IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
2. Results
2.1. Blocking of IL-6 Signaling Did Not Ameliorate EAE Clinical Outcome in Aged Mice
2.2. EAE Clinical Parameters at 28 Days Postimmunization in Young and Aged Mice
2.3. IL-6 Inhibition Enhances Peripheral Immunosuppressive Response in EAE Aged Mice
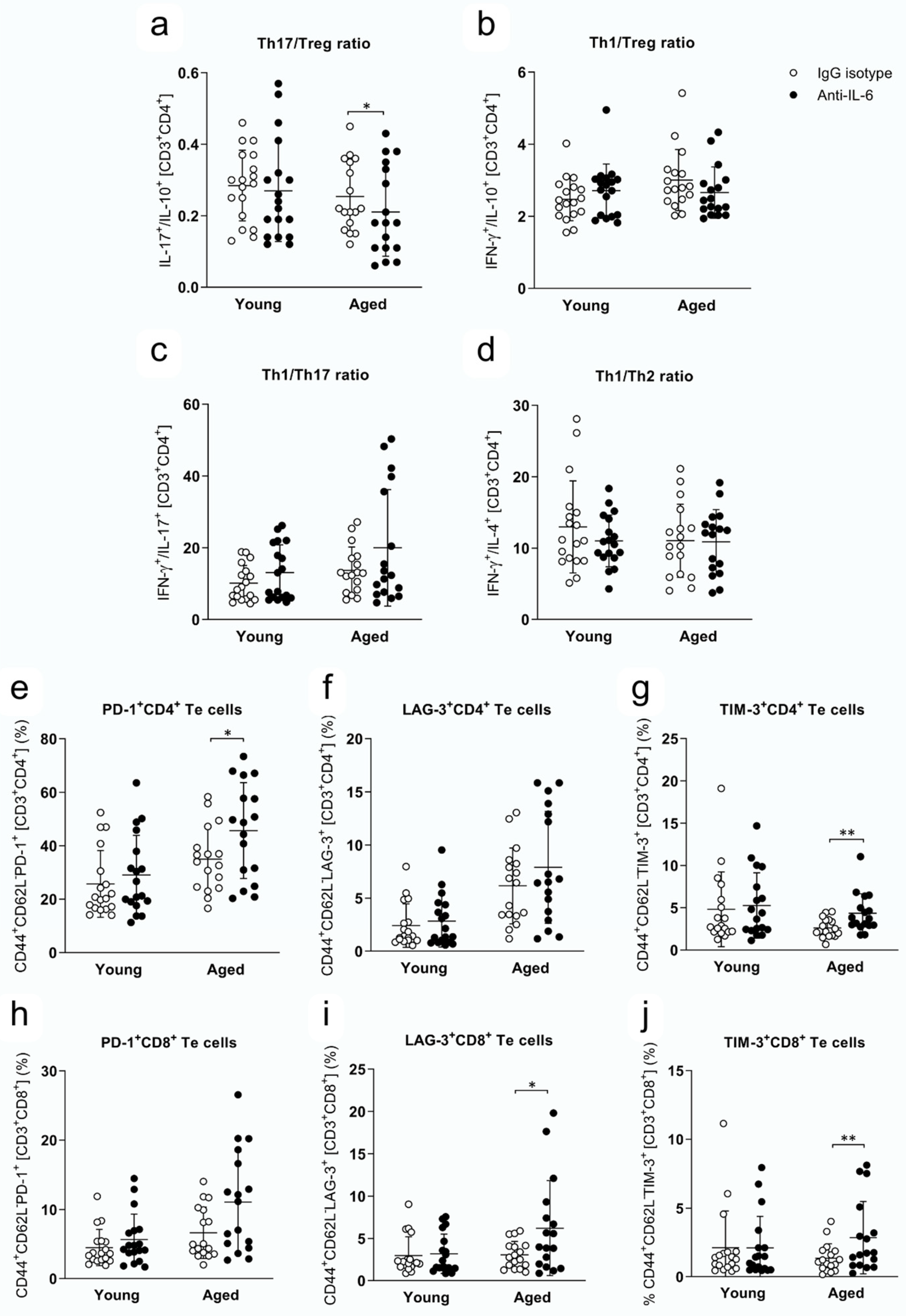
2.4. Th Responses and Expression of Immune Checkpoint Inhibitors in Te Cells at 28 dpi in Young and Aged Mice Treated with Anti-IL-6 Antibody or IgG Isotype
2.5. CD8+ T, NKT and NK Cell Cytotoxic Capacity at 28 dpi in Young and Aged Mice Treated with IgG Isotype or Anti-IL-6 Antibody
3. Discussion
4. Materials and Methods
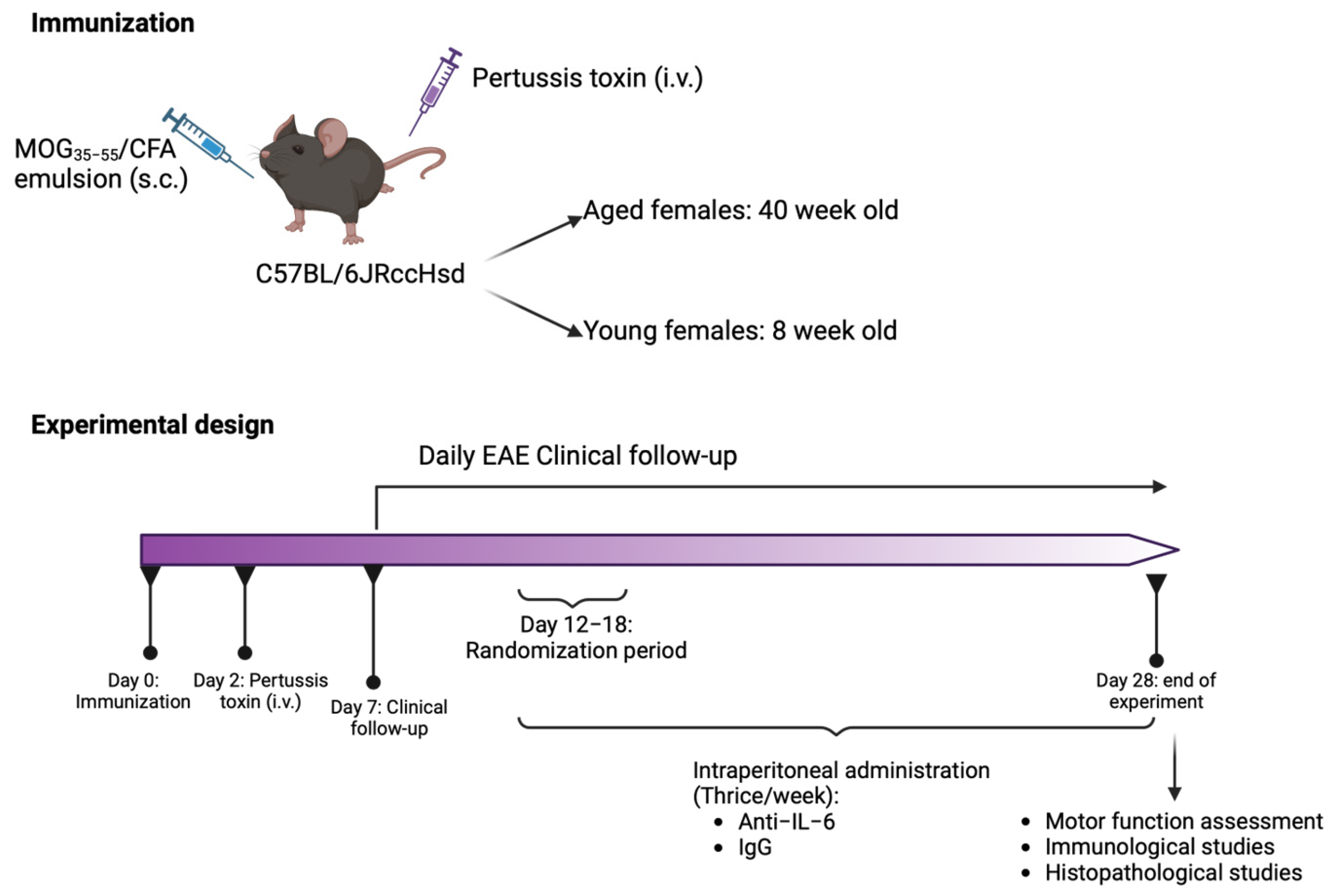
4.1. Mice
4.2. EAE Induction and Clinical Follow-Up
4.3. Experimental Treatment
4.4. Motor Function Assessment
4.5. Histopathological Analysis
4.6. Peripheral Immune Response Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Morrisette-Thomas, V.; Cohen, A.A.; Fulop, T.; Riesco, E.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Belanger, F.; Ferrucci, L. Inflammaging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef]
- Ireland, S.J.; Monson, N.L.; Davis, L.S. Seeking balance: Potentiation and inhibition of multiple sclerosis autoimmune responses by IL-6 and IL-10. Cytokine 2015, 73, 236–244. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Daumer, M.; Ebers, G.C.; Muraro, P.A. Age and disability accumulation in multiple sclerosis. Neurology 2011, 77, 1246–1252. [Google Scholar] [CrossRef]
- Vaughn, C.B.; Jakimovski, D.; Kavak, K.S.; Ramanathan, M.; Benedict, R.H.B.; Zivadinov, R.; Weinstock-Guttman, B. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat. Rev. Neurol. 2019, 15, 329–342. [Google Scholar] [CrossRef]
- Dema, M.; Eixarch, H.; Villar, L.M.; Montalban, X.; Espejo, C. Immunosenescence in multiple sclerosis: The identification of new therapeutic targets. Autoimmun. Rev. 2021, 20, 102893. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2016, 36, 115–127. [Google Scholar] [CrossRef]
