Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor
Abstract
1. Introduction
2. Results
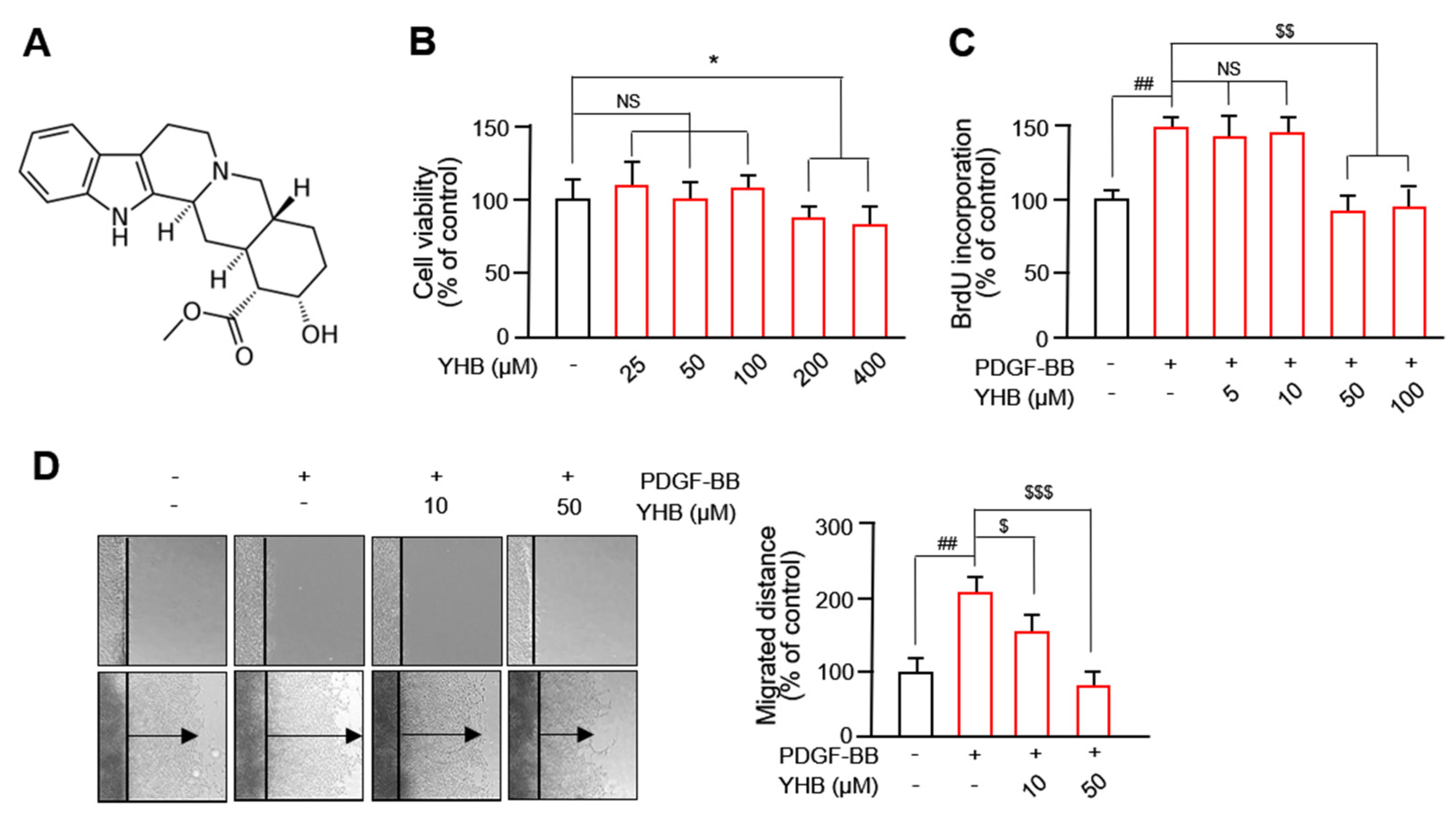
2.1. YHB Inhibits PDGF-BB-Induced VSMC Proliferation and Migration
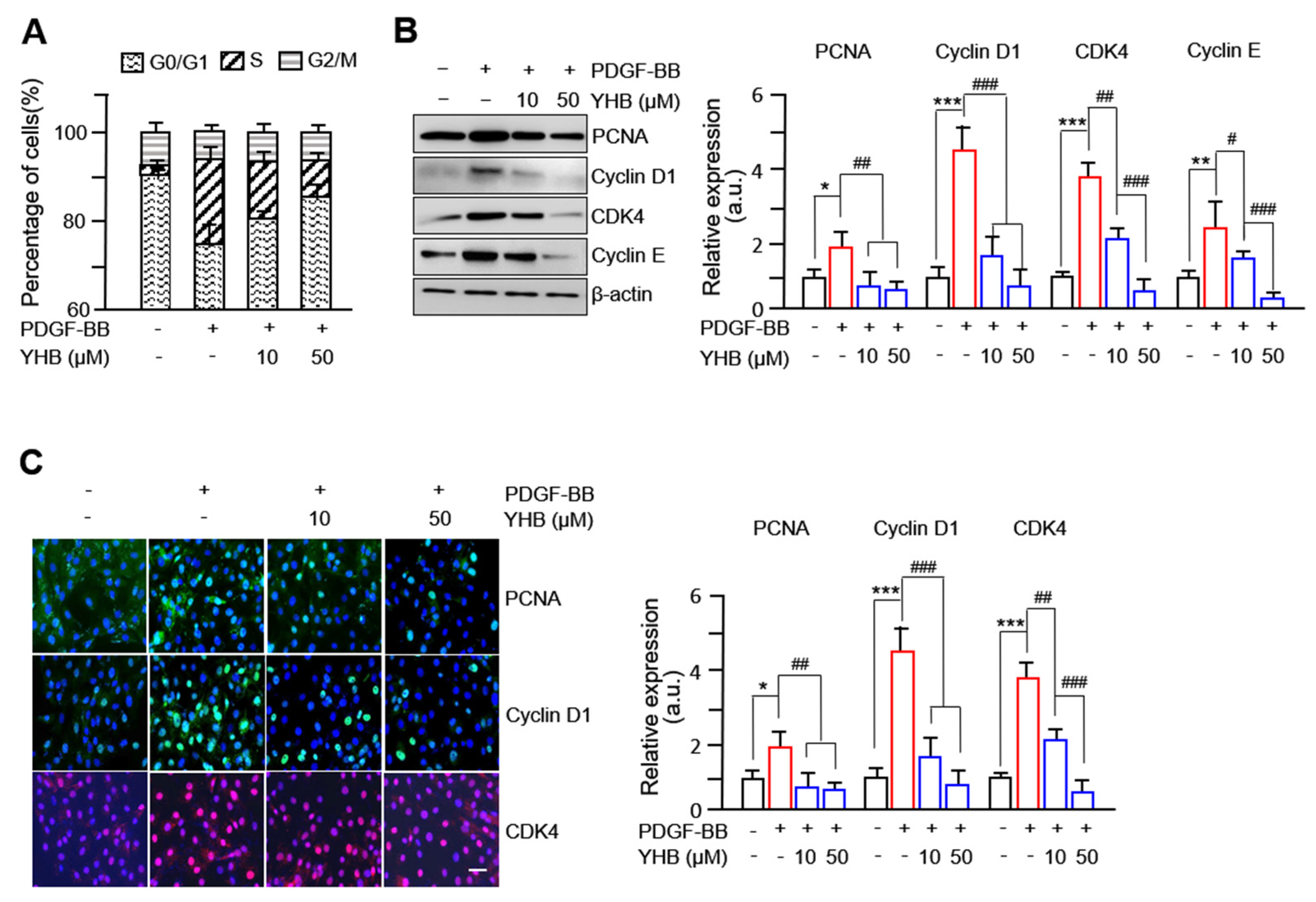
2.2. YHB Suppresses PDGF-BB-Induced Expression of Cell Cycle Regulatory Proteins in VSMCs
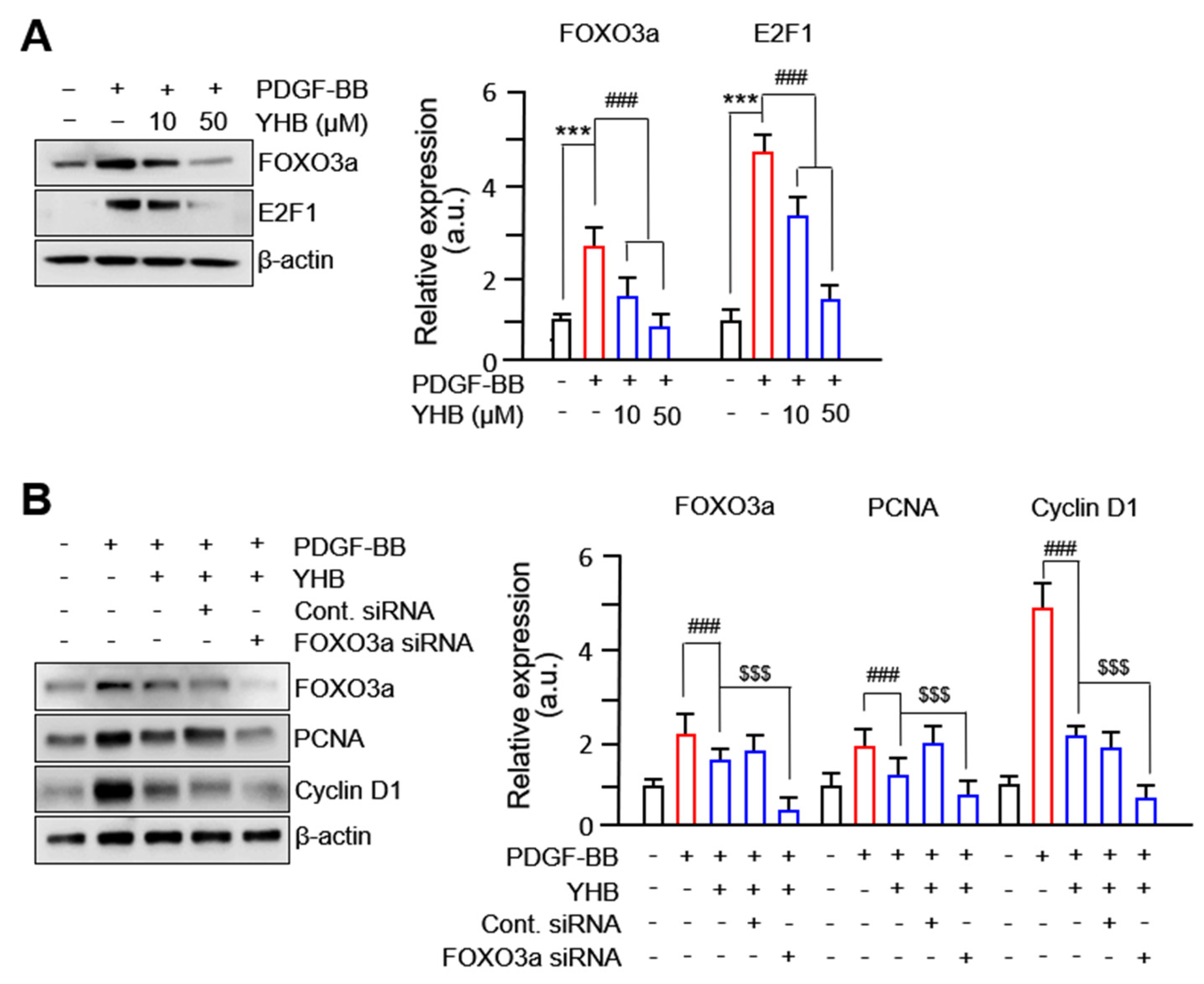
2.3. YHB Inhibits PDGF-BB-Induced VSMC Proliferation via Regulation of FOXO3a Factor
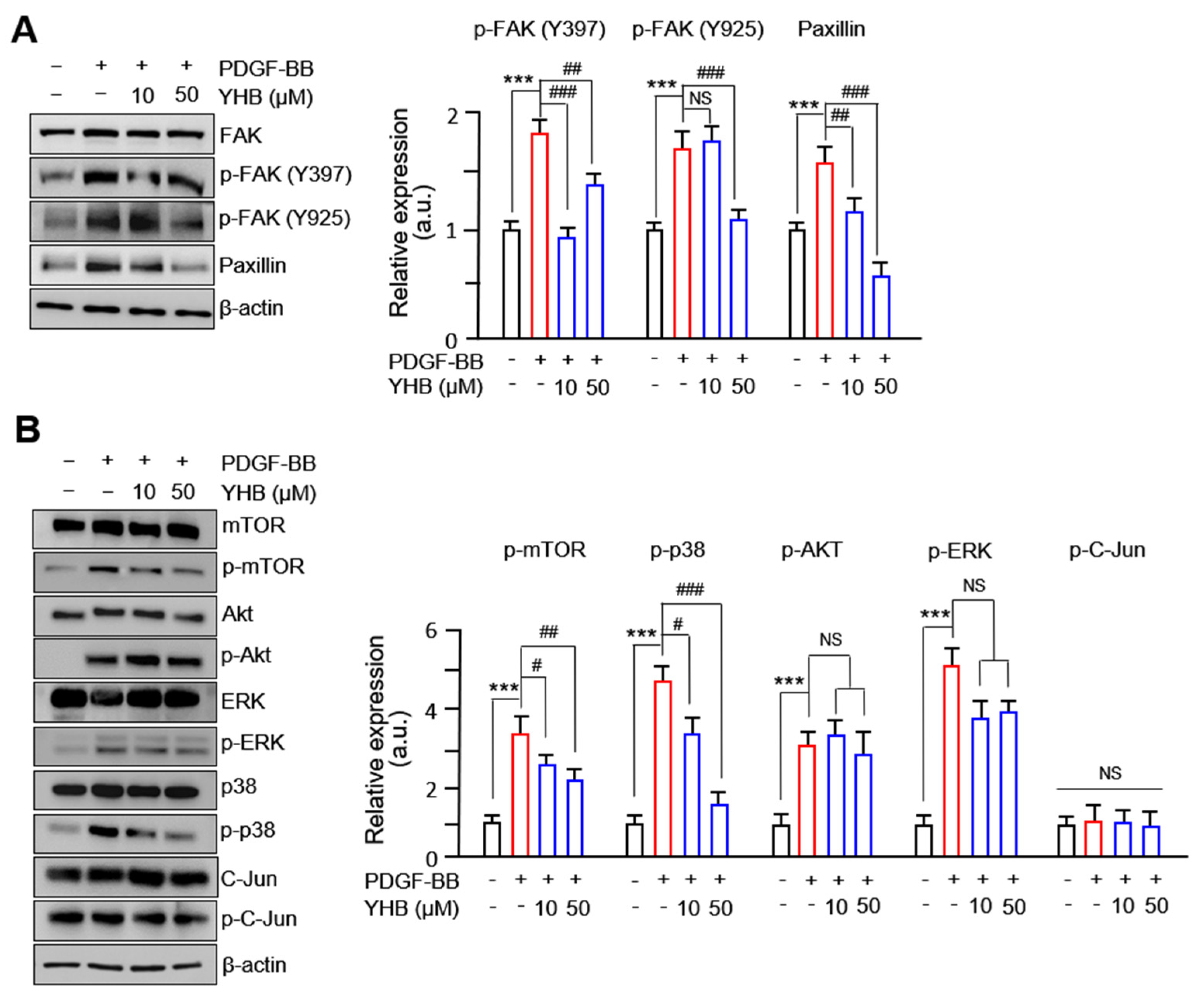
2.4. YHB Modulates FAK-Related and mTOR/p38 Signaling Pathway in PDGF-BB-Stimulated VSMCs
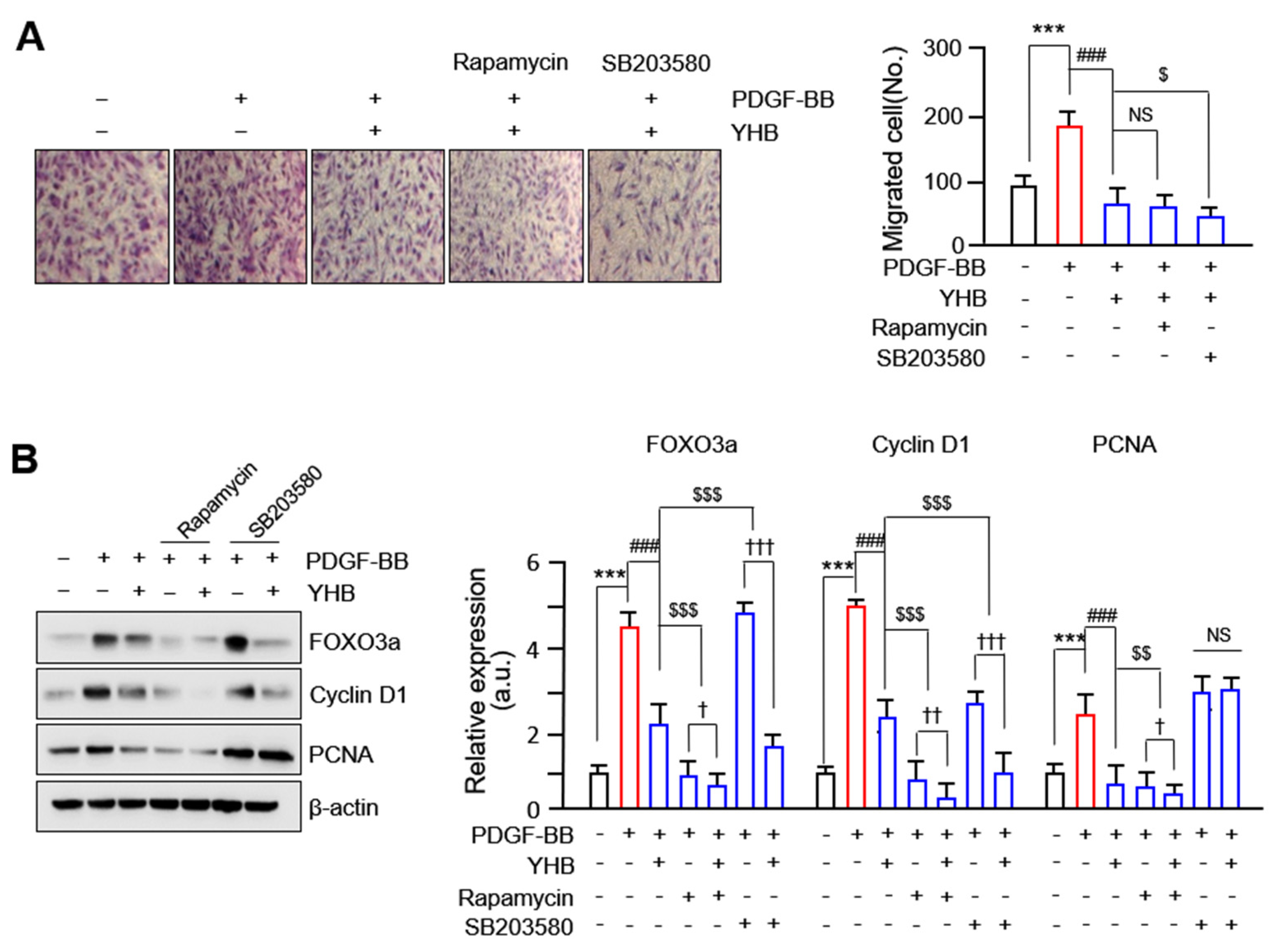
2.5. YHB Inhibits PDGF-BB-Induced VSMCs Proliferation and Migration Synergistically with mTOR/p38 Signaling Inhibition
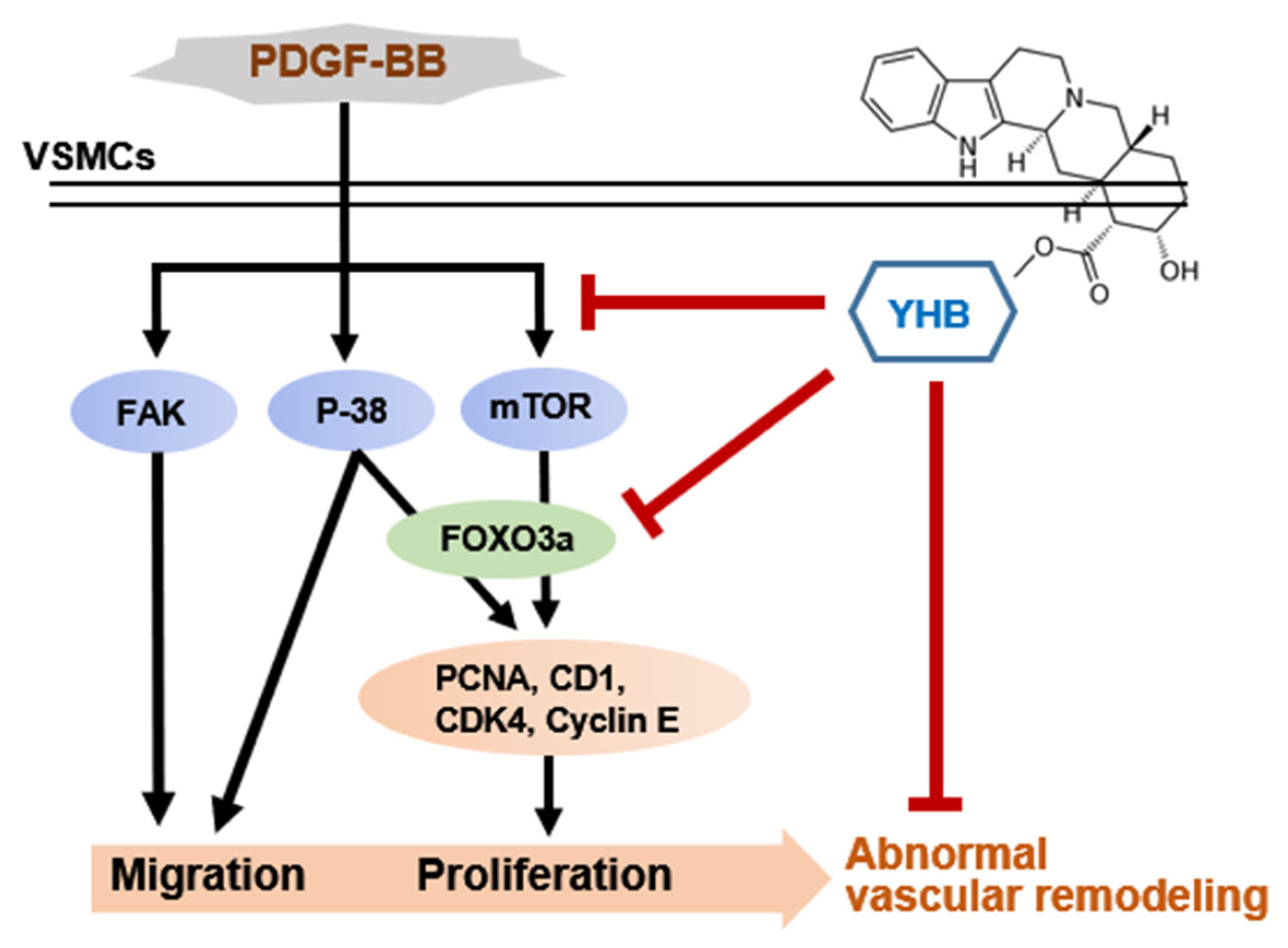
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of VSMCs
4.2. Cell Viability Assay
4.3. Cell Proliferation Assay
4.4. Cell Cycle Analysis
4.5. Cell Migration Assay
4.6. Immunocytochemistry
4.7. Immunoblot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015, 214, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Raines, E.W.; Ferri, N. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J. Lipid Res. 2005, 46, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.Y.; Karki, R.; Paudel, K.R.; Sharma, B.R.; Adhikari, D.; Kim, D.W. Alkaloid rich fraction from Nelumbo nucifera targets VSMC proliferation and migration to suppress restenosis in balloon-injured rat carotid artery. Atherosclerosis 2016, 248, 179–189. [Google Scholar] [CrossRef]
