Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats
Abstract
1. Introduction
2. Results
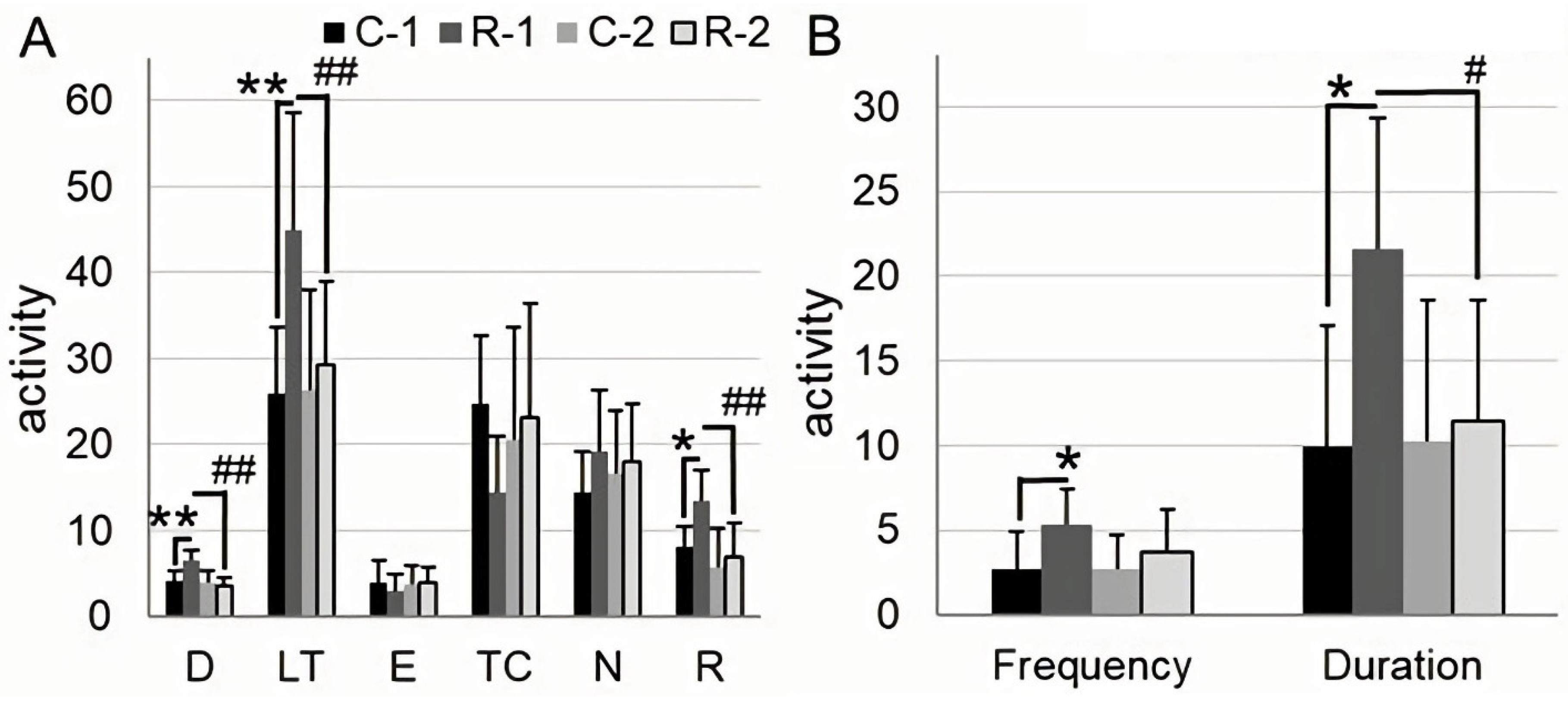
2.1. Irradiation Increases Locomotor Activity, Anxiety, and Exploratory Behavior
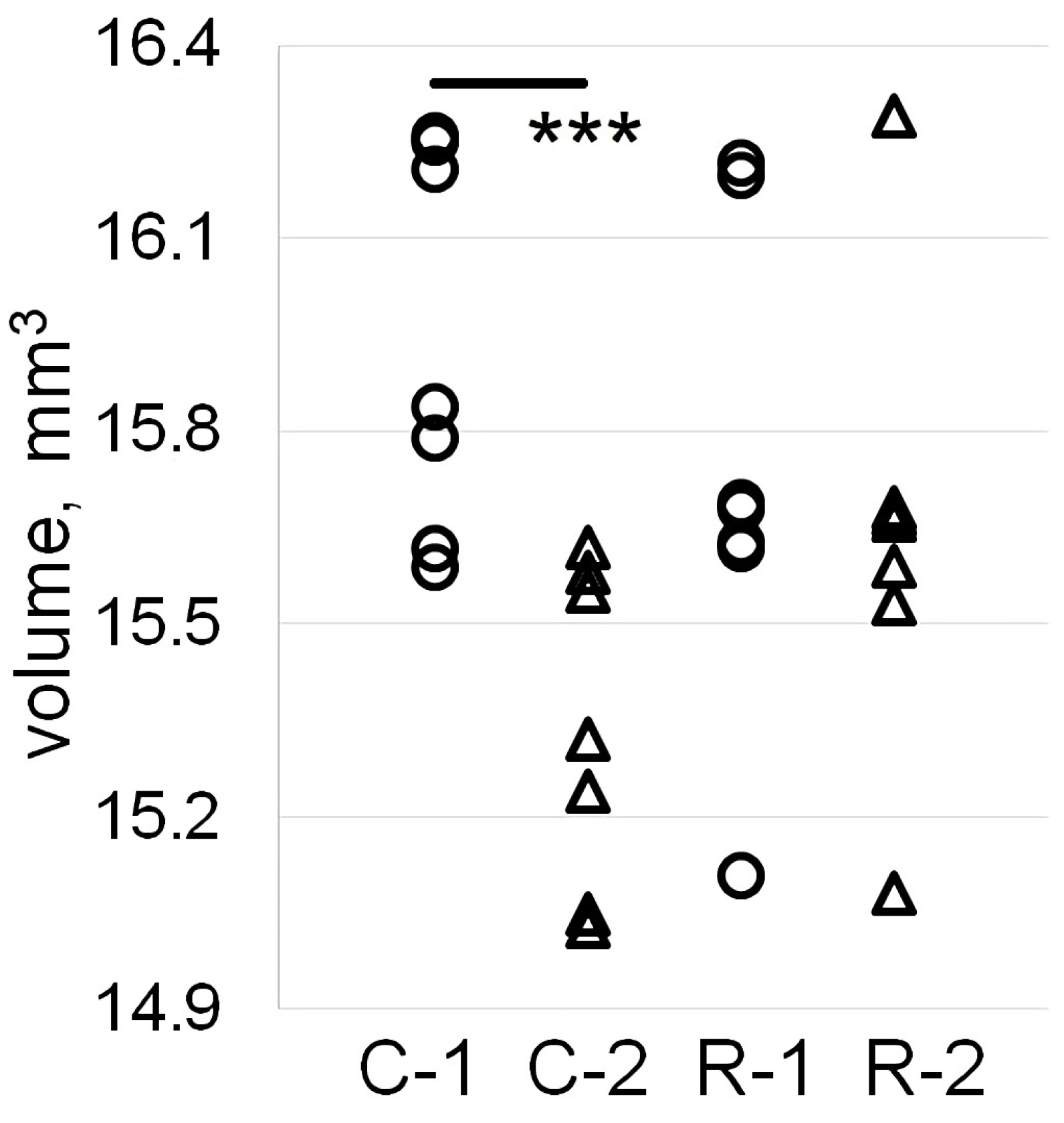
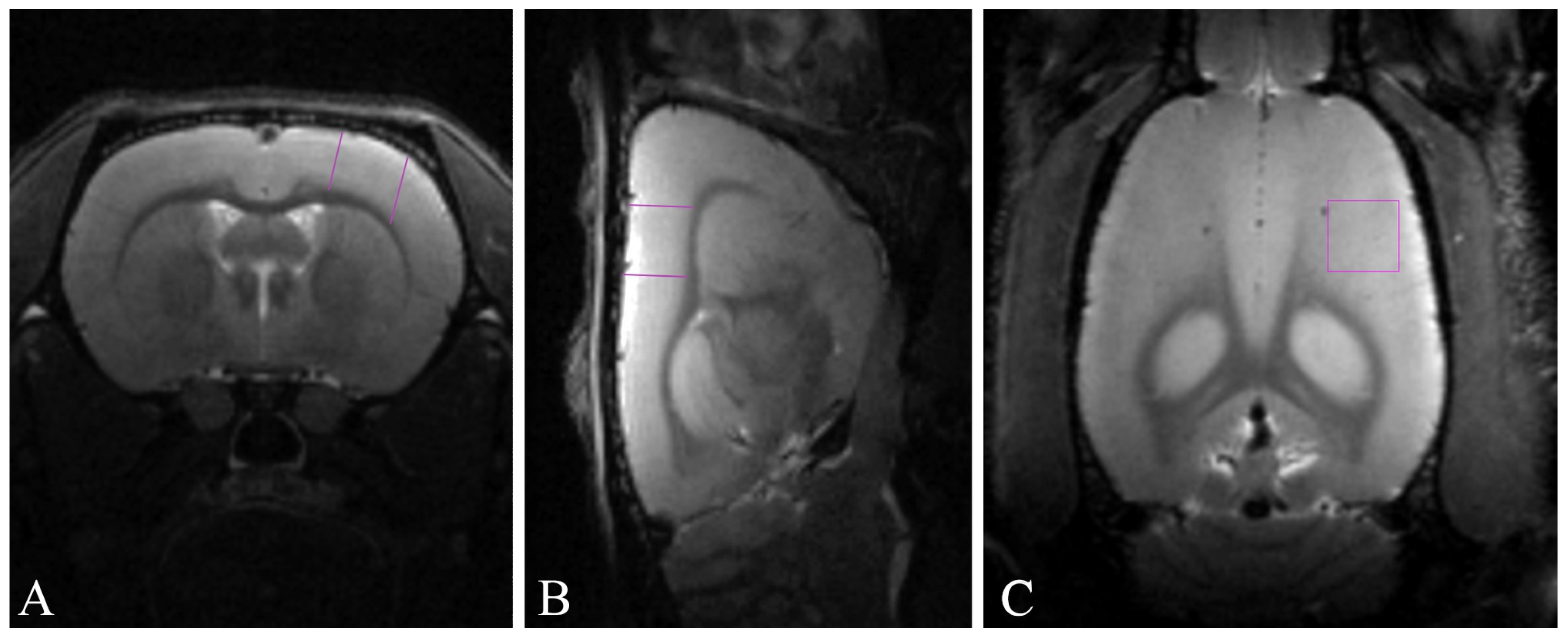
2.2. Sensorimotor Cortex Volume Is Reduced in the Intact, but Not in the Irradiated Rats through Aging
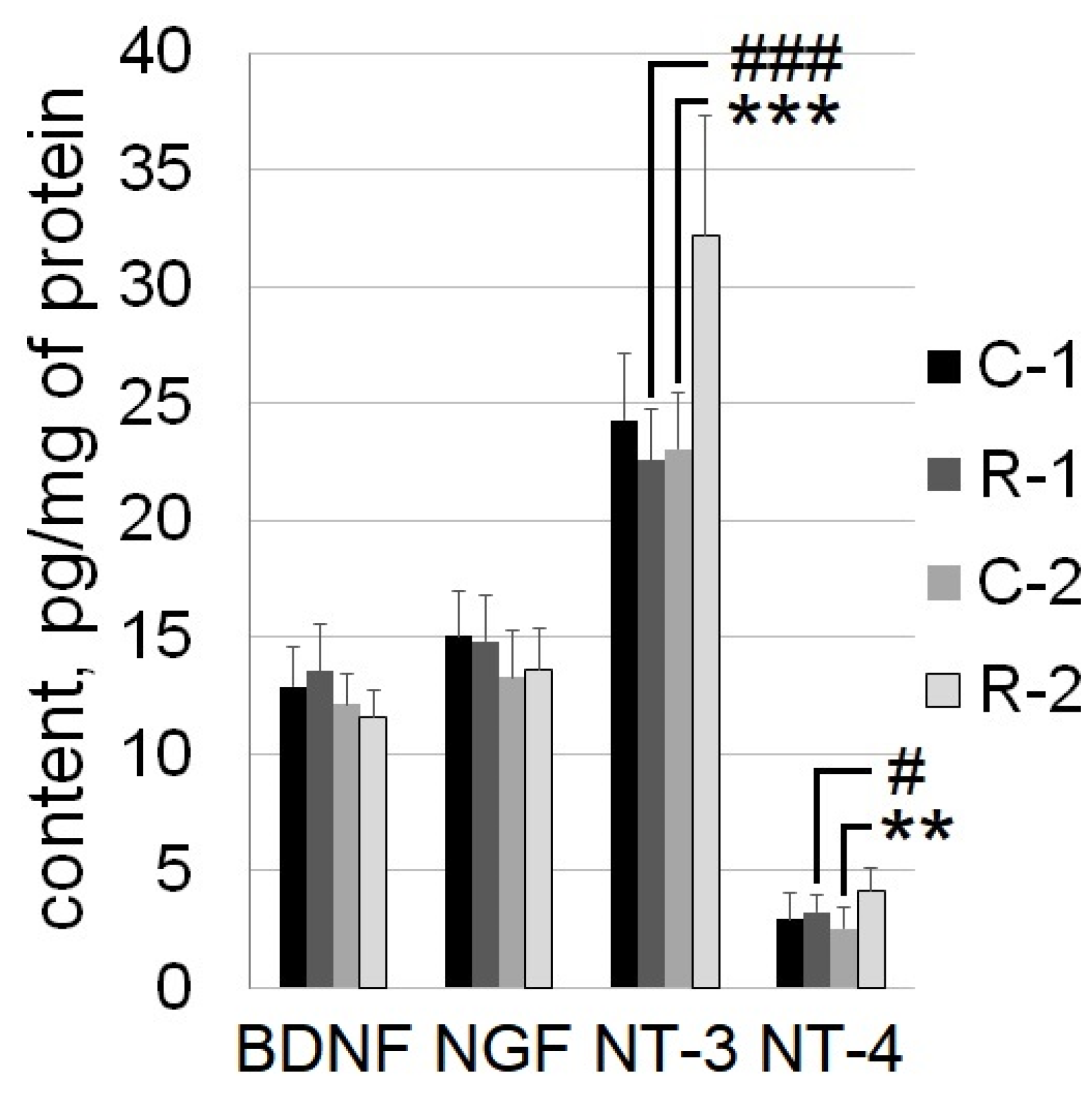
2.3. The Content of NT-3 and NT-4 in the Sensorimotor Cortex Increased 7 Months after the Irradiation of Rats
3. Discussion
4. Materials and Methods
4.1. Animals
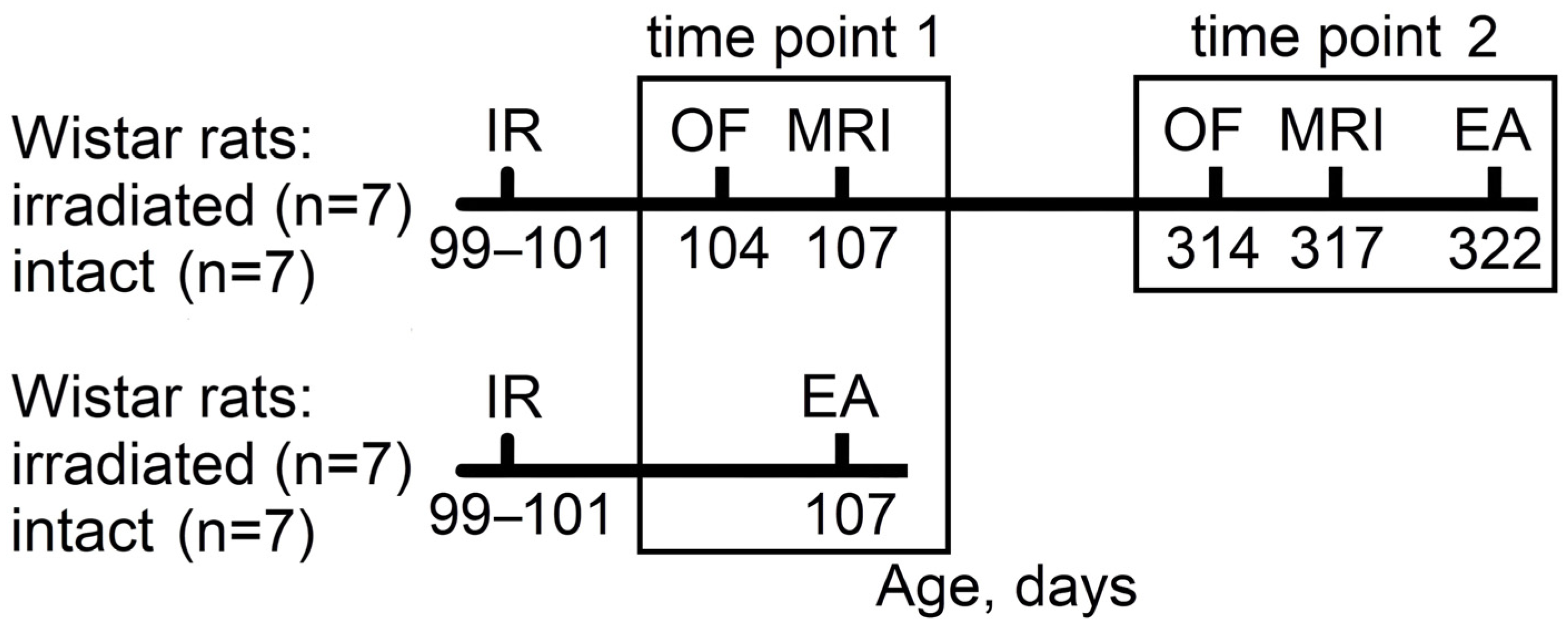
4.2. Study Timeline
4.3. Irradiation Procedures and Dosimetry
4.4. Open Field Test with Object Exploration
4.5. Magnetic Resonance Imaging-Based Morphometry
4.6. Tissue Collection
4.7. Multiplex Assay
4.8. Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokhan, V.S.; Dobynde, M.I. The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter. Biology 2023, 12, 400. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Shakhbazian, E.V.; Markova, N.A. Psycho-emotional status but not cognition are changed under the combined effect of ionizing radiations at doses related to deep space missions. Behav. Brain Res. 2019, 362, 311–318. [Google Scholar] [CrossRef]
- Belov, O.V.; Belokopytova, K.V.; Bazyan, A.S.; Kudrin, V.S.; Narkevich, V.B.; Ivanov, A.A.; Severiukhin, Y.S.; Timoshenko, G.N.; Krasavin, E.A. Exposure to (12)C particles alters the normal dynamics of brain monoamine metabolism and behaviour in rats. Phys. Medica 2016, 32, 1088–1094. [Google Scholar] [CrossRef]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Lebedeva-Georgievskaya, K.B.; Kudrin, V.S.; Bazyan, A.S.; Maltsev, A.V.; Shtemberg, A.S. An investigation of the single and combined effects of hypogravity and ionizing radiation on brain monoamine metabolism and rats’ behavior. Life Sci. Space Res. 2019, 20, 12–19. [Google Scholar] [CrossRef]