- Dema, M.; Eixarch, H.; Castillo, M.; Villar, L.M.; Montalban, X.; Espejo, C. Disease aggravation with age in an experimental model of multiple sclerosis: Role of immunosenescence. Aging Cell, 2024; submitted. [Google Scholar]
- Awad, A.; Stuve, O. Multiple sclerosis in the elderly patient. Drugs Aging 2010, 27, 283–294. [Google Scholar] [CrossRef]
- Okuda, Y.; Sakoda, S.; Bernard, C.C.; Fujimura, H.; Saeki, Y.; Kishimoto, T.; Yanagihara, T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998, 10, 703–708. [Google Scholar] [CrossRef]
- Eugster, H.P.; Frei, K.; Kopf, M.; Lassmann, H.; Fontana, A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 1998, 28, 2178–2187. [Google Scholar] [CrossRef]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Moller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. Nat. Immunol. 2017, 18, 74–85. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef]
- Molnarfi, N.; Schulze-Topphoff, U.; Weber, M.S.; Patarroyo, J.C.; Prod’homme, T.; Varrin-Doyer, M.; Shetty, A.; Linington, C.; Slavin, A.J.; Hidalgo, J.; et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 2013, 210, 2921–2937. [Google Scholar] [CrossRef]
- Sanchis, P.; Fernandez-Gayol, O.; Comes, G.; Escrig, A.; Giralt, M.; Palmiter, R.D.; Hidalgo, J. Interleukin-6 Derived from the Central Nervous System May Influence the Pathogenesis of Experimental Autoimmune Encephalomyelitis in a Cell-Dependent Manner. Cells 2020, 9, 330. [Google Scholar] [CrossRef]
- Gijbels, K.; Brocke, S.; Abrams, J.S.; Steinman, L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1995, 1, 795–805. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Mihara, M.; Koike, N.; Ohsugi, Y.; Nomura, S.; Yoshida, H.; Nishikawa, T.; Terabe, F.; Ohkawara, T.; et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9041–9046. [Google Scholar] [CrossRef]
- Brod, S.A.; Bauer, V.L. Ingested (oral) tocilizumab inhibits EAE. Cytokine 2014, 68, 86–93. [Google Scholar] [CrossRef]