- Ding, X.; Yan, Y.; Zhang, C.; Xu, X.; Yang, F.; Liu, Y.; Wang, G.; Qin, Y. OCT4 regulated neointimal formation in injured mouse arteries by matrix metalloproteinase 2-mediated smooth muscle cells proliferation and migration. J. Cell Physiol. 2021, 236, 5421–5431. [Google Scholar] [CrossRef]
- Kanda, Y.; Nishio, E.; Kuroki, Y.; Mizuno, K.; Watanabe, Y. Thrombin activates p38 mitogen-activated protein kinase in vascular smooth muscle cells. Life Sci. 2001, 68, 1989–2000. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Mayr, M.; Xu, Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J. Biol. Chem. 1999, 274, 25273–25280. [Google Scholar] [CrossRef]
- Ohashi, N.; Matsumori, A.; Furukawa, Y.; Ono, K.; Okada, M.; Iwasaki, A.; Miyamoto, T.; Nakano, A.; Sasayama, S. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2521–2526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahanshahi, M.; Nikmahzar, E.; Elyasi, L.; Babakordi, F.; Hooshmand, E. α(2)-Adrenoceptor-ir neurons’ density changes after single dose of clonidine and yohimbine administration in the hippocampus of male rat. Int. J. Neurosci. 2018, 128, 404–411. [Google Scholar] [CrossRef]
