Antineoplastic Effect of ALK Inhibitor Crizotinib in Primary Human Anaplastic Thyroid Cancer Cells with STRN–ALK Fusion In Vitro
Abstract
1. Introduction
2. Results
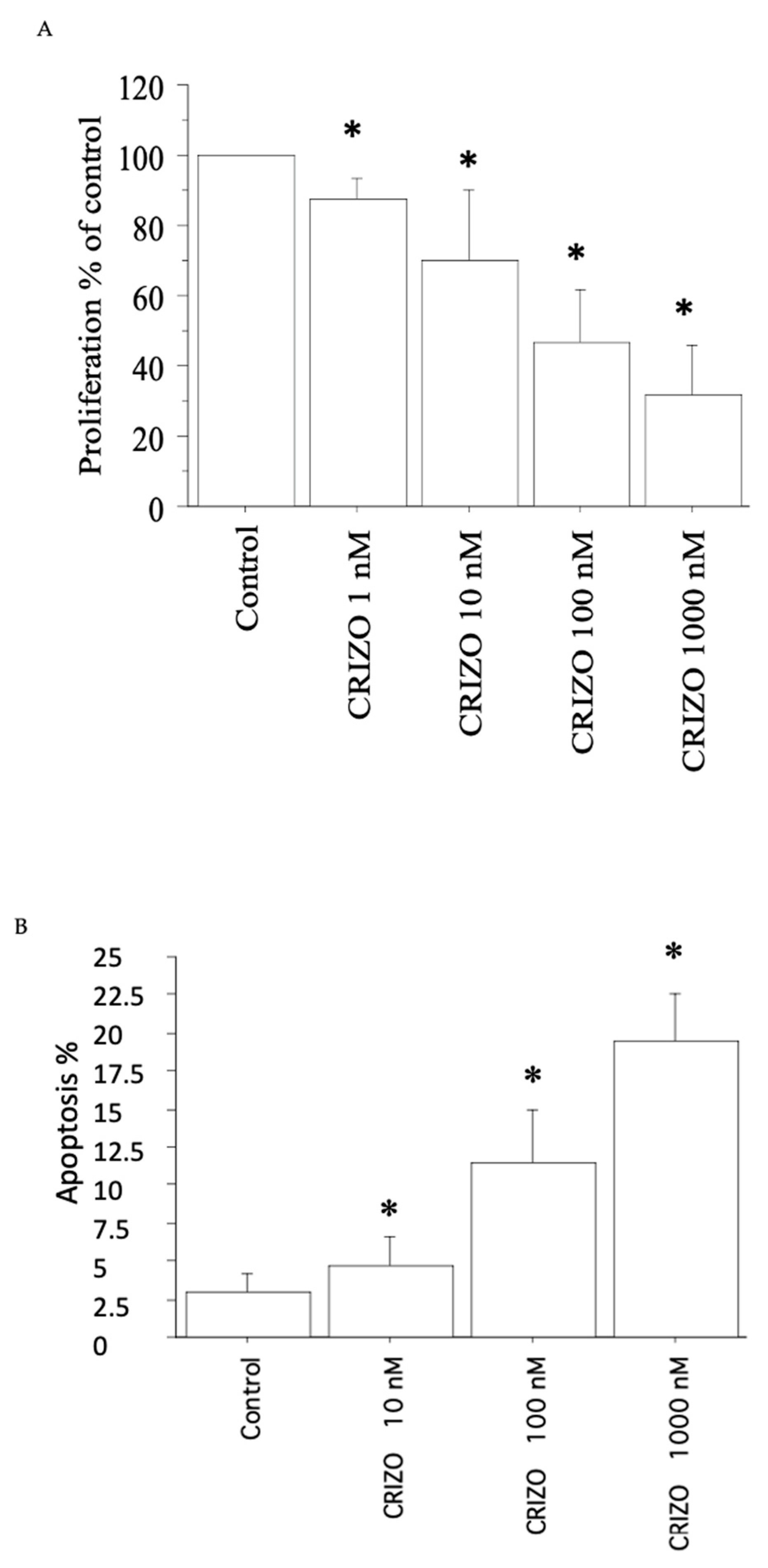
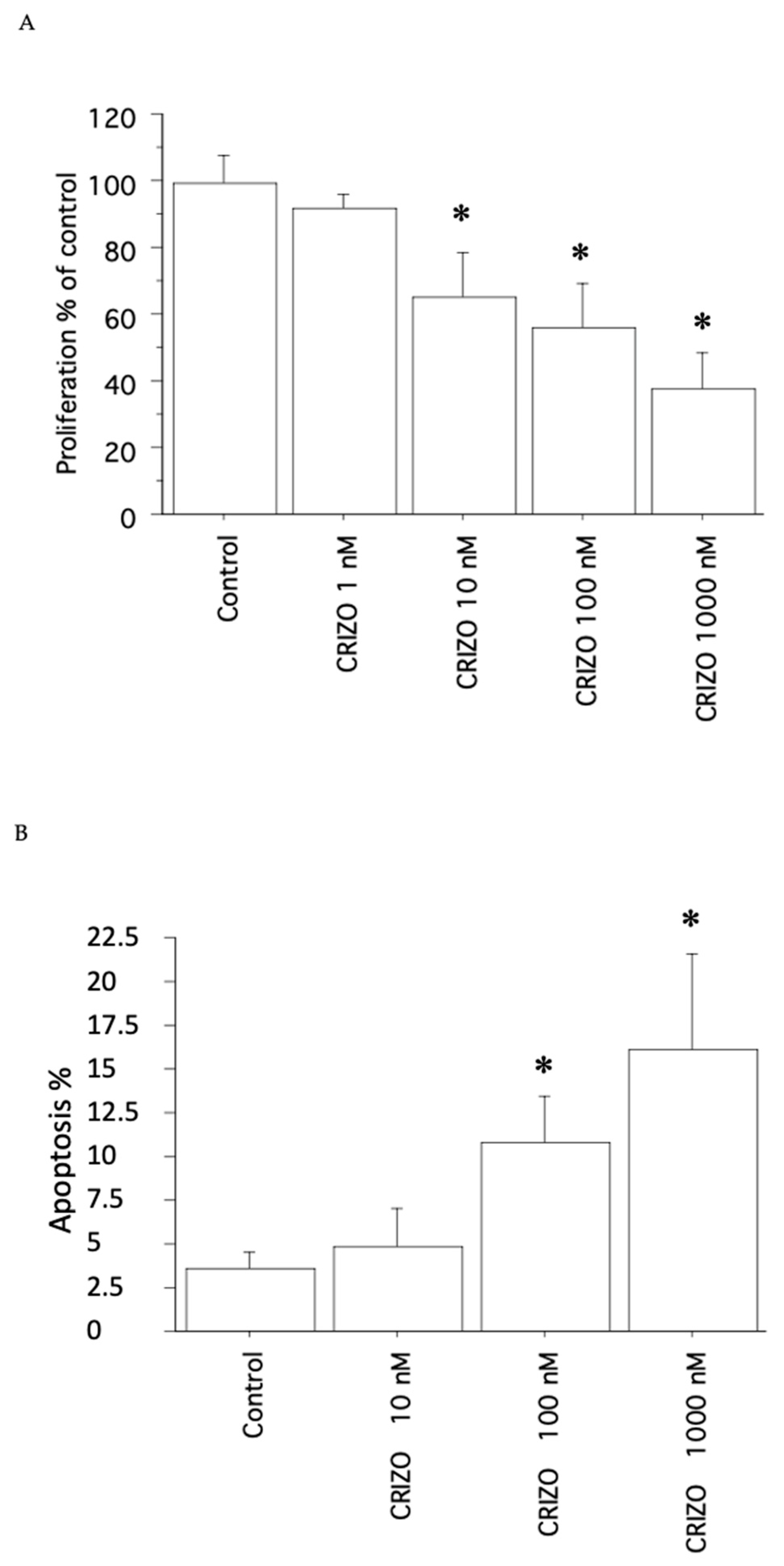
2.1. Proliferation, Cell Viability, and Cytotoxicity Assay
2.2. Apoptosis
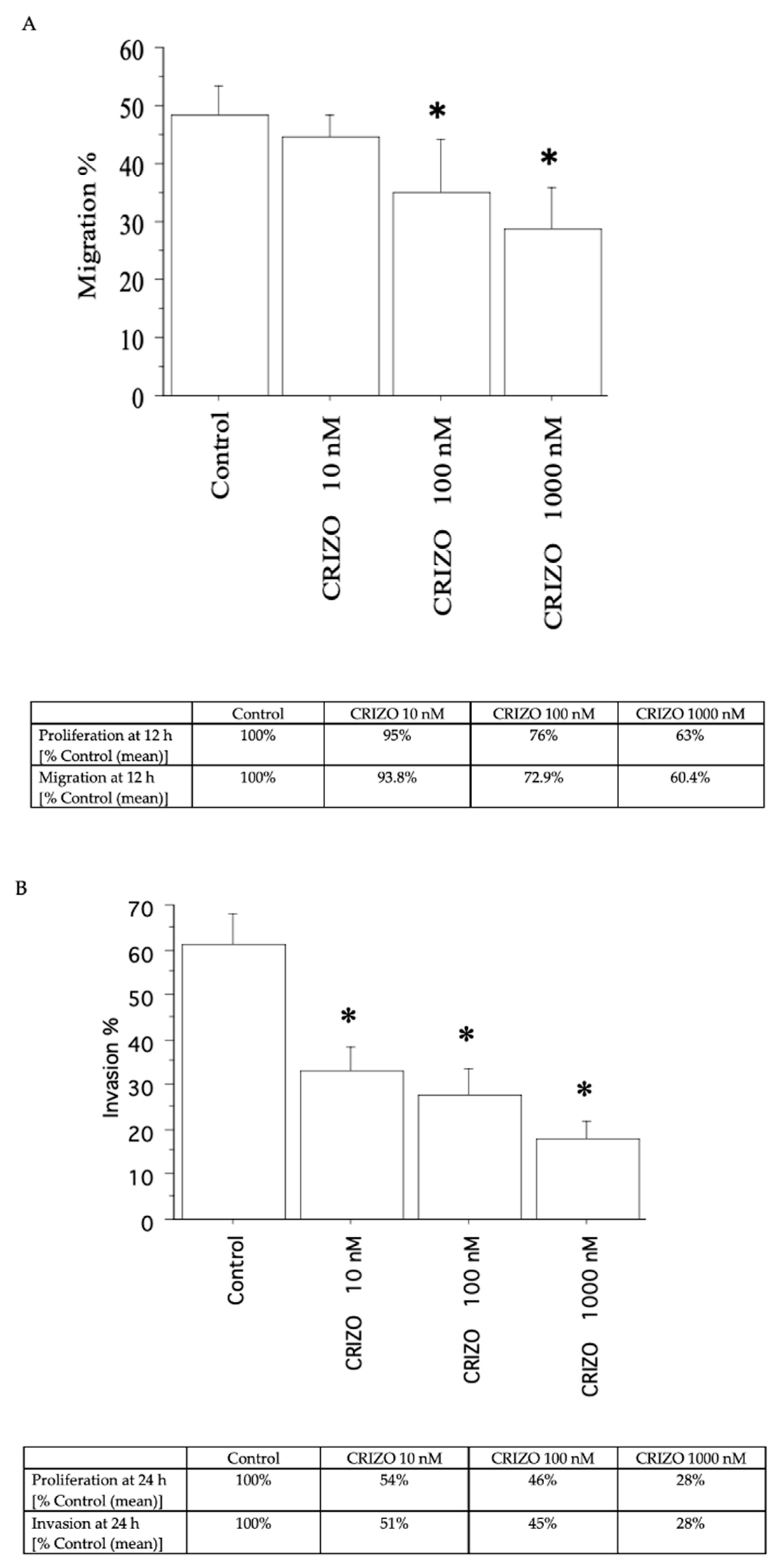
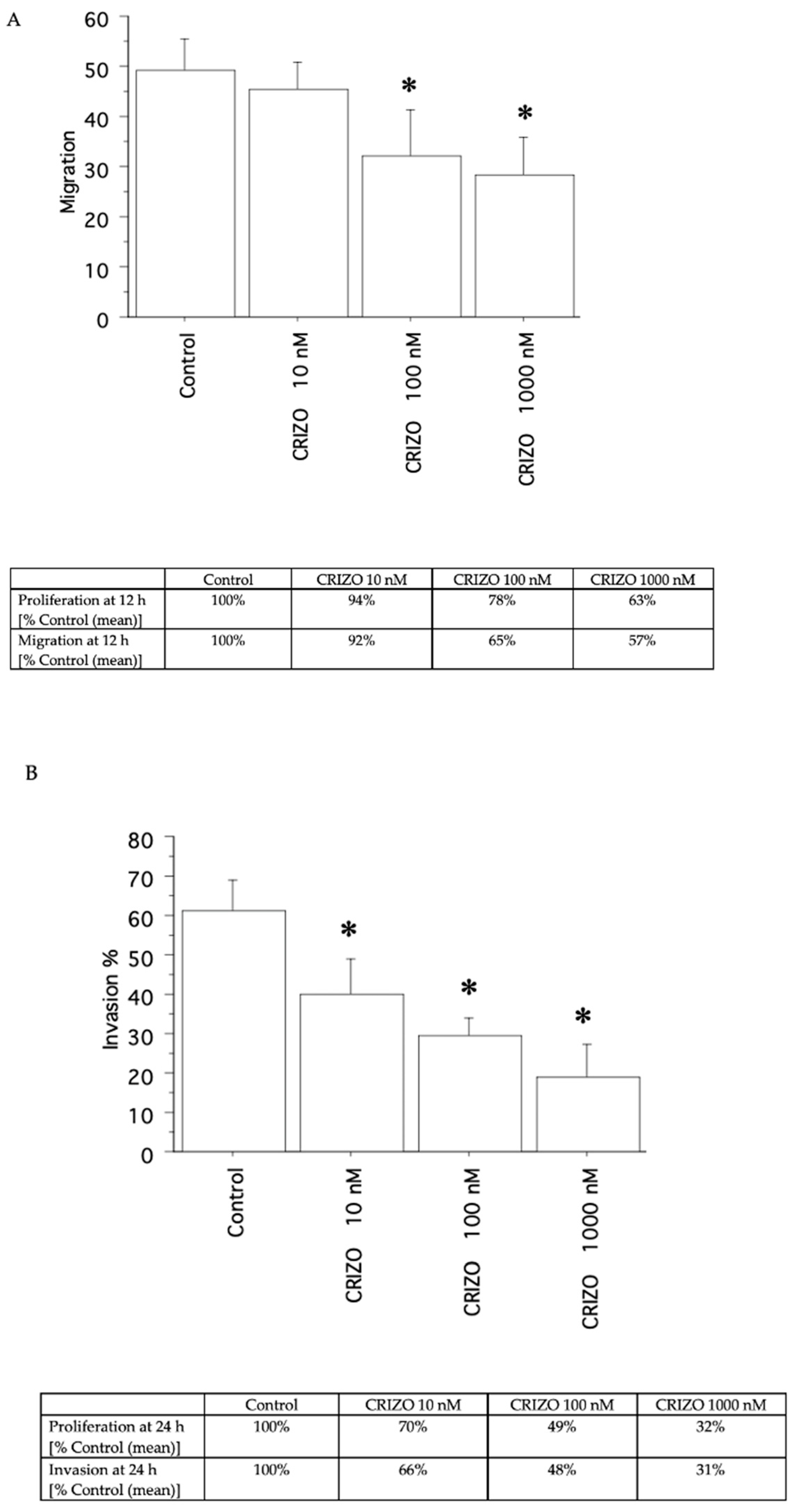
2.3. Migration and Invasion Assays
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.1.1. Tissue Samples
4.1.2. Characterization of ATC Samples
4.1.3. Primary Cell Cultures
4.1.4. AF Cell Line
4.2. Proliferation, Cell Viability, and Cytotoxicity Assay
4.3. Cell Counting
4.4. Apoptosis
4.4.1. Hoechst 33342 Uptake
4.4.2. Annexin V
4.5. Migration and Invasion Tests
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernandez-Prera, J.C. The evolving concept of aggressive histological variants of differentiated thyroid cancer. Semin. Diagn. Pathol. 2020, 37, 228–233. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Galdiero, M.R.; Varricchi, G.; Elia, G.; Ragusa, F.; Paparo, S.R.; Benvenga, S.; Antonelli, A. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin. Cancer Biol. 2022, 79, 180–196. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, X.; Wang, L.; Xiong, D.; Wu, C.; Cen, L.; Xie, L.; Li, X. Modifiable risk factors for thyroid cancer: Lifestyle and residence environment. Endokrynol. Pol. 2024, 75, 119–129. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 2307. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Carling, T.; Udelsman, R. Thyroid cancer. Annu. Rev. Med. 2014, 65, 125–137. [Google Scholar] [CrossRef]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur. Thyroid. J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Gild, M.L.; Clifton-Bligh, R.J.; Wirth, L.J.; Robinson, B.G. Medullary Thyroid Cancer: Updates and Challenges. Endocr. Rev. 2023, 44, 934–946. [Google Scholar] [CrossRef]