- Thiolat, A.; Semerano, L.; Pers, Y.M.; Biton, J.; Lemeiter, D.; Portales, P.; Quentin, J.; Jorgensen, C.; Decker, P.; Boissier, M.C.; et al. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014, 66, 273–283. [Google Scholar] [CrossRef]
- Samson, M.; Greigert, H.; Ciudad, M.; Gerard, C.; Ghesquiere, T.; Trad, M.; Corbera-Bellalta, M.; Genet, C.; Ouandji, S.; Cladiere, C.; et al. Improvement of Treg immune response after treatment with tocilizumab in giant cell arteritis. Clin. Transl. Immunol. 2021, 10, e1332. [Google Scholar] [CrossRef]
- Chandran, S.; Leung, J.; Hu, C.; Laszik, Z.G.; Tang, Q.; Vincenti, F.G. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: A randomized controlled trial. Am. J. Transplant. 2021, 21, 2543–2554. [Google Scholar] [CrossRef]
- Kikuchi, J.; Hashizume, M.; Kaneko, Y.; Yoshimoto, K.; Nishina, N.; Takeuchi, T. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: Increase in regulatory T cells correlates with clinical response. Arthritis Res. Ther. 2015, 17, 10. [Google Scholar] [CrossRef]
- Yoshida, H.; Hashizume, M.; Suzuki, M.; Mihara, M. Anti-IL-6 receptor antibody suppressed T cell activation by inhibiting IL-2 production and inducing regulatory T cells. Eur. J. Pharmacol. 2010, 634, 178–183. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, Q.; Gao, L.; Hou, N.; Zhao, D.; Liu, X.; Zhang, X.; Xu, L.; Yue, X.; Zhu, F.; et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin. Immunol. 2010, 137, 288–295. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Baker, D.; Amor, S. Publication guidelines for refereeing and reporting on animal use in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2012, 242, 78–83. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Edo, A.; Calvo-Barreiro, L.; Eixarch, H.; Bosch, A.; Chillon, M.; Espejo, C. Therapeutic Effect of IL-21 Blockage by Gene Therapy in Experimental Autoimmune Encephalomyelitis. Neurotherapeutics 2022, 19, 1617–1633. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, K.; Guo, J.; Wroblewska, J.; Fu, Y.X.; Peng, H. Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication. Sci. Rep. 2015, 5, 10406. [Google Scholar] [CrossRef]