- Guay, A.T.; Spark, R.F.; Jacobson, J.; Murray, F.T.; Geisser, M.E. Yohimbine treatment of organic erectile dysfunction in a dose-escalation trial. Int. J. Impot. Res. 2002, 14, 25–31. [Google Scholar] [CrossRef][Green Version]
- Tasleem, M.; Alrehaily, A.; Almeleebia, T.M.; Alshahrani, M.Y.; Ahmad, I.; Asiri, M.; Alabdallah, N.M.; Saeed, M. Investigation of Antidepressant Properties of Yohimbine by Employing Structure-Based Computational Assessments. Curr. Issues Mol. Biol. 2021, 43, 1805–1827. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.A.; Newaz, M.; Hercule, H.; Saleh, M.; Bode, C.O.; Oyekan, A.O. Endothelin-like action of Pausinystalia yohimbe aqueous extract on vascular and renal regional hemodynamics in Sprague Dawley rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, P.; Zhang, Z.; Li, K.; Liu, J.; Li, Q. Analysis of yohimbine alkaloid from Pausinystalia yohimbe by non-aqueous capillary electrophoresis and gas chromatography-mass spectrometry. J. Sep. Sci. 2008, 31, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Qin, X.; Ma, Y.; Yuan, X.; Wu, S.; Ye, X.; Dang, Y. Yohimbine hydrochloride inhibits skin melanin synthesis by regulating wnt/β-catenin and p38/MAPK signal pathways. J. Dermatol. Sci. 2022, 107, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Zhang, H.; Jia, B.; Wang, D.; Li, H.; Lu, D.; Qi, R.; Yan, Y.; Wang, H. Yohimbine enhances protection of berberine against LPS-induced mouse lethality through multiple mechanisms. PLoS ONE 2012, 7, e52863. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Yoneda, K.; Yamagata, M.; Hayashi, K.; Tomita, S. Yohimbine ameliorates lipopolysaccharide-induced acute kidney injury in rats. Eur. J. Pharmacol. 2020, 871, 172917. [Google Scholar] [CrossRef] [PubMed]
- Neha; Ansari, M.M.; Khan, H.A. Yohimbine hydrochloride ameliorates collagen type-II-induced arthritis targeting oxidative stress and inflammatory cytokines in Wistar rats. Environ. Toxicol. 2017, 32, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Tong, J.H.; Zhou, Z.Q.; Duan, M.L.; Xu, J.G.; Zeng, H.J.; Wang, S.H. Tramadol inhibits proliferation, migration and invasion via α2-adrenoceptor signaling in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 157–165. [Google Scholar] [PubMed]
- Shen, S.G.; Zhang, D.; Hu, H.T.; Li, J.H.; Wang, Z.; Ma, Q.Y. Effects of alpha-adrenoreceptor antagonists on apoptosis and proliferation of pancreatic cancer cells in vitro. World J. Gastroenterol. 2008, 14, 2358–2363. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Wang, F.; Wang, Y.; Wang, Y.; Li, H.; Lv, X.; Lu, D.; Wang, H. Yohimbine promotes cardiac NE release and prevents LPS-induced cardiac dysfunction via blockade of presynaptic α2A-adrenergic receptor. PLoS ONE 2013, 8, e63622. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, L.; Tang, J.; Zheng, J.; Witman, N.; Jakob, P.; Tan, Y.; Liu, M.; Chen, Y.; Wang, H.; et al. Yohimbine Directly Induces Cardiotoxicity on Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cardiovasc. Toxicol. 2022, 22, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.W.; Hsieh, C.Y.; Yang, C.H.; Tsai, J.H.; Huang, S.Y.; Sheu, J.R. Yohimbine, an α2-Adrenoceptor Antagonist, Suppresses PDGF-BB-Stimulated Vascular Smooth Muscle Cell Proliferation by Downregulating the PLCγ1 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8049. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fellows, A.; Foote, K.; Yang, Z.; Figg, N.; Littlewood, T.; Bennett, M. FOXO3a (Forkhead Transcription Factor O Subfamily Member 3a) Links Vascular Smooth Muscle Cell Apoptosis, Matrix Breakdown, Atherosclerosis, and Vascular Remodeling Through a Novel Pathway Involving MMP13 (Matrix Metalloproteinase 13). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, Y.; Zeng, S.; Zhong, L.; Zhou, S.; Song, H.; Ding, R.; Zhong, G.; Li, Q.; Hu, Y.; et al. Loss of FoxO3a prevents aortic aneurysm formation through maintenance of VSMC homeostasis. Cell Death Dis. 2021, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Kim, J.H.; Murphy, J.M.; Park, H.; Kim, S.J.; Rodriguez, Y.A.R.; Kong, H.; Choi, C.; Guan, J.L.; Taylor, J.M.; et al. Nuclear Focal Adhesion Kinase Controls Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia Through GATA4-Mediated Cyclin D1 