- Appetecchia, M.; Lauretta, R.; Barnabei, A.; Pieruzzi, L.; Terrenato, I.; Cavedon, E.; Mian, C.; Castagna, M.G.; Elisei, R.; SIE (Italian Society of Endocrinology) Working Group. Epidemiology of Simultaneous Medullary and Papillary Thyroid Carcinomas (MTC/PTC): An Italian Multicenter Study. Cancers 2019, 11, 1516. [Google Scholar] [CrossRef]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef]
- Lamartina, L.; Cooper, D.S. Radioiodine remnant ablation in low-risk differentiated thyroid cancer: The “con” point of view. Endocrine 2015, 50, 67–71. [Google Scholar] [CrossRef]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214, Erratum in Thyroid 2010, 20, 674–675. [Google Scholar] [CrossRef]
- Cirello, V.; Gambale, C.; Nikitski, A.V.; Masaki, C.; Roque, J.; Colombo, C. Poorly differentiated thyroid carcinoma: Molecular, clinico-pathological hallmarks and therapeutic perspectives. Panminerva Med. 2024, 66, 155–173. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef]
- Gruber, J.J.; Colevas, A.D. Differentiated thyroid cancer: Focus on emerging treatments for radioactive iodine-refractory patients. Oncologist 2015, 20, 113–126. [Google Scholar] [CrossRef]
- Durante, C.; Montesano, T.; Torlontano, M.; Attard, M.; Monzani, F.; Tumino, S.; Costante, G.; Meringolo, D.; Bruno, R.; Trulli, F.; et al. Papillary thyroid cancer: Time course of recurrences during postsurgery surveillance. J. Clin. Endocrinol. Metab. 2013, 98, 636–642. [Google Scholar] [CrossRef]
- D’Agostino, M.; Sponziello, M.; Puppin, C.; Celano, M.; Maggisano, V.; Baldan, F.; Biffoni, M.; Bulotta, S.; Durante, C.; Filetti, S.; et al. Different expression of TSH receptor and NIS genes in thyroid cancer: Role of epigenetics. J. Mol. Endocrinol. 2014, 52, 121–131. [Google Scholar] [CrossRef]
