Serotonin Influences Insulin Secretion in Rat Insulinoma INS-1E Cells
Abstract
:1. Introduction
2. Results
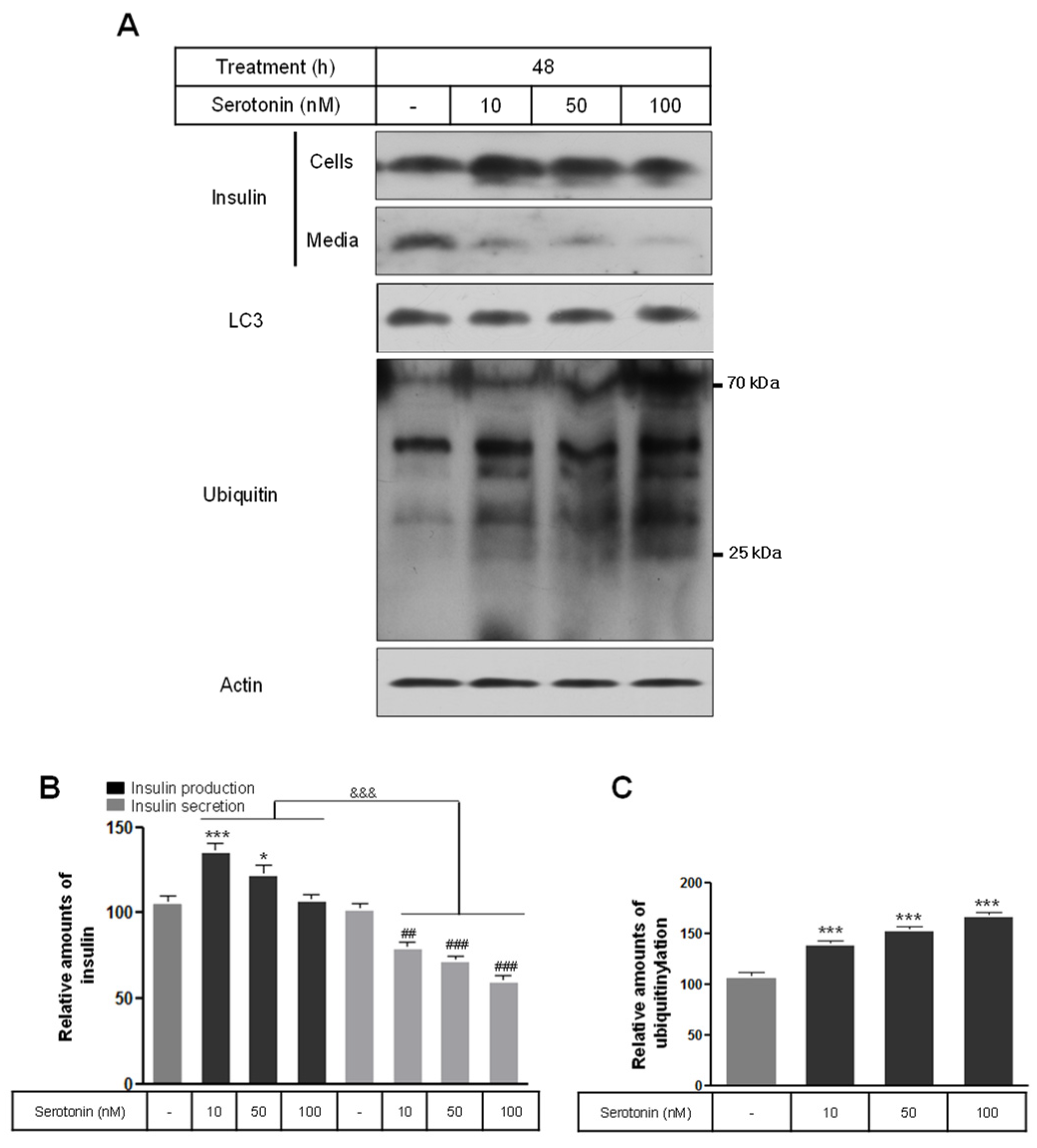
2.1. Insulin Synthesis and Secretion by Nanomolar Serotonin
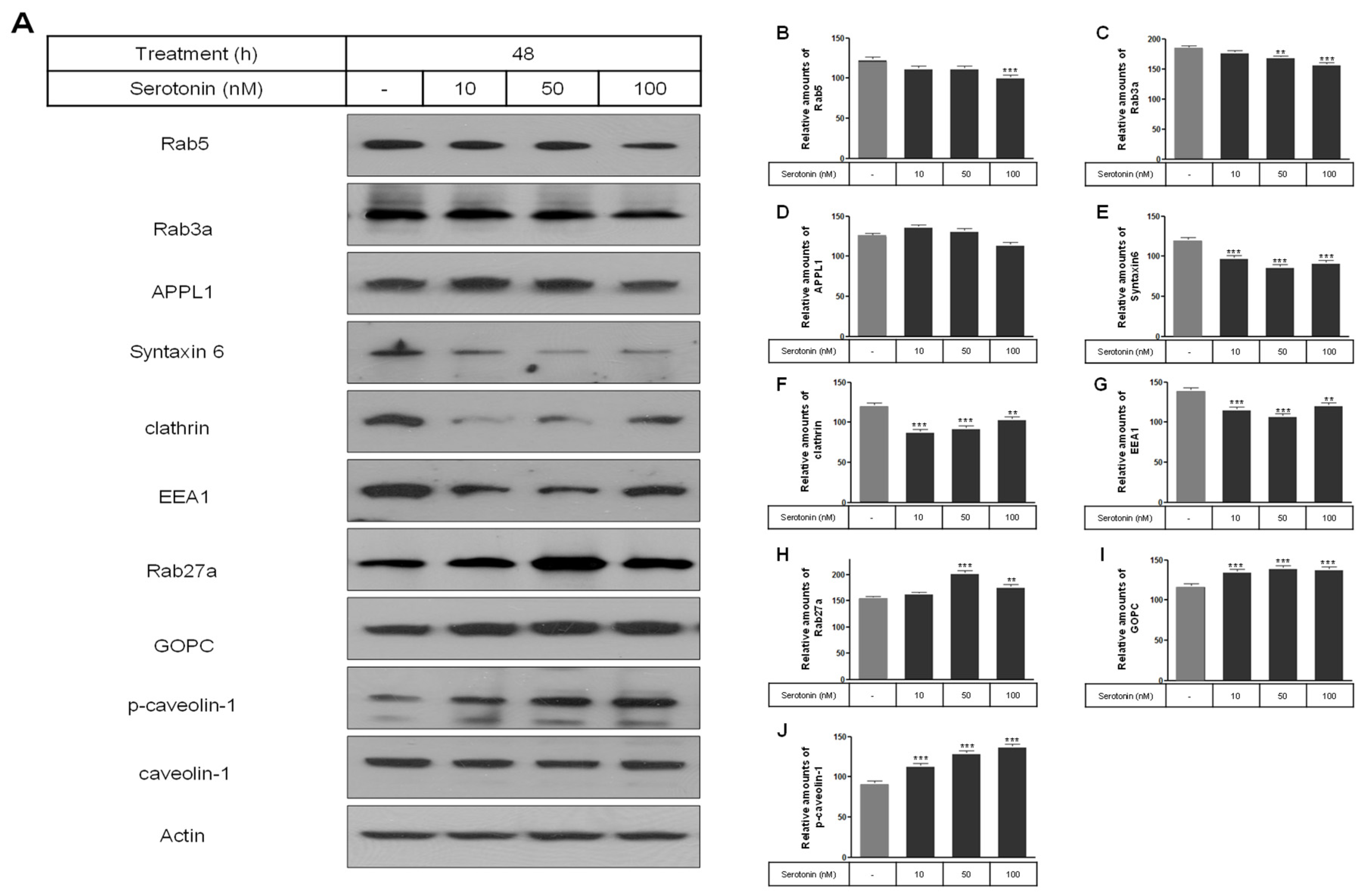
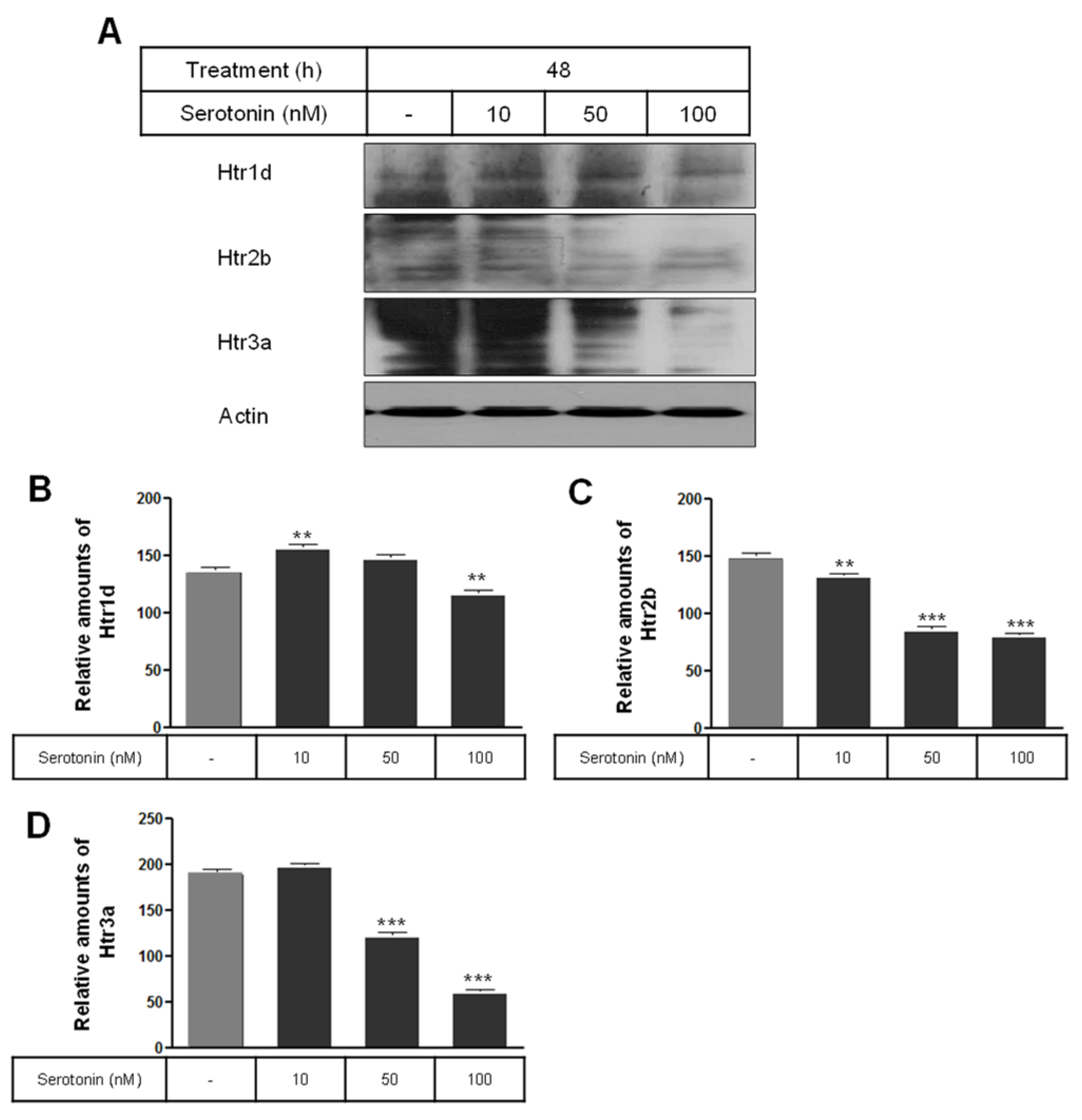
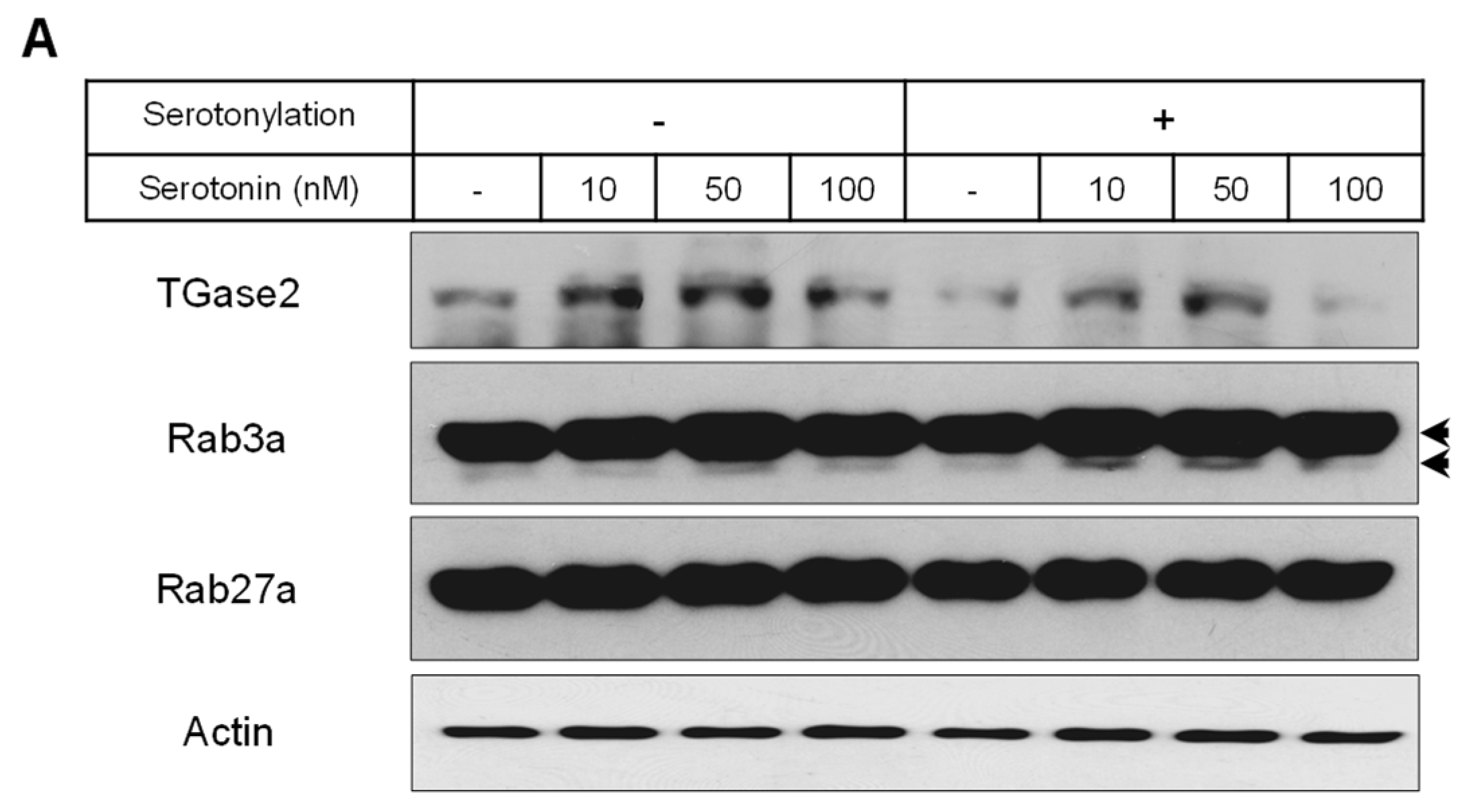
2.2. Levels of Membrane Vesicle Trafficking-Related Proteins, Serotonin Receptors, and the Serotonylation of Rab3a and Rab27a
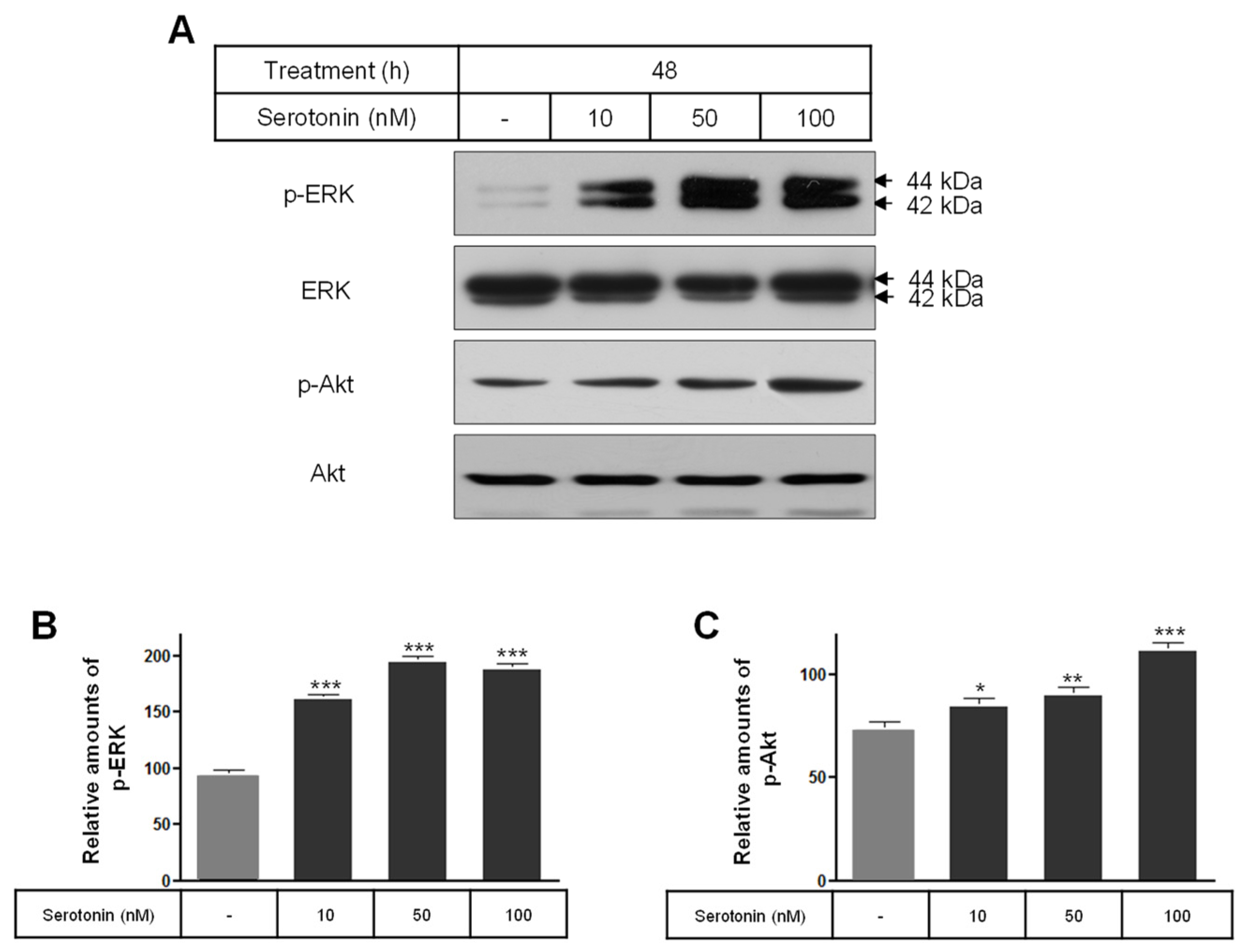
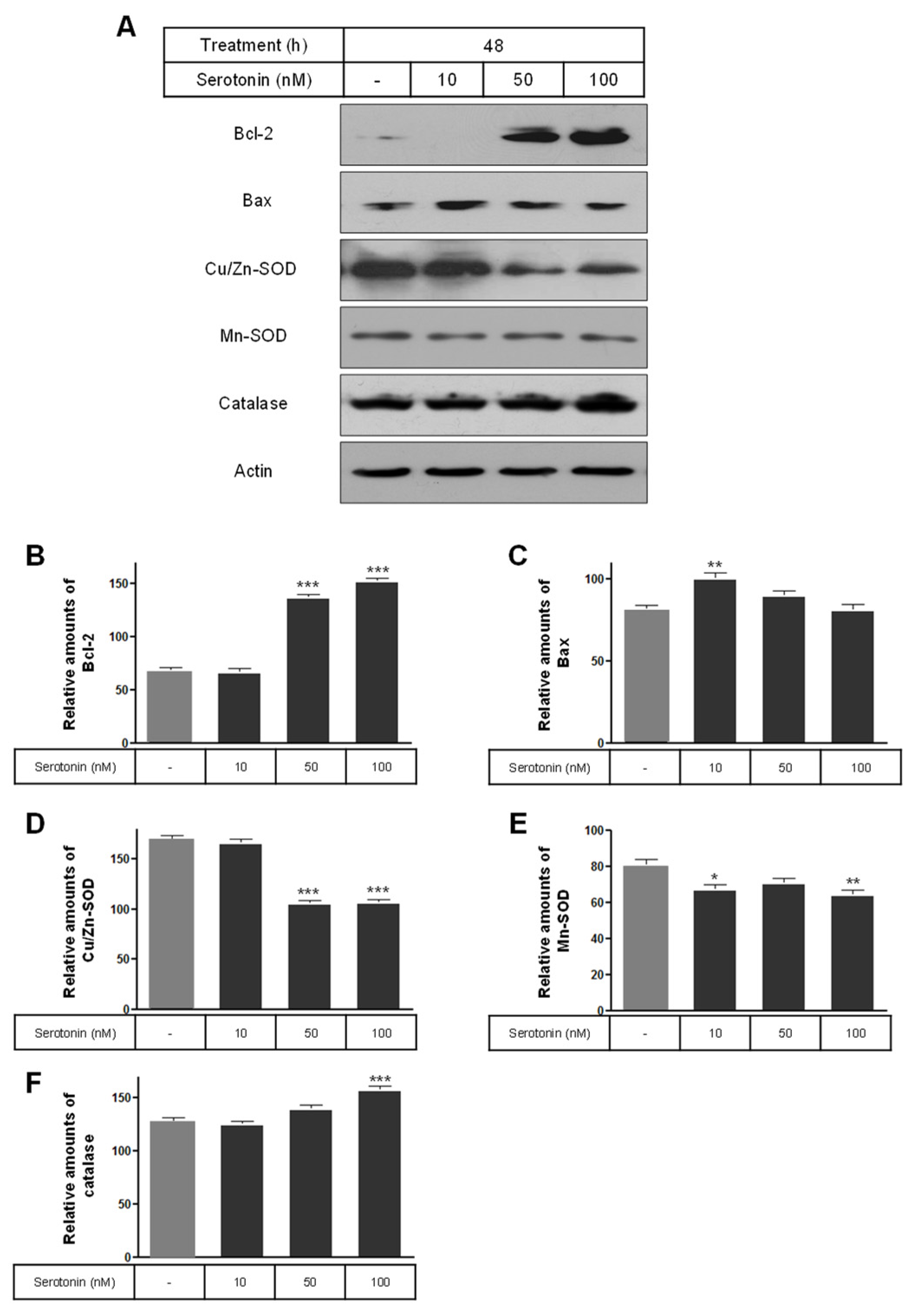
2.3. The Levels of Phospho-ERK, Phospho-Akt, Bcl-2, Bax, and SOD
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blot Analysis
4.3. Serotonylation of Rab3a and Rab27a Proteins
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Nichols, C.; Sanders-Bush, E. Serotonin receptor signaling and hallucinogenic drug action. Heffer Rev. Psychedelic Res. 2001, 2, 73–79. [Google Scholar]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Bennet, H.; Balhuizen, A.; Medina, A.; Dekker Nitert, M.; Ottosson Laakso, E.; Essén, S.; Spégel, P.; Storm, P.; Krus, U.; Wierup, N.; et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides 2015, 71, 113–120. [Google Scholar] [CrossRef]
- Falk, B.; Hellman, B. Evidence for the presence of biogenic amines in the pancreatic islets. Experientia 1963, 19, 139–140. [Google Scholar] [CrossRef]
- Falk, B.; Hellman, B. A fluorescent reaction for monoamines in the insulin producing cells of the guinea-pig. Acta Endocrinol. 1964, 45, 133–139. [Google Scholar] [CrossRef]
- Sundler, F.; Hakanson, R.; Loren, I.; Lundquist, I. Amine storage and function in peptide hormone-producing cells. Investig. Cell Pathol. 1980, 3, 87–103. [Google Scholar]