- Liang, B.; Gardner, D.B.; Griswold, D.E.; Bugelski, P.J.; Song, X.Y. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology 2006, 119, 296–305. [Google Scholar] [CrossRef]
- Barber, D.L.; Andrade, B.B.; McBerry, C.; Sereti, I.; Sher, A. Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome. J. Immunol. 2014, 192, 676–682. [Google Scholar] [CrossRef]

| Parameter | Anti-IL-6 | IgG Isotype | p-Value |
|---|---|---|---|
| Maximum clinical score | young: 3.6 ± 0.8 | young: 4.1 ± 0.3 | 0.1016 |
| aged: 4.2 ± 0.6 | aged: 4.4 ± 0.5 | 1.0000 | |
| Motor coordination (sec) | young: 26.9 ± 18.5 | young: 19.0 ± 8.6 | 0.8482 |
| aged: 8.0 ± 7.1 | aged: 6.4 ± 5.3 | 1.0000 | |
| Clinical score (AUC) | young: 42.3 ± 13.4 | young: 47.9 ± 10.1 | 0.5078 |
| aged: 52.6 ± 11.6 | aged: 56.0 ± 9.3 | 1.0000 | |
| Weight loss (AUC) | young: 38.6 ± 135.7 | young: 58.7 ± 141.7 | 1.0000 |
| aged: −301.7 ± 114.2 | aged: −290.9 ± 124.2 | 1.0000 | |
| Time to score 3 | young: 14/18 (77.78%) | young: 18/18 (100%) | 0.2381 |
| aged: 18/18 (100%) | aged: 18/18 (100%) | 0.8146 | |
| Time to score 4 | young: 14/18 (77.78%) | young: 17/18 (94.44%) | 0.2913 |
| aged: 16/18 (88.89%) | aged: 18/18 (100%) | 0.5464 |
| Population | Anti-IL-6 | IgG Isotype | p-Value |
|---|---|---|---|
| Th1 response | young: 16.05 ± 6.82 | young: 15.13 ± 7.27 | 0.9448 |
| aged: 24.45 ± 8.66 | aged: 19.50 ± 6.52 | 0.2272 | |
| Th2 response | young: 1.71 ± 1.19 | young: 1.47 ± 1.30 | 0.2769 |
| aged: 2.63 ± 1.48 | aged: 2.01 ± 0.80 | 0.2900 | |
| Th17 response | young: 1.51 ± 0.67 | young: 1.63 ± 0.58 | 0.7276 |
| aged: 1.74 ± 0.79 | aged: 1.61 ± 0.59 | 0.7399 | |
| Treg response | young: 6.08 ± 2.32 | young: 6.26 ± 2.88 | 1.0000 |
| aged: 9.50 ± 3.52 | aged: 6.76 ± 2.55 | 0.0387 | |
| Th1/Th17 ratio | young: 13.06 ± 7.72 | young: 10.12 ± 4.97 | 0.2184 |
| aged: 19.22 ± 16.05 | aged: 13.69 ± 6.51 | 0.2875 | |
| Th1/Treg ratio | young: 2.71 ± 0.74 | young: 2.47 ± 0.60 | 0.7924 |
| aged: 2.66 ± 0.71 | aged: 3.00 ± 0.85 | 1.0000 | |
| Th17/Treg ratio | young: 0.27 ± 0.14 | young: 0.28 ± 0.10 | 0.7686 |
| aged: 0.21 ± 0.12 | aged: 0.25 ± 0.10 | 0.0387 | |
| Th1/Th2 ratio | young: 11.01 ± 3.62 | young: 12.97 ± 6.45 | 0.3342 |
| aged: 10.88 ± 4.51 | aged: 11.03 ± 5.12 | 0.1290 | |
| PD-1+CD4+ Te cells | young: 29.03 ± 14.81 | young: 25.68 ± 12.47 | 0.6461 |
| aged: 45.64 ± 17.93 | aged: 34.96 ± 12.13 | 0.0466 | |
| LAG-3+CD4+ Te cells | young: 2.85 ± 2.45 | young: 2.41 ± 2.05 | 1.0000 |
| aged: 7.90 ± 5.27 | aged: 6.15 ± 3.56 | 0.7331 | |
| TIM-3+CD4+ Te cells | young: 5.27 ± 3.87 | young: 4.82 ± 4.42 | 0.9006 |
| aged: 4.34 ± 2.31 | aged: 2.61 ± 1.12 | 0.0052 | |
| PD-1+CD8+ Te cells | young: 5.65 ± 3.68 | young: 4.49 ± 2.63 | 0.6900 |
| aged: 11.10 ± 7.27 | aged: 6.64 ± 3.72 | 0.0563 | |
| LAG-3+CD8+ Te cells | young: 3.18 ± 2.33 | young: 2.97 ± 2.24 | 1.0000 |
| aged: 6.22 ± 5.62 | aged: 3.06 ± 1.60 | 0.0252 | |
| TIM-3+CD8+ Te cells | young: 2.10 ± 2.30 | young: 2.12 ± 2.68 | 1.0000 |
| aged: 2.85 ± 2.64 | aged: 1.36 ± 1.05 | 0.0294 |
| Population | Anti-IL-6 | IgG Isotype | p-Value |
|---|---|---|---|
| Prf+CD8+ T cells | young: 70.97 ± 12.47 | young: 72.24 ± 13.72 | 1.0000 |
| aged: 65.68 ± 19.26 | aged: 70.69 ± 16.75 | 0.3293 | |
| GzmB+CD8+ T cells | young: 41.22 ± 12.07 | young: 48.09 ± 8.95 | 0.1504 |
| aged: 42.72 ± 15.00 | aged: 47.47 ± 11.84 | 0.3689 | |
| Prf+ NKT cells | young: 73.63 ± 14.96 | young: 75.42 ± 15.28 | 1.0000 |
| aged: 78.94 ± 10.34 | aged: 82.17 ± 11.98 | 0.9413 | |
| GzmB+ NKT cells | young: 46.36 ± 13.56 | young: 56.43 ± 14.98 | 0.0129 |
| aged: 49.50 ± 15.10 | aged: 54.26 ± 16.14 | 0.4372 | |
| Prf+ NK cells | young: 96.64 ± 2.92 | young: 97.72 ± 1.39 | 0.6017 |
| aged: 93.66 ± 4.80 | aged: 95.17 ± 3.67 | 0.2974 | |