- Valerio, L.; Pieruzzi, L.; Giani, C.; Agate, L.; Bottici, V.; Lorusso, L.; Cappagli, V.; Puleo, L.; Matrone, A.; Viola, D.; et al. Targeted therapy in thyroid Cancer: State of the art. Clin. Oncol. 2017, 29, 316–324. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel treatments for anaplastic thyroid carcinoma. Gland. Surg. 2020, 9 (Suppl. 1), S28–S42. [Google Scholar] [CrossRef]
- Abdalla, A.S.; Rahman, M.; Khan, S.A. Promising Therapeutic Targets for Recurrent/Metastatic Anaplastic Thyroid Cancer. Curr. Treat. Options Oncol. 2024. [Google Scholar] [CrossRef]
- Yoo, S.K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.J.; Cho, S.W.; Won, J.K.; et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Ravi, N.; Yang, M.; Gretarsson, S.; Jansson, C.; Mylona, N.; Sydow, S.R.; Woodward, E.L.; Ekblad, L.; Wennerberg, J.; Paulsson, K. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers 2019, 11, 402. [Google Scholar] [CrossRef]
- Khan, S.A.; Ci, B.; Xie, Y.; Gerber, D.E.; Beg, M.S.; Sherman, S.I.; Cabanillas, M.E.; Busaidy, N.L.; Burtness, B.A.; Heilmann, A.M.; et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck. 2019, 41, 1928–1934. [Google Scholar] [CrossRef]
- Nylén, C.; Mechera, R.; Maréchal-Ross, I.; Tsang, V.; Chou, A.; Gill, A.J.; Clifton-Bligh, R.J.; Robinson, B.G.; Sywak, M.S.; Sidhu, S.B.; et al. Molecular Markers Guiding Thyroid Cancer Management. Cancers 2020, 12, 2164. [Google Scholar] [CrossRef]
- Singh, A.; Ham, J.; Po, J.W.; Niles, N.; Roberts, T.; Lee, C.S. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Elia, G.; Patrizio, A.; Ragusa, F.; Paparo, S.R.; Mazzi, V.; Balestri, E.; Botrini, C.; Rugani, L.; Benvenga, S.; Materazzi, G.; et al. Molecular features of aggressive thyroid cancer. Front. Oncol. 2022, 12, 1099280. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 6496570. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Du, X.; Shao, Y.; Qin, H.F.; Tai, Y.H.; Gao, H.J. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef]
- Tennstedt, P.; Strobel, G.; Bölch, C.; Grob, T.; Minner, S.; Masser, S.; Simon, R. Patterns of ALK expression in different human cancer types. J. Clin. Pathol. 2014, 67, 477–481. [Google Scholar] [CrossRef]