- Jaim-Etcheverry, G.; Zieher, L.M. Electron microscopic cytochemistry of 5-hydroxytryptamine (5-HT) in the beta cells of guinea pig endocrine pancreas. Endocrinology 1968, 83, 917–923. [Google Scholar] [CrossRef]
- Gylfe, E. Association between 5-hydroxytryptamine release and insulin secretion. J. Endocrinol. 1978, 78, 239–248. [Google Scholar] [CrossRef]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef]
- Kim, K.; Oh, C.M.; Ohara-Imaizumi, M.; Park, S.; Namkung, J.; Yadav, V.K.; Tamarina, N.A.; Roe, M.W.; Philipson, L.H.; Karsenty, G.; et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology 2015, 156, 444–452. [Google Scholar] [CrossRef]
- Ohara-Imaizumi, M.; Kim, H.; Yoshida, M.; Fujiwara, T.; Aoyagi, K.; Toyofuku, Y.; Nakamichi, Y.; Nishiwaki, C.; Okamura, T.; Uchida, T.; et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proc. Natl. Acad. Sci. USA 2013, 110, 19420–19425. [Google Scholar] [CrossRef]
- Marco, J.; Hedo, J.A.; Villanueva, M.L. Inhibition of glucagon release by serotonin in mouse pancreatic islets. Diabetologia 1977, 13, 585–588. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Micossi, P.; Foá, P.P. Effects of serotonin, of its biosynthetic precursors and of the anti-serotonin agent metergoline on the release of glucagon and insulin from rat pancreas. Horm. Metab. Res. 1978, 10, 200–203. [Google Scholar] [CrossRef]
- Almaça, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.O.; Caicedo, A. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016, 17, 3281–3291. [Google Scholar] [CrossRef]
- Bennet, H.; Mollet, I.G.; Balhuizen, A.; Medina, A.; Nagorny, C.; Bagge, A.; Fadista, J.; Ottosson-Laakso, E.; Vikman, P.; Dekker-Nitert, M.; et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia 2016, 59, 744–754. [Google Scholar] [CrossRef]