Transcription. Circ. Res. 2019, 125, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R.; Norris, R.A.; Zhang, L.; Yu, S.; Wu, F.; Markwald, R.R.; Nanda, A.; Conway, S.J.; Smyth, S.S.; et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 2010, 208, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Chu, B.; Choi, D.H.; Lim, L.; Song, H. ETV2 Enhances CXCL5 Secretion from Endothelial Cells, Leading to the Promotion of Vascular Smooth Muscle Cell Migration. Int. J. Mol. Sci. 2023, 24, 9904. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor. Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Pan, D.; Yang, J.; Lu, F.; Xu, D.; Zhou, L.; Shi, A.; Cao, K. Platelet-derived growth factor BB modulates PCNA protein synthesis partially through the transforming growth factor beta signalling pathway in vascular smooth muscle cells. Biochem. Cell Biol. 2007, 85, 606–615. [Google Scholar] [CrossRef]
- Li, Y.Q.; Li, Y.L.; Li, X.T.; Lv, J.Y.; Gao, Y.; Li, W.N.; Gong, Q.H.; Yang, D.L. Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression. Front. Physiol. 2020, 11, 514494. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Zhou, Y.; Su, Q.; Liu, T.; Wang, X.T.; Li, L. Atorvastatin Inhibits Myocardial Apoptosis in a Swine Model of Coronary Microembolization by Regulating PTEN/PI3K/Akt Signaling Pathway. Cell Physiol. Biochem. 2016, 38, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Jayaraman, T.; Go, L.O.; Marks, A.R. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ. Res. 1995, 76, 412–417. [Google Scholar] [CrossRef]