- Devarakonda, S.; Morgensztern, D.; Govindan, R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015, 16, e342–e351. [Google Scholar] [CrossRef]
- Panebianco, F.; Nikitski, A.V.; Nikiforova, M.N.; Kaya, C.; Yip, L.; Condello, V.; Wald, A.I.; Nikiforov, Y.E.; Chiosea, S.I. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr. Relat. Cancer 2019, 26, 803–814. [Google Scholar] [CrossRef]
- Bastos, A.U.; de Jesus, A.C.; Cerutti, J.M. ETV6-NTRK3 and STRN-ALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. Eur. J. Endocrinol. 2018, 178, 83–91. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Kelly, L.M.; Barila, G.; Liu, P.; Evdokimova, V.N.; Trivedi, S.; Panebianco, F.; Gandhi, M.; Carty, S.E.; Hodak, S.P.; Luo, J.; et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4233–4238. [Google Scholar] [CrossRef]
- Chou, A.; Fraser, S.; Toon, C.W.; Clarkson, A.; Sioson, L.; Farzin, M.; Cussigh, C.; Aniss, A.; O’Neill, C.; Watson, N.; et al. A detailed clinicopathologic study of ALK-translocated papillary thyroid carcinoma. Am. J. Surg. Pathol. 2015, 39, 652–659. [Google Scholar] [CrossRef]
- Nikitski, A.V.; Rominski, S.L.; Wankhede, M.; Kelly, L.M.; Panebianco, F.; Barila, G.; Altschuler, D.L.; Nikiforov, Y.E. Mouse Model of Poorly Differentiated Thyroid Carcinoma Driven by STRN-ALK Fusion. Am. J. Pathol. 2018, 188, 2653–2661. [Google Scholar] [CrossRef]
- Godbert, Y.; Henriques de Figueiredo, B.; Bonichon, F.; Chibon, F.; Hostein, I.; Pérot, G.; Dupin, C.; Daubech, A.; Belleannée, G.; Gros, A.; et al. Remarkable Response to Crizotinib in Woman With Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2015, 33, e84–e87. [Google Scholar] [CrossRef]
- van der Tuin, K.; Ventayol Garcia, M.; Corver, W.E.; Khalifa, M.N.; Ruano Neto, D.; Corssmit, E.P.M.; Hes, F.J.; Links, T.P.; Smit, J.W.A.; Plantinga, T.S.; et al. Targetable gene fusions identified in radioactive iodine refractory advanced thyroid carcinoma. Eur. J. Endocrinol. 2019, 180, 235–241. [Google Scholar] [CrossRef]