- Schroeder, M.K.; Liu, B.; Hinshaw, R.G.; Park, M.-A.; Wang, S.; Dubey, S.; Liu, G.G.; Shi, Q.; Holton, P.; Reiser, V.; et al. Long-Term Sex- and Genotype-Specific Effects of 56Fe Irradiation on Wild-Type and APPswe/PS1dE9 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 13305. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Cantuti-Castelvetri, I.; Rabin, B.M.; Joseph, J.A. The effects of heavy particle irradiation on exploration and response to environmental change. Adv. Space Res. 2004, 33, 1340–1346. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Haerich, P.; Miller, C.N.; Smith, A.L.; Zendejas, E.D.; Nelson, G.A. The effects of low-dose, high-LET radiation exposure on three models of behavior in C57BL/6 mice. Radiat. Res. 2004, 162, 148–156. [Google Scholar] [CrossRef]
- Kolesnikova, I.A.; Budennay, N.N.; Severiukhin, Y.S.; Lyakhova, K.N.; Utina, D.M. Analysis of morphofunctional state of experimental animals brain fields under proton irradiation over the long period. J. New Med. Technol. 2018, 25, 177–181. [Google Scholar]
- Kokhan, V.S.; Matveeva, M.I.; Bazyan, A.S.; Kudrin, V.S.; Mukhametov, A.; Shtemberg, A.S. Combined effects of antiorthostatic suspension and ionizing radiation on the behaviour and neurotransmitters changes in different brain structures of rats. Behav. Brain Res. 2017, 320, 473–483. [Google Scholar] [CrossRef]
- Ung, M.-C.; Garrett, L.; Dalke, C.; Leitner, V.; Dragosa, D.; Hladik, D.; Neff, F.; Wagner, F.; Zitzelsberger, H.; Miller, G.; et al. Dose-dependent long-term effects of a single radiation event on behaviour and glial cells. Int. J. Radiat. Biol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Rappaport, M.B.; Corbally, C.J. Neuroplasticity as a Foundation for Decision-Making in Space. NeuroSci 2022, 3, 457–475. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Fan, Q.; Zhao, S.; Wu, X.; Wang, J.; Liu, Y.; Li, Y.; Lu, W. Long-term spaceflight composite stress induces depression and cognitive impairment in astronauts—Insights from neuroplasticity. Transl. Psychiatry 2023, 13, 342. [Google Scholar] [CrossRef]
- Pearson-Fuhrhop, K.M.; Cramer, S.C. Genetic Influences on Neural Plasticity. PMR 2010, 2, S227–S240. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef]
- Koppelmans, V.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain structural plasticity with spaceflight. npj Microgravity 2016, 2, 2. [Google Scholar] [CrossRef]