| GzmB+ NK cells | young: 86.35 ± 8.66 | young: 90.36 ± 6.57 | 0.4755 |
| aged: 76.34 ± 16.38 | aged: 76.30 ± 15.11 | 1.0000 |
| Age | Treatment | Clinical Onset | Days of Disease | Accumulated Score |
|---|---|---|---|---|
| Young I | IgG isotype (n = 9) | 12.9 ± 1.6 | 2.0 ± 0.5 | 3.4 ± 2.0 |
| Anti-IL-6 (n = 9) | 12.9 ± 1.6 | 2.0 ± 0.5 | 3.3 ± 2.3 | |
| Aged I | IgG isotype (n = 9) | 14.3 ± 1.8 | 1.4 ± 0.5 | 2.4 ± 0.7 |
| Anti-IL-6 (n = 9) | 14.1 ± 2.1 | 1.7 ± 0.7 | 2.6 ± 0.8 | |
| Young II | IgG isotype (n = 9) | 13.1 ± 1.5 | 1.8 ± 0.4 | 2.2 ± 0.9 |
| Anti-IL-6 (n = 9) | 12.9 ± 1.5 | 1.9 ± 0.6 | 2.3 ± 0.9 | |
| Aged II | IgG isotype (n = 9) | 13.3 ± 1.1 | 1.3 ± 0.5 | 2.6 ± 0.5 |
| Anti-IL-6 (n = 9) | 13.2 ± 1.0 | 1.4 ± 0.5 | 2.8 ± 1.1 |
| Panel | Antibody | Fluorochrome | Clone | Reference | Manufacturer | Isotype |
|---|---|---|---|---|---|---|
| Proinflammatory cytokine-producing T cells | IFN-γ | BV421 | XMG1.2 | 563376 | BD | Rat IgG1k |
| FVS | BV510 | - | 564406 | BD | - | |
| CD8 | BV605 | 53-6.7 | 563152 | BD | Rat IgG2ak | |
| CD3 | FITC | 145-2C11 | 561827 | BD | Hamster IgG1k | |
| CD4 | PerCP-eF710 | RM4-5 | 46-0042 | Thermo Fisher Scientific | Rat IgG2ak | |
| IL-17a | PE | TC11-18H10 | 561020 | BD | Rat IgG1k | |
| CD69 | APC | H1.2F3 | 560689 | BD | Hamster IgG1λ1 | |
| Anti-inflammatory cytokine-producing T cells | IL-10 | BV421 | JES5-16E3 | 563276 | BD | Rat IgG2bk |
| FVS | BV510 | - | 564406 | BD | - | |
| CD8 | BV605 | 53-6.7 | 563152 | BD | Rat IgG2ak | |
| CD3 | FITC | 145-2C11 | 561827 | BD | Hamster IgG1k | |
| CD4 | PerCP-eF710 | RM4-5 | 46-0042 | Thermo Fisher Scientific | Rat IgG2ak | |
| IL-4 | APC | 11B11 | 554436 | BD | Rat IgG1k | |
| Treg cells | FVS | BV510 | - | 564406 | BD | - |
| CD8 | BV605 | 53-6.7 | 563152 | BD | Rat IgG2ak | |
| CD3 | FITC | 145-2C11 | 561827 | BD | Hamster IgG1k | |
| CD25 | PE | PC61 | 12-0251 | BioLegend | Rat IgG1λ | |
| CD39 | PE-Cy7 | 24DMS1 | 25-0391 | Thermo Fisher Scientific | Rat IgG2bk | |