- Abe, I.; Lam, A.K. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr. Oncol. Rep. 2021, 23, 31. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Mazzi, V.; Patrizio, A.; Piaggi, S.; Baldini, E.; Centanni, M.; La Motta, C.; et al. Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture. Int. J. Mol. Sci. 2023, 24, 2398. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Nair, A.; Lemery, S.J.; Yang, J.; Marathe, A.; Zhao, L.; Zhao, H.; Jiang, X.; He, K.; Ladouceur, G.; Mitra, A.K.; et al. FDA Approval Summary: Lenvatinib for Progressive, Radio-iodine-Refractory Differentiated Thyroid Cancer. Clin. Cancer Res. 2015, 21, 5205–5208. [Google Scholar] [CrossRef]
- FDA Approves Cabozantinib for Patients with Previously Treated Radioactive Iodine–Refractory Differentiated Thyroid Cancer. Available online: https://ascopost.com/issues/october-10-2021/fda-approves-cabozantinib-for-patients-with-previously-treated-radioactive-iodine-refractory-differentiated-thyroid-cancer/ (accessed on 15 March 2024).
- Kurzrock, R.; Sherman, S.I.; Ball, D.W.; Forastiere, A.A.; Cohen, R.B.; Mehra, R.; Pfister, D.G.; Cohen, E.E.; Janisch, L.; Nauling, F.; et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011, 29, 2660–2666. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Gosnell, J.E.; Gagel, R.F.; Moley, J.; Pfister, D.; Sosa, J.A.; Skinner, M.; Krebs, A.; Vasselli, J.; Schlumberger, M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 2010, 28, 767–772. [Google Scholar] [CrossRef]
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2012, 18, 3722–3730. [Google Scholar] [CrossRef]