- Roberts, D.R.; Zhu, X.; Tabesh, A.; Duffy, E.W.; Ramsey, D.A.; Brown, T.R. Structural Brain Changes following Long-Term 6° Head-Down Tilt Bed Rest as an Analog for Spaceflight. Am. J. Neuroradiol. 2015, 36, 2048–2054. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Matveeva, M.I.; Mukhametov, A.; Shtemberg, A.S. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef]
- Sweet, T.B.; Panda, N.; Hein, A.M.; Das, S.L.; Hurley, S.D.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Central nervous system effects of whole-body proton irradiation. Radiat. Res. 2014, 182, 18–34. [Google Scholar] [CrossRef]
- Zanni, G.; Deutsch, H.M.; Rivera, P.D.; Shih, H.Y.; LeBlanc, J.A.; Amaral, W.Z.; Lucero, M.J.; Redfield, R.L.; DeSalle, M.J.; Chen, B.P.C.; et al. Whole-Body (12)C Irradiation Transiently Decreases Mouse Hippocampal Dentate Gyrus Proliferation and Immature Neuron Number, but Does Not Change New Neuron Survival Rate. Int. J. Mol. Sci. 2018, 19, 3078. [Google Scholar] [CrossRef]
- Rola, R.; Fishman, K.; Baure, J.; Rosi, S.; Lamborn, K.R.; Obenaus, A.; Nelson, G.A.; Fike, J.R. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat. Res. 2008, 169, 626–632. [Google Scholar] [CrossRef]
- Raber, J.; Allen, A.R.; Sharma, S.; Allen, B.; Rosi, S.; Olsen, R.H.; Davis, M.J.; Eiwaz, M.; Fike, J.R.; Nelson, G.A. Effects of Proton and Combined Proton and (56)Fe Radiation on the Hippocampus. Radiat. Res. 2016, 185, 20–30. [Google Scholar] [CrossRef]
- Whoolery, C.W.; Walker, A.K.; Richardson, D.R.; Lucero, M.J.; Reynolds, R.P.; Beddow, D.H.; Clark, K.L.; Shih, H.Y.; LeBlanc, J.A.; Cole, M.G.; et al. Whole-Body Exposure to (28)Si-Radiation Dose-Dependently Disrupts Dentate Gyrus Neurogenesis and Proliferation in the Short Term and New Neuron Survival and Contextual Fear Conditioning in the Long Term. Radiat. Res. 2017, 188, 532–551. [Google Scholar] [CrossRef]
- Eom, H.S.; Park, H.R.; Jo, S.K.; Kim, Y.S.; Moon, C.; Kim, S.H.; Jung, U. Ionizing Radiation Induces Altered Neuronal Differentiation by mGluR1 through PI3K-STAT3 Signaling in C17.2 Mouse Neural Stem-Like Cells. PLoS ONE 2016, 11, e0147538. [Google Scholar] [CrossRef]