| FoxP3 | APC | FJK-16s | 17-5773 | BD | Rat IgG2ak | |
| CD4 | APC-H7 | GK1.5 | 560181 | BD | Rat IgG2ak | |
| CD8+ T, NKT and NK cell cytotoxicity | Granzyme B | eF-450 | NGZB | 48-8898 | Thermo Fisher Scientific | Rat IgG2ak |
| CD8 | BV605 | 53-6.7 | 563152 | BD | Rat IgG2ak | |
| CD3 | FITC | 145-2C11 | 561827 | BD | Hamster IgG1k | |
| CD4 | PerCP-eF710 | RM4-5 | 46-0042 | Thermo Fisher Scientific | Rat IgG2ak | |
| Perforin | PE | S16009A | 154306 | BioLegend | Rat IgG2ak | |
| NK1.1 | APC | PK136 | 550627 | BioLegend | Mouse IgG2ak | |
| FVS | APC-700 | - | 564997 | BD | - | |
| Immune checkpoint inhibitors | CD279 | BV421 | MIH4 | 564323 | BD | Rat IgG2ak |
| CD8 | BV510 | 53-6.7 | 563068 | BD | Rat IgG2ak | |
| CD62L | BV605 | MEL-14 | 563252 | BD | Rat IgG2ak | |
| CD3 | FITC | 145-2C11 | 561827 | BD | Hamster IgG1k | |
| CD44 | PerCP-Cy5.5 | IM7 | 560570 | BD | Rat IgG2bk | |
| CD366 | PE-Cy7 | RMT3-23 | 25-5870 | BD | Rat IgG2ak | |
| CD223 | APC | C9B7W | 562346 | BD | Rat IgG2ak | |
| FVS | APC-700 | - | 564997 | BD | - | |
| CD4 | APC-H7 | GK1.5 | 560181 | BD | Rat IgG2ak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dema, M.; Eixarch, H.; Castillo, M.; Montalban, X.; Espejo, C. IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2024, 25, 6732. https://doi.org/10.3390/ijms25126732
Dema M, Eixarch H, Castillo M, Montalban X, Espejo C. IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences. 2024; 25(12):6732. https://doi.org/10.3390/ijms25126732
Chicago/Turabian StyleDema, María, Herena Eixarch, Mireia Castillo, Xavier Montalban, and Carmen Espejo. 2024. "IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis" International Journal of Molecular Sciences 25, no. 12: 6732. https://doi.org/10.3390/ijms25126732
APA StyleDema, M., Eixarch, H., Castillo, M., Montalban, X., & Espejo, C. (2024). IL-6 Inhibition as a Therapeutic Target in Aged Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences, 25(12), 6732. https://doi.org/10.3390/ijms25126732