- Iyer, P.C.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Habra, M.A.; Zafereo, M.; Gross, N.; Hess, K.R.; Gule-Monroe, M.; Williams, M.D.; et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 79–87. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, S.; Lee, S.H.; Kim, T.H.; Kim, S.W.; Ahn, M.J.; Jung, H.A.; Chung, J.H. Combination of Dabrafenib and Trametinib in Patients with Metastatic BRAFV600E-Mutated Thyroid Cancer. Cancer Res. Treat. 2024. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Elia, G.; Ruffilli, I.; Ragusa, F.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; Piaggi, S.; et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol. Rep. 2018, 39, 2225–2234. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Ruffilli, I.; Elia, G.; Ragusa, F.; Fioravanti, A.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; et al. Vandetanib has antineoplastic activity in anaplastic thyroid cancer, in vitro and in vivo. Oncol. Rep. 2018, 39, 2306–2314. [Google Scholar] [CrossRef]
- Ferrari, S.M.; La Motta, C.; Elia, G.; Ragusa, F.; Ruffilli, I.; Quattrini, L.; Paparo, S.R.; Piaggi, S.; Patrizio, A.; Ulisse, S.; et al. Antineoplastic Effect of Lenvatinib and Vandetanib in Primary Anaplastic Thyroid Cancer Cells Obtained From Biopsy or Fine Needle Aspiration. Front. Endocrinol. 2018, 9, 764. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; Fallahi, P.; La Motta, C.; Ferrari, S.M.; Mancusi, C.; Fioravanti, A.; Di Desidero, T.; Sartini, S.; Corti, A.; et al. CLM3, a multitarget tyrosine kinase inhibitor with antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J. Clin. Endocrinol. Metab. 2014, 99, E572–E581. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; La Motta, C.; Materazzi, G.; Bocci, G.; Da Settimo, F.; Miccoli, P.; Antonelli, A. CLM29 and CLM24, pyrazolopyrimidine derivatives, have antitumoral activity in vitro in anaplastic thyroid cancer, with or without BRAF mutation. Endocrine 2016, 53, 136–144. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; La Motta, C.; Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Di Domenicantonio, A.; Fioravanti, A.; Sartini, S.; Minuto, M.; et al. CLM94, a novel cyclic amide with anti-VEGFR-2 and antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J. Clin. Endocrinol. Metab. 2012, 97, E528–E536. [Google Scholar] [CrossRef]
- Newell, D.R. Flasks fibres and flanks—Pre-clinical tumour models for predicting clinical antitumour activity. Br. J. Cancer 2001, 84, 1289–1290. [Google Scholar] [CrossRef]
- Dollner, R.; Granzow, C.; Werner, J.A.; Dietz, A. Is there a role for chemosensitivity tests in head and neck cancer? Onkologie 2004, 27, 310–315. [Google Scholar] [CrossRef]
- Schroyens, W.; Tueni, E.; Dodion, P.; Bodecker, R.; Stoessel, F.; Klastersky, J. Validation of clinical predictive value of in vitro colorimetric chemosensitivity assay in head and neck cancer. Eur. J. Cancer 1990, 26, 834–838. [Google Scholar] [CrossRef]
- Lowe, E.; Mossé, Y.P. Podcast on Emerging Treatment Options for Pediatric Patients with ALK-Positive Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumors. Oncol. Ther. 2024. [Google Scholar] [CrossRef]
- Demeure, M.J.; Aziz, M.; Rosenberg, R.; Gurley, S.D.; Bussey, K.J.; Carpten, J.D. Whole-genome sequencing of an aggressive BRAF wild-type papillary thyroid cancer identified EML4-ALK translocation as a therapeutic target. World J. Surg. 2014, 38, 1296–1305. [Google Scholar] [CrossRef]
- Molnar, T.F.; Szipocs, A.; Szalai, Z. Neoadjuvant Crizotinib for ALK Re-arranged NSCLC? J. Thorac. Oncol. 2019, 14, 574–576. [Google Scholar] [CrossRef]
- Califano, I.; Smulever, A.; Jerkovich, F.; Pitoia, F. Advances in the management of anaplastic thyroid carcinoma: Transforming a life-threatening condition into a potentially treatable disease. Rev. Endocr. Metab. Disord. 2024, 25, 123–147. [Google Scholar] [CrossRef]
- An Investigational Drug, Crizotinib (PF-02341066), Is Being Studied in Tumors, Except Non-Small Cell Lung Cancer, That Are Positive for Anaplastic Lymphoma Kinase (ALK). Available online: https://clinicaltrials.gov/study/NCT01121588 (accessed on 15 March 2024).