- Katoh, S.; Kobayashi, J.; Umeda, T.; Kobayashi, Y.; Izumo, N.; Suzuki, T. Xray Irradiation Promotes Nerve Growth Factor-induced Neurite Extension in PC12 Cells. Radioisotopes 2016, 65, 137–143. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic Potential of Neurotrophins for Repair After Brain Injury: A Helping Hand From Biomaterials. Front. Neurosci. 2019, 13, 790. [Google Scholar] [CrossRef]
- Dimberg, Y.; Vazquez, M.; Soderstrom, S.; Ebendal, T. Effects of X-irradiation on nerve growth factor in the developing mouse brain. Toxicol. Lett. 1997, 90, 35–43. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Kawa, M.P.; Klos, P.; Roginska, D.; Rudnicki, M.; Boehlke, M.; Waloszczyk, P.; Machalinski, B. Alteration of Selected Neurotrophic Factors and their Receptor Expression in Mouse Brain Response to Whole-Brain Irradiation. Radiat. Res. 2016, 186, 489–507. [Google Scholar] [CrossRef]
- Raber, J.; Fuentes Anaya, A.; Torres, E.R.S.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.D.; Sharpton, T.J.; Turker, M.S.; et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kulikov, A.V.; Kondaurova, E.M.; Tsybko, A.S.; Kulikova, E.A.; Krasnov, I.B.; Shenkman, B.S.; Sychev, V.N.; Bazhenova, E.Y.; Sinyakova, N.A.; et al. Effect of actual long-term spaceflight on BDNF, TrkB, p75, BAX and BCL-XL genes expression in mouse brain regions. Neuroscience 2015, 284, 730–736. [Google Scholar] [CrossRef]
- Sandi, C.; Toledo-Rodriguez, M. Stress during Adolescence Increases Novelty Seeking and Risk-Taking Behavior in Male and Female Rats. Front. Behav. Neurosci. 2011, 5, 9893. [Google Scholar] [CrossRef]
- Minassian, A.; Kelsoe, J.R.; Miranda, A.; Young, J.W.; Perry, W. The relationship between novelty-seeking traits and behavior: Establishing construct validity for the human Behavioral Pattern Monitor. Psychiatry Res. 2022, 316, 114776. [Google Scholar] [CrossRef]
- Kabbaj, M.; Devine, D.P.; Savage, V.R.; Akil, H. Neurobiological Correlates of Individual Differences in Novelty-Seeking Behavior in the Rat: Differential Expression of Stress-Related Molecules. J. Neurosci. 2000, 20, 6983–6988. [Google Scholar] [CrossRef]