- Poon, M.; Marx, S.O.; Gallo, R.; Badimon, J.J.; Taubman, M.B.; Marks, A.R. Rapamycin inhibits vascular smooth muscle cell migration. J. Clin. Investig. 1996, 98, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.; Pinney, S.; Burkhoff, D.; LaManca, J.; Itescu, S.; Burke, E.; Edwards, N.; Oz, M.; Marks, A.R. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation 2003, 108, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Rzucidlo, E.M.; Merenick, B.L.; Fingar, D.C.; Brown, D.J.; Wagner, R.J.; Powell, R.J. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am. J. Physiol. Cell Physiol. 2004, 286, C507–C517. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nada, S.; Kimura, H.; Tajima, S.; Takahashi, Y.; Kitamura, A.; Oneyama, C.; Okada, M. The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PLoS ONE 2014, 9, e88891. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wen, J.; Zhou, Y.; Li, L.; Cui, X.; Wang, J.; Pan, L.; Ye, Z.; Liu, P.; Wu, L. Apelin induces vascular smooth muscle cells migration via a PI3K/Akt/FoxO3a/MMP-2 pathway. Int. J. Biochem. Cell Biol. 2015, 69, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Tesio, M.; Golan, K.; Corso, S.; Giordano, S.; Schajnovitz, A.; Vagima, Y.; Shivtiel, S.; Kalinkovich, A.; Caione, L.; Gammaitoni, L.; et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 2011, 117, 419–428. [Google Scholar] [CrossRef]