- Deeney, J.T.; Bränström, R.; Corkey, B.E.; Larsson, O.; Berggren, P.O. 3H-serotonin as a marker of oscillatory insulin secretion in clonal beta-cells (INS-1). FEBS Lett. 2007, 581, 4080–4084. [Google Scholar] [CrossRef]
- Paulmann, N.; Grohmann, M.; Voigt, J.P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevsek, M.; Fink, H.; Rupnik, M.; et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef]
- Ekholm, R.; Ericson, L.E.; Lundquist, I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia 1971, 7, 339–348. [Google Scholar] [CrossRef]
- Richmond, J.E.; Codignola, A.; Cooke, I.M.; Sher, E. Calcium- and barium-dependent exocytosis from the rat insulinoma cell line RINm5F assayed using membrane capacitance measurements and serotonin release. Pflug. Arch. 1996, 432, 258–269. [Google Scholar] [CrossRef]
- Oh, C.M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 6794. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, L.; Lemaire, K.; Arijs, I.; Auffret, J.; Granvik, M.; Van Lommel, L.; Binart, N.; in’t Veld, P.; Schuit, F.; Schraenen, A. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes. PLoS ONE 2015, 10, e0121868. [Google Scholar] [CrossRef]
- Schraenen, A.; Lemaire, K.; de Faudeur, G.; Hendrickx, N.; Granvik, M.; Van Lommel, L.; Mallet, J.; Vodjdani, G.; Gilon, P.; Binart, N.; et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 2010, 53, 2589–2599. [Google Scholar] [CrossRef]
- Georgescu, T.; Lyons, D.; Heisler, L.K. Role of serotonin in body weight, insulin secretion and glycaemic control. J. Neuroendocrinol. 2021, 33, e12960. [Google Scholar] [CrossRef]
- Xie, K.Y.; Chien, S.J.; Tan, B.C.; Chen, Y.W. RNA editing of 5-HT2C R impairs insulin secretion of pancreatic beta cells via altered store-operated calcium entry. FASEB J. 2021, 35, e21929. [Google Scholar] [CrossRef] [PubMed]
- Kitiphongspattana, K.; Mathews, C.E.; Leiter, E.H.; Gaskins, H.R. Proteasome inhibition alters glucose-stimulated (pro)insulin secretion and turnover in pancreatic β-cells. J. Biol. Chem. 2005, 280, 15727–15734. [Google Scholar] [CrossRef]
- Hartley, T.; Brumell, J.; Volchuk, A. Emerging roles for the ubiquitin-proteasome system and autophagy in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Minami, K.; Nagashima, K.; Seino, S. Essential role of ubiquitin-proteasome system in normal regulation of insulin secretion. J. Biol. Chem. 2006, 281, 13015–13020. [Google Scholar] [CrossRef]
- López-Avalos, M.D.; Duvivier-Kali, V.F.; Xu, G.; Bonner-Weir, S.; Sharma, A.; Weir, G.C. Evidence for a role of the ubiquitin-proteasome pathway in pancreatic islets. Diabetes 2006, 55, 1223–1231. [Google Scholar] [CrossRef]
- Charest, A.; Lane, K.; McMahon, K.; Housman, D.E. Association of a novel PDZ domain-containing peripheral Golgi protein with the Q-SNARE (Q-soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptor) protein syntaxin 6. J. Biol. Chem. 2001, 276, 29456–29465. [Google Scholar] [CrossRef]
- Simonsen, A.; Gaullier, J.M.; D’Arrigo, A.; Stenmark, H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999, 274, 28857–28860. [Google Scholar] [CrossRef] [PubMed]
- Kuliawat, R.; Kalinina, E.; Bock, J.; Fricker, L.; McGraw, T.E.; Kim, S.R.; Zhong, J.; Scheller, R.; Arvan, P. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol. Biol. Cell 2004, 15, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Perera, R.M.; Balkin, D.M.; Pirruccello, M.; Toomre, D.; De Camilli, P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 2009, 136, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Lippé, R.; Christoforidis, S.; Gaullier, J.M.; Brech, A.; Callaghan, J.; Toh, B.H.; Murphy, C.; Zerial, M.; Stenmark, H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 1998, 394, 494–498. [Google Scholar] [CrossRef]
- Christoforidis, S.; McBride, H.M.; Burgoyne, R.D.; Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. Nature 1999, 397, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Molinete, M.; Dupuis, S.; Brodsky, F.M.; Halban, P.A. Role of clathrin in the regulated secretory pathway of pancreatic beta-cells. J. Cell Sci. 2001, 114 Pt 16, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Veluthakal, R.; Chvyrkova, I.; Tannous, M.; McDonald, P.; Amin, R.; Hadden, T.; Thurmond, D.C.; Quon, M.J.; Kowluru, A. Essential role for membrane lipid rafts in interleukin-1beta-induced nitric oxide release from insulin-secreting cells: Potential regulation by caveolin-1+. Diabetes 2005, 54, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Nevins, A.K.; Thurmond, D.C. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J. Biol. Chem. 2006, 281, 18961–18972. [Google Scholar] [CrossRef] [PubMed]
- Kepner, E.M.; Yoder, S.M.; Oh, E.; Kalwat, M.A.; Wang, Z.; Quilliam, L.A.; Thurmond, D.C. Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1072–E1080. [Google Scholar] [CrossRef]
- Pojoga, L.H.; Underwood, P.C.; Goodarzi, M.O.; Williams, J.S.; Adler, G.K.; Jeunemaitre, X.; Hopkins, P.N.; Raby, B.A.; Lasky-Su, J.; Sun, B.; et al. Variants of the caveolin-1 gene: A translational investigation linking insulin resistance and hypertension. J. Clin. Endocrinol. Metab. 2011, 96, E1288–E1292. [Google Scholar] [CrossRef]