- Sara, S.J.; Dyon-Laurent, C.; Hervé, A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Cogn. Brain Res. 1995, 2, 181–187. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Mariasina, S.; Pikalov, V.A.; Abaimov, D.A.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Neurokinin-1 Receptor Antagonist Reverses Functional CNS Alteration Caused by Combined gamma-rays and Carbon Nuclei Irradiation. CNS Neurol. Disord. Drug Targets 2022, 21, 278–289. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Yaxley, R.; Paniagua, B.; Johnson, G.A.; Crews, F.T. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addict. Biol. 2017, 22, 712–723. [Google Scholar] [CrossRef]
- Demertzi, A.; Van Ombergen, A.; Tomilovskaya, E.; Jeurissen, B.; Pechenkova, E.; Di Perri, C.; Litvinova, L.; Amico, E.; Rumshiskaya, A.; Rukavishnikov, I.; et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct. Funct. 2016, 221, 2873–2876. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Anokhin, P.K.; Belov, O.V.; Gulyaev, M.V. Cortical Glutamate/GABA Imbalance after Combined Radiation Exposure: Relevance to Human Deep-Space Missions. Neuroscience 2019, 416, 295–308. [Google Scholar] [CrossRef]
- Bashir, S.; Al-Sultan, F.; Jamea, A.A.; Almousa, A.; Alnafisah, M.; Alzahrani, M.; Abualait, T.; Yoo, W.-K. Physical exercise keeps the brain connected by increasing white matter integrity in healthy controls. Medicine 2021, 100, e27015. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35, S20–S28. [Google Scholar] [CrossRef]
- Sumiyoshi, A.; Taki, Y.; Nonaka, H.; Takeuchi, H.; Kawashima, R. Regional gray matter volume increases following 7 days of voluntary wheel running exercise: A longitudinal VBM study in rats. NeuroImage 2014, 98, 82–90. [Google Scholar] [CrossRef]
- Eyme, K.; Domin, M.; Lotze, M. PB 9 Exercise increases right motor cortex volume in a VBM-study of a representative cohort. Clin. Neurophysiol. 2017, 128, e317–e318. [Google Scholar] [CrossRef]
- Saha, B.; Peron, S.; Murray, K.; Jaber, M.; Gaillard, A. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013, 11, 965–977. [Google Scholar] [CrossRef]
- Chang, E.H.; Adorjan, I.; Mundim, M.V.; Sun, B.; Dizon, M.L.; Szele, F.G. Traumatic Brain Injury Activation of the Adult Subventricular Zone Neurogenic Niche. Front. Neurosci. 2016, 10, 332. [Google Scholar] [CrossRef]