- Pan, Q.; Xie, X.; Guo, Y.; Wang, H. Simvastatin promotes cardiac microvascular endothelial cells proliferation, migration and survival by phosphorylation of p70 S6K and FoxO3a. Cell Biol. Int. 2014, 38, 599–609. [Google Scholar] [CrossRef]
- Zhang, X.; Campreciós, G.; Rimmelé, P.; Liang, R.; Yalcin, S.; Mungamuri, S.K.; Barminko, J.; D’Escamard, V.; Baron, M.H.; Brugnara, C.; et al. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am. J. Hematol. 2014, 89, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.F.; Kelpke, S.S.; Oparil, S.; Thompson, J.A. Estrogen attenuates integrin-beta(3)-dependent adventitial fibroblast migration after inhibition of osteopontin production in vascular smooth muscle cells. Circulation 2000, 101, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Z.; Yue, L. Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway. Cancer Biomark. 2019, 25, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Tan, Y.; Deng, H.; Wu, W.; Mei, L.; Huang, X.; Qin, Y.; Zhu, H.; Liu, C. SRPX2 Promotes Tumor Proliferation and Migration via the FAK Pathway in Papillary Thyroid Carcinoma. J. Oncol. 2022, 2022, 5821545. [Google Scholar] [CrossRef]
- Li, J.J.; Han, M.; Wen, J.K.; Li, A.Y. Osteopontin stimulates vascular smooