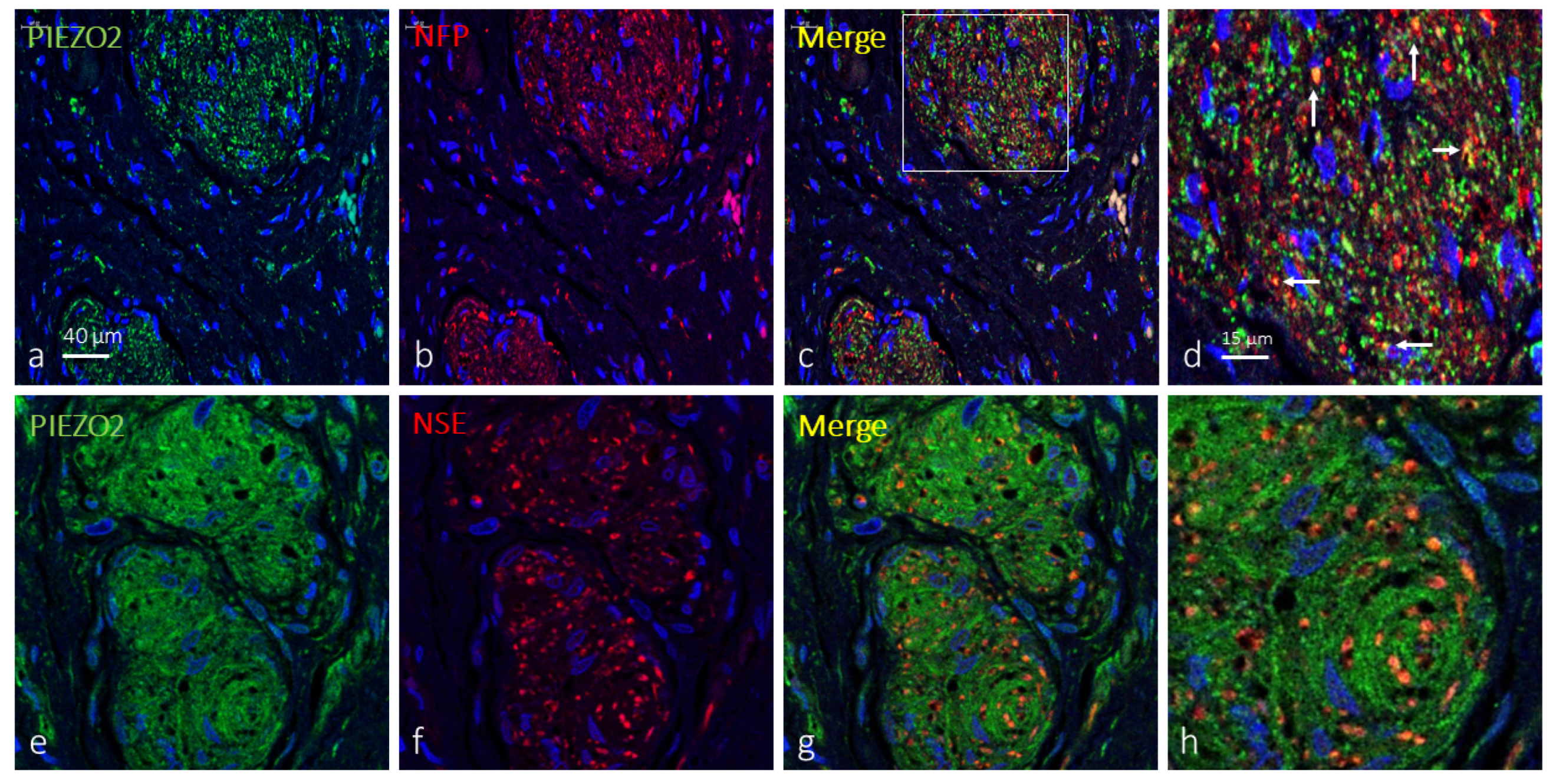
2.3. Clitoral Krause’s Corpuscles Display Axonal and Glial PIEZO2 Immunoreactivity
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Simple Immunohistochemistry
4.3. Double Immunofluorescence
4.4. Quantitative Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cobo, R.; García-Piqueras, J.; Cobo, J.; Vega, J.A. The Human Cutaneous Sensory Corpuscles: An Update. J. Clin. Med. 2021, 10, 227. [Google Scholar] [CrossRef]
- Handler, A.; Ginty, D.D. The mechanosensory neurons of touch and their mechanisms of activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef]
- Abraira, V.E.; Kuehn, E.D.; Chirila, A.M.; Springel, M.W.; Toliver, A.A.; Zimmerman, A.L.; Orefice, L.L.; Boyle, K.A.; Bai, L.; Song, B.J.; et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 2017, 168, 295–310.e19. [Google Scholar] [CrossRef]
- Suazo, I.; Vega, J.A.; García-Mesa, Y.; García-Piqueras, J.; García-Suárez, O.; Cobo, T. The Lamellar Cells of Vertebrate Meissner and Pacinian Corpuscles: Development, Characterization, and Functions. Front. Neurosci. 2022, 16, 790130. [Google Scholar] [CrossRef]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Cárcaba, L.; Coronado, C.; Cobo, R.; Martín-Cruces, J.; García-Piqueras, J.; Feito, J.; García-Suárez, O.; Vega, J.A. Glans clitoris innervation: PIEZO2 and sexual mechanosensitivity. J. Anat. 2021, 238, 446–454. [Google Scholar] [CrossRef]
- Qi, L.; Iskols, M.; Handler, A.; Ginty, D.D. Krause corpuscles of the genitalia are vibrotactile sensors required for normal sexual behavior. bioRxiv 2023. Preprint. [Google Scholar] [CrossRef]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Roh, J.; Hwang, S.M.; Lee, S.H.; Lee, K.; Kim, Y.H.; Park, C.K. Functional Expression of Piezo1 in Dorsal Root Ganglion (DRG) Neurons. Int. J. Mol. Sci. 2020, 21, 3834. [Google Scholar] [CrossRef]
- Shin, S.M.; Moehring, F.; Itson-Zoske, B.; Fan, F.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Piezo2 mechanosensitive ion channel is located to sensory neurons and nonneuronal cells in rat peripheral sensory pathway: Implications in pain. Pain 2021, 162, 2750–2768. [Google Scholar] [CrossRef]
- Shin, S.M.; Itson-Zoske, B.; Fan, F.; Gani, U.; Rahman, M.; Hogan, Q.H.; Yu, H. Peripheral sensory neurons and non-neuronal cells express functional Piezo1 channels. Mol. Pain 2023, 19, 17448069231174315. [Google Scholar] [CrossRef]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef]
- García-Mesa, Y.; García-Piqueras, J.; García, B.; Feito, J.; Cabo, R.; Cobo, J.; Vega, J.A.; García-Suárez, O. Merkel cells and Meissner’s corpuscles in human digital skin display Piezo2 immunoreactivity. J. Anat. 2017, 231, 978–989. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Feito, J.; Cuendias, P.; García-Piqueras, J.; Germanà, A.; García-Suárez, O.; Martín-Biedma, B.; Vega, J.A. The acquisition of mechanoreceptive competence by human digital Merkel cells and sensory corpuscles during development: An immunohistochemical study of PIEZO2. Ann. Anat. Anat. Anz. 2022, 243, 151953. [Google Scholar] [CrossRef]
- Handler, A.; Zhang, Q.; Pang, S.; Nguyen, T.M.; Iskols, M.; Nolan-Tamariz, M.; Cattel, S.; Plumb, R.; Sanchez, B.; Ashjian, K.; et al. Three-dimensional reconstructions of mechanosensory end organs suggest a unifying mechanism underlying dynamic, light touch. Neuron 2023, 111, 3211–3229.e9. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Cuendias, P.; Alonso-Guervós, M.; García-Piqueras, J.; Martín-Biedma, B.; Cobo, T.; García-Suárez, O.; Vega, J.A. Immunohistochemical detection of PIEZO1 and PIEZO2 in human digital Meissner’s corpuscles. Ann. Anat. Anat. Anz. 2024, 252, 152200. [Google Scholar] [CrossRef]
- Yamanishi, H.; Iwabuchi, T. Three-dimensional correlative light and focused ion beam scanning electron microscopy reveals the distribution and ultrastructure of lanceolate nerve endings surrounding terminal hair follicles in human scalp skin. J. Anat. 2023, 242, 1012–1028. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Feketa, V.V.; Anderson, E.O.; Schneider, E.R.; Gracheva, E.O.; Bagriantsev, S.N. Lamellar cells in Pacinian and Meissner corpuscles are touch sensors. Sci. Adv. 2020, 6, eabe6393. [Google Scholar] [CrossRef]
- Ojeda-Alonso, J.; Calvo-Enrique, L.; Paricio-Montesinos, R.; Kumar, R.; Zhang, M.D.; Poulet, J.F.A.; Ernfors, P.; Lewin, G.R. Sensory Schwann cells set perceptual thresholds for touch and selectively regulate mechanical nociception. Nat. Commun. 2024, 15, 898. [Google Scholar] [CrossRef]
- Lam, R.M.; von Buchholtz, L.J.; Falgairolle, M.; Osborne, J.; Frangos, E.; Servin-Vences, M.R.; Nagel, M.; Nguyen, M.Q.; Jayabalan, M.; Saade, D.; et al. PIEZO2 and perineal mechanosensation are essential for sexual function. Science 2023, 381, 906–910. [Google Scholar] [CrossRef]
- García-Mesa, Y.; García-Piqueras, J.; Cobo, R.; Martín-Cruces, J.; Suazo, I.; García-Suárez, O.; Feito, J.; Vega, J.A. Sensory innervation of the human male prepuce: Meissner’s corpuscles predominate. J. Anat. 2021, 239, 892–902. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Eid, E.S.; Kurban, M.S. A Piez-o the jigsaw: The Piezo1 channel in skin biology. Clin. Exp. Dermatol. 2022, 47, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cruces, J.; Martín-Biedma, B.; García-Mesa, Y.; Cuendias, P.; Gaite, J.J.; García-Suárez, O.; Cobo, J.L.; Vega, J.A. Exploring somatosensory innervation of the human lip: A focus con the vermilion. Ann. Anat. Anat. Anz. 2023, 250, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, Y.; Duenas-Bianchi, L.F.; Lumpkin, E.A. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci. Rep. 2018, 8, 9975. [Google Scholar] [CrossRef]
- Holt, J.R.; Zeng, W.Z.; Evans, E.L.; Woo, S.H.; Ma, S.; Abuwarda, H.; Loud, M.; Patapoutian, A.; Pathak, M.M. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. Elife 2021, 10, 65415. [Google Scholar] [CrossRef]
- Mikesell, A.R.; Isaeva, O.; Moehring, F.; Sadler, K.E.; Menzel, A.D.; Stucky, C.L. Keratinocyte PIEZO1 modulates cutaneous mechanosensation. Elife 2022, 11, 65987. [Google Scholar] [CrossRef]
- Johnson, K.O. The roles and functions of cutaneous mechanoreceptors. Curr. Opin. Neurobiol. 2001, 11, 455–461. [Google Scholar] [CrossRef]
- Jones, L.A.; Smith, A.M. Tactile sensory system: Encoding from the periphery to the cortex. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 279–287. [Google Scholar] [CrossRef]
- Hastings, R.L.; Valdez, G. Origin, identity, and function of terminal Schwann cells. Trends Neurosci. 2024, 47, 432–446. [Google Scholar] [CrossRef]
- Cobo, R.; García-Piqueras, J.; García-Mesa, Y.; Feito, J.; García-Suárez, O.; Vega, J.A. Peripheral Mechanobiology of Touch–Studies on Vertebrate Cutaneous Sensory Corpuscles. Int. J. Mol. Sci. 2020, 21, 6221. [Google Scholar] [CrossRef]
- Jannini, E.A.; Rubio-Casillas, A.; Whipple, B.; Buisson, O.; Komisaruk, B.R.; Brody, S. Female orgasm(s): One, two, several. J. Sex. Med. 2011, 9, 956–965. [Google Scholar] [CrossRef]
- Pauls, R.N. Anatomy of the clitoris and the female sexual response. Clin. Anat. 2015, 28, 376–384. [Google Scholar] [CrossRef]
- Song, Y.B.; Hwang, K.; Kim, D.J.; Han, S.H. Innervation of vagina: Microdissection and immunohistochemical study. J. Sex. Marital. Ther. 2009, 35, 144–153. [Google Scholar] [CrossRef]