- Ohira, K. Regulation of adult neurogenesis in the cerebral cortex. J. Neurol. Neuromedicine 2018, 3, 59–64. [Google Scholar] [CrossRef]
- Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.K.; Sun, W.; Yu, S.W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, H.; Gleichman, A.; Machnicki, M.; Telang, S.; Tang, S.; Rshtouni, M.; Ruddell, J.; Carmichael, S.T. Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 13621–13630. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Miller, F.D. Endogenously Produced Neurotrophins Regulate Survival and Differentiation of Cortical Progenitors via Distinct Signaling Pathways. J. Neurosci. 2003, 23, 5149–5160. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Opposing Roles for Endogenous BDNF and NT-3 in Regulating Cortical Dendritic Growth. Neuron 1997, 18, 767–778. [Google Scholar] [CrossRef]
- Turnley, A.M.; Basrai, H.S.; Christie, K.J. Is integration and survival of newborn neurons the bottleneck for effective neural repair by endogenous neural precursor cells? Front. Neurosci. 2014, 8, 29. [Google Scholar] [CrossRef]
- Ghosh, A.; Greenberg, M.E. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron 1995, 15, 89–103. [Google Scholar] [CrossRef]
- Miyashita, T.; Wintzer, M.; Kurotani, T.; Konishi, T.; Ichinohe, N.; Rockland, K.S. Neurotrophin-3 is involved in the formation of apical dendritic bundles in cortical layer 2 of the rat. Cereb Cortex 2010, 20, 229–240. [Google Scholar] [CrossRef]
- Kakanos, S.G.; Moon, L.D.F. Delayed peripheral treatment with neurotrophin-3 improves sensorimotor recovery after central nervous system injury. Neural Regen. Res. 2019, 14, 1703–1704. [Google Scholar] [CrossRef]
- Duricki, D.A.; Drndarski, S.; Bernanos, M.; Wood, T.; Bosch, K.; Chen, Q.; Shine, H.D.; Simmons, C.; Williams, S.C.R.; McMahon, S.B.; et al. Stroke Recovery in Rats after 24-Hour-Delayed Intramuscular Neurotrophin-3 Infusion. Ann. Neurol. 2019, 85, 32–46. [Google Scholar] [CrossRef]
- Kim, H.G.; Wang, T.; Olafsson, P.; Lu, B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 12341–12345. [Google Scholar] [CrossRef]