- Asterholm, I.W.; Mundy, D.I.; Weng, J.; Anderson, R.G.; Scherer, P.E. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, B.P.; Kao, A.W.; Santeler, S.R.; Pessin, J.E. Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol. Cell. Biol. 1998, 18, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Boothe, T.; Lim, G.E.; Cen, H.; Skovsø, S.; Piske, M.; Li, S.N.; Nabi, I.R.; Gilon, P.; Johnson, J.D. Inter-domain tagging implicates caveolin-1 in insulin receptor trafficking and Erk signaling bias in pancreatic beta-cells. Mol. Metab. 2016, 5, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Kajio, H.; Olszewski, S.; Rosner, P.J.; Donelan, M.J.; Geoghegan, K.F.; Rhodes, C.J. A low-affinity Ca2+-dependent association of calmodulin with the Rab3A effector domain inversely correlates with insulin exocytosis. Diabetes 2001, 50, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Ohara-Imaizumi, M.; Takahashi, N.; Mizutani, S.; Zhao, S.; Kikuta, T.; Kasai, H.; Nagamatsu, S.; Gomi, H.; Izumi, T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Investig. 2005, 115, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Joo, S.S.; Yoo, Y.M. Nanomolar melatonin influences insulin synthesis and secretion in rat insulinoma INS-1E cells. J. Physiol. Pharmacol. 2020, 71, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [PubMed]
- Amisten, S.; Salehi, A.; Rorsman, P.; Jones, P.M.; Persaud, S.J. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 2013, 139, 359–391. [Google Scholar] [CrossRef]
- Cavaghan, M.K.; Ehrmann, D.A.; Polonsky, K.S. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J. Clin. Investig. 2000, 106, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Clarke, D.D.; Waelsch, H. An enzymatically catalyzed incorporation of amines into proteins. Biochim. Biophys. Acta 1957, 25, 451–452. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Lin, C.J.; Greenberg, C.S. Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 2017, 49, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. Serotonylation: Serotonin Signaling and Epigenetics. Front. Mol. Neurosci. 2019, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Rolli-Derkinderen, M.; Tharaux, P.L.; Melino, G.; Pacaud, P.; Loirand, G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J. Biol. Chem. 2007, 282, 2918–2928. [Google Scholar] [CrossRef] [PubMed]
- Regazzi, R.; Ravazzola, M.; Iezzi, M.; Lang, J.; Zahraoui, A.; Andereggen, E.; Morel, P.; Takai, Y.; Wollheim, C.B. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J. Cell Sci. 1996, 109, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Yokota, H.; Torii, S.; Aoki, T.; Hosaka, M.; Zhao, S.; Takata, K.; Takeuchi, T.; Izumi, T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell Biol. 2002, 22, 1858–1867. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Qu, Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 2012, 586, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Errico, M.; Lin, S.L.; Cowen, D.S. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J. Neurochem. 2000, 75, 934–938. [Google Scholar] [CrossRef]
- Gallwitz, B. Therapies for the treatment of type 2 diabetes mellitus based on incretin action. Minerva Endocrinol. 2006, 31, 133–147. [Google Scholar] [PubMed]
- Halperin, F.; Lopez, X.; Manning, R.; Kahn, C.R.; Kulkarni, R.N.; Goldfine, A.B. Insulin augmentation of glucose-stimulated insulin secretion is impaired in insulin-resistant humans. Diabetes 2012, 61, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A.; Kowluru, R.A. Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem. Pharmacol. 2014, 88, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.A. The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β-cell failure. Curr. Diabetes Rev. 2012, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lee, J.S.; Rodgers, M.; Min, C.K.; Lee, J.Y.; Kim, H.J.; Lee, K.H.; Kim, C.J.; Oh, B.; Zandi, E.; et al. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 2012, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Herance, R.; Rovira, S.; Hernández-Mijares, A.; Victor, V.M. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect. Disord. Drug Targets 2012, 12, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Blandino-Rosano, M.; Chen, A.Y.; Scheys, J.O.; Alejandro, E.U.; Gould, A.P.; Taranukha, T.; Elghazi, L.; Cras-Méneur, C.; Bernal-Mizrachi, E. mTORC1 signaling and regulation of pancreatic β-cell mass. Cell Cycle 2012, 11, 1892–1902. [Google Scholar] [CrossRef]
- Berggreen, C.; Gormand, A.; Omar, B.; Degerman, E.; Göransson, O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E635–E646. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-M.; Joo, S.S. Serotonin Influences Insulin Secretion in Rat Insulinoma INS-1E Cells. Int. J. Mol. Sci. 2024, 25, 6828. https://doi.org/10.3390/ijms25136828
Yoo Y-M, Joo SS. Serotonin Influences Insulin Secretion in Rat Insulinoma INS-1E Cells. International Journal of Molecular Sciences. 2024; 25(13):6828. https://doi.org/10.3390/ijms25136828
Chicago/Turabian StyleYoo, Yeong-Min, and Seong Soo Joo. 2024. "Serotonin Influences Insulin Secretion in Rat Insulinoma INS-1E Cells" International Journal of Molecular Sciences 25, no. 13: 6828. https://doi.org/10.3390/ijms25136828