- Baskin, L.S.; Erol, A.; Li, Y.W.; Liu, W.H.; Kurzrock, E.; Cunha, G.R. Anatomical studies of the human clitoris. J. Urol. 1999, 162 Pt 2, 1015–1020. [Google Scholar] [CrossRef]
- Catania, L.; Abdulcadir, O.; Puppo, V.; Verde, J.B.; Abdulcadir, J.; Abdulcadir, D. Pleasure and orgasm in women with Female Genital Mutilation/Cutting (FGM/C). J. Sex. Med. 2007, 4, 1666–1678. [Google Scholar] [CrossRef]
- O’Connell, H.E.; Anderson, C.R.; Plenter, R.J.; Hutson, J.M. The clitoris: A unified structure. Histology of the clitoral glans, body, crura and bulbs. Urodiynamics. 2004, 14, 127–132. [Google Scholar]
- Basson, R. Human sexual response. Handb. Clin. Neurol. 2015, 130, 11–18. [Google Scholar] [CrossRef]
- Yeung, J.; Pauls, R.N. Anatomy of the Vulva and the Female Sexual Response. Obstet. Gynecol. Clin. N. Am. 2016, 43, 27–44. [Google Scholar] [CrossRef]
- Granville, L.; Pregler, J. Women’s Sexual Health and Aging. J. Am. Geriatr. Soc. 2018, 66, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Cold, C.J.; Yang, C.C. Cutaneous corpuscular receptors of the human glans clitoris: Descriptive characteristics and comparison with the glans penis. J. Sex. Med. 2013, 10, 1783–1789. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuendias, P.; Vega, J.A.; García-Suárez, O.; Suazo, I.; Cobo, R.; García-Piqueras, J.; García-Mesa, Y. Axonal and Glial PIEZO1 and PIEZO2 Immunoreactivity in Human Clitoral Krause’s Corpuscles. Int. J. Mol. Sci. 2024, 25, 6722. https://doi.org/10.3390/ijms25126722
Cuendias P, Vega JA, García-Suárez O, Suazo I, Cobo R, García-Piqueras J, García-Mesa Y. Axonal and Glial PIEZO1 and PIEZO2 Immunoreactivity in Human Clitoral Krause’s Corpuscles. International Journal of Molecular Sciences. 2024; 25(12):6722. https://doi.org/10.3390/ijms25126722
Chicago/Turabian StyleCuendias, Patricia, José A. Vega, Olivia García-Suárez, Iván Suazo, Ramón Cobo, Jorge García-Piqueras, and Yolanda García-Mesa. 2024. "Axonal and Glial PIEZO1 and PIEZO2 Immunoreactivity in Human Clitoral Krause’s Corpuscles" International Journal of Molecular Sciences 25, no. 12: 6722. https://doi.org/10.3390/ijms25126722
APA StyleCuendias, P., Vega, J. A., García-Suárez, O., Suazo, I., Cobo, R., García-Piqueras, J., & García-Mesa, Y. (2024). Axonal and Glial PIEZO1 and PIEZO2 Immunoreactivity in Human Clitoral Krause’s Corpuscles. International Journal of Molecular Sciences, 25(12), 6722. https://doi.org/10.3390/ijms25126722