- Fan, G.; Egles, C.; Sun, Y.; Minichiello, L.; Renger, J.J.; Klein, R.; Liu, G.; Jaenisch, R. Knocking the NT4 gene into the BDNF locus rescues BDNF deficient mice and reveals distinct NT4 and BDNF activities. Nat. Neurosci. 2000, 3, 350–357. [Google Scholar] [CrossRef]
- Proenca, C.C.; Song, M.; Lee, F.S. Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors. J. Neurochem. 2016, 138, 397–406. [Google Scholar] [CrossRef]
- Wirth, M.J.; Brun, A.; Grabert, J.; Patz, S.; Wahle, P. Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development 2003, 130, 5827–5838. [Google Scholar] [CrossRef]
- Gao, W.Q.; Zheng, J.L.; Karihaloo, M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J. Neurosci. 1995, 15, 2656–2667. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Li, P.; Ding, Y.; Tang, J.; Chen, G.; Zhang, J.H. NT-4 attenuates neuroinflammation via TrkB/PI3K/FoxO1 pathway after germinal matrix hemorrhage in neonatal rats. J. Neuroinflamm. 2020, 17, 158. [Google Scholar] [CrossRef]
- Bendlin, B.B.; Newman, L.M.; Ries, M.L.; Puglielli, L.; Carlsson, C.M.; Sager, M.A.; Rowley, H.A.; Gallagher, C.L.; Willette, A.A.; Alexander, A.L.; et al. NSAIDs may protect against age-related brain atrophy. Front. Aging Neurosci. 2010, 2, 1867. [Google Scholar] [CrossRef]
- Pitts, A.F.; Miller, M.W. Expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 in the somatosensory cortex of the mature rat: Coexpression with high-affinity neurotrophin receptors. J. Comp. Neurol. 2000, 418, 241–254. [Google Scholar] [CrossRef]
- Pariset, E.; Malkani, S.; Cekanaviciute, E.; Costes, S.V. Ionizing radiation-induced risks to the central nervous system and countermeasures in cellular and rodent models. Int. J. Radiat. Biol. 2020, 97, S132–S150. [Google Scholar] [CrossRef]
- Marshall, G.P., 2nd; Scott, E.W.; Zheng, T.; Laywell, E.D.; Steindler, D.A. Ionizing radiation enhances the engraftment of transplanted in vitro-derived multipotent astrocytic stem cells. Stem Cells 2005, 23, 1276–1285. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef]
- Bevelacqua, J.J.; Welsh, J.; Mortazavi, S.M.J. Comments on “New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose Rate, Neutron Radiation”. eNeuro 2020, 7, ENEURO.0329-19.2019. [Google Scholar] [CrossRef]
- Lezniak, J.A.; Webber, W.R. The charge composition and energy spectra of cosmic-ray nuclei from 3000 MeV per nucleon to 50 GeV per nucleon. Astrophys. J. 1978, 223, 676–696. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Aunon-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018, 4, 8. [Google Scholar] [CrossRef]
- Al Zaman, M.A.; Nizam, Q.M.R. Study on Shielding Effectiveness of a Combined Radiation Shield for Manned Long Termed Interplanetary Expeditions. J. Space Saf. Eng. 2022, 9, 83–89. [Google Scholar] [CrossRef]
- Task Group on Radiation Protection in Space, I.C.; Dietze, G.; Bartlett, D.T.; Cool, D.A.; Cucinotta, F.A.; Jia, X.; McAulay, I.R.; Pelliccioni, M.; Petrov, V.; Reitz, G.; et al. ICRP, 123. Assessment of radiation exposure of astronauts in space. ICRP Publication 123. Ann ICRP 2013, 42, 1–339. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schwarz, A.J.; Danckaert, A.; Reese, T.; Gozzi, A.; Paxinos, G.; Watson, C.; Merlo-Pich, E.V.; Bifone, A. A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: Application to pharmacological MRI. NeuroImage 2006, 32, 538–550. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Amsterdam, The Netherlands; Elsevier: Boston, MA, USA, 2007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhan, V.S.; Pikalov, V.A.; Chaprov, K.; Gulyaev, M.V. Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats. Int. J. Mol. Sci. 2024, 25, 6725. https://doi.org/10.3390/ijms25126725
Kokhan VS, Pikalov VA, Chaprov K, Gulyaev MV. Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats. International Journal of Molecular Sciences. 2024; 25(12):6725. https://doi.org/10.3390/ijms25126725
Chicago/Turabian StyleKokhan, Viktor S., Vladimir A. Pikalov, Kirill Chaprov, and Mikhail V. Gulyaev. 2024. "Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats" International Journal of Molecular Sciences 25, no. 12: 6725. https://doi.org/10.3390/ijms25126725
APA StyleKokhan, V. S., Pikalov, V. A., Chaprov, K., & Gulyaev, M. V. (2024). Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats. International Journal of Molecular Sciences, 25(12), 6725. https://doi.org/10.3390/ijms25126725