- Gambacorti-Passerini, C.; Orlov, S.; Zhang, L.; Braiteh, F.; Huang, H.; Esaki, T.; Horibe, K.; Ahn, J.S.; Beck, J.T.; Edenfield, W.J.; et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am. J. Hematol. 2018, 93, 607–614. [Google Scholar] [CrossRef]
- Nikitski, A.V.; Condello, V.; Divakaran, S.S.; Nikiforov, Y.E. Inhibition of ALK-Signaling Overcomes STRN-ALK-Induced Downregulation of the Sodium Iodine Symporter and Restores Radioiodine Uptake in Thyroid Cells. Thyroid 2023, 33, 464–473. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Marone, G.; Galdiero, M.R.; Guglielmi, G.; Foddis, R.; et al. Primary cell cultures for the personalized therapy in aggressive thyroid cancer of follicular origin. Semin. Cancer Biol. 2022, 79, 203–216. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Phay, J.E.; Shen, R.; Pellegata, N.S.; Saji, M.; Ringel, M.D.; de la Chapelle, A.; He, H. Primary Cell Culture Systems for Human Thyroid Studies. Thyroid 2016, 26, 1131–1140. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Minuto, M.; Giannini, R.; Marchetti, I.; Barani, L.; Basolo, F.; et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin. Endocrinol. 2009, 70, 946–953. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Marchetti, I.; Ugolini, C.; Basolo, F.; Miccoli, P.; Ferrannini, E. Evaluation of the sensitivity to chemotherapeutics or thiazolidinediones of primary anaplastic thyroid cancer cells obtained by fine-needle aspiration. Eur. J. Endocrinol. 2008, 159, 283–291. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Barani, L.; Marchetti, I.; Ferrannini, E.; Miccoli, P. Primary cell cultures from anaplastic thyroid cancer obtained by fine-needle aspiration used for chemosensitivity tests. Clin. Endocrinol. 2008, 69, 148–152. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Frascerra, S.; Piaggi, S.; Gelmini, S.; Lupi, C.; Minuto, M.; Berti, P.; Benvenga, S.; et al. Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by peroxisome proliferator-activated receptor-gamma agonists. Endocr. Relat. Cancer 2009, 16, 1299–1311. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; LeBeau, S.O.; Ferris, R.L.; Yip, L.; Seethala, R.R.; Tublin, M.E.; Stang, M.T.; et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; La Motta, C.; Ferrari, S.M.; Fallahi, P.; Fioravanti, A.; Sartini, S.; Minuto, M.; Piaggi, S.; Corti, A.; et al. Novel pyrazolopyrimidine derivatives as tyrosine kinase inhibitors with antitumoral activity in vitro and in vivo in papillary dedifferentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, E288–E296. [Google Scholar] [CrossRef]



| Control | Crizotinib 1 nM | Crizotinib 10 nM | Crizotinib 100 nM | Crizotinib 1000 nM | p (* by ANOVA) | IC50 | |
|---|---|---|---|---|---|---|---|
| First pATC culture with STRN–ALK fusion | 19,994 ± 9870 (100%) | 17,195 ± 998 (86%) | 10,997 ± 943 (55%) | 9797 ± 1005 (49%) | 4998 ± 1018 (25%) | <0.01 | 72 nM |
| Second pATC culture with STRN–ALK fusion | 20,005 ± 9745 (100%) | 18,005 ± 1102 (90%) | 13,605 ± 988 (68%) | 9202 ± 878 (46%) | 6602 ± 988 (33%) | <0.01 | 85 nM |
| pATC culture without STRN–ALK fusion | 19,898 ± 9470 (100%) | 18,903 ± 1114 (95%) | 12,734 ± 990 (64%) | 11,540 ± 998 (58%) | 7959 ± 974 (40%) | <0.01 | 112 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, S.M.; Ragusa, F.; Elia, G.; Mazzi, V.; Balestri, E.; Botrini, C.; Rugani, L.; Patrizio, A.; Piaggi, S.; La Motta, C.; et al. Antineoplastic Effect of ALK Inhibitor Crizotinib in Primary Human Anaplastic Thyroid Cancer Cells with STRN–ALK Fusion In Vitro. Int. J. Mol. Sci. 2024, 25, 6734. https://doi.org/10.3390/ijms25126734
Ferrari SM, Ragusa F, Elia G, Mazzi V, Balestri E, Botrini C, Rugani L, Patrizio A, Piaggi S, La Motta C, et al. Antineoplastic Effect of ALK Inhibitor Crizotinib in Primary Human Anaplastic Thyroid Cancer Cells with STRN–ALK Fusion In Vitro. International Journal of Molecular Sciences. 2024; 25(12):6734. https://doi.org/10.3390/ijms25126734
Chicago/Turabian StyleFerrari, Silvia Martina, Francesca Ragusa, Giusy Elia, Valeria Mazzi, Eugenia Balestri, Chiara Botrini, Licia Rugani, Armando Patrizio, Simona Piaggi, Concettina La Motta, and et al. 2024. "Antineoplastic Effect of ALK Inhibitor Crizotinib in Primary Human Anaplastic Thyroid Cancer Cells with STRN–ALK Fusion In Vitro" International Journal of Molecular Sciences 25, no. 12: 6734. https://doi.org/10.3390/ijms25126734
APA StyleFerrari, S. M., Ragusa, F., Elia, G., Mazzi, V., Balestri, E., Botrini, C., Rugani, L., Patrizio, A., Piaggi, S., La Motta, C., Ulisse, S., Virili, C., Antonelli, A., & Fallahi, P. (2024). Antineoplastic Effect of ALK Inhibitor Crizotinib in Primary Human Anaplastic Thyroid Cancer Cells with STRN–ALK Fusion In Vitro. International Journal of Molecular Sciences, 25(12), 6734. https://doi.org/10.3390/ijms25126734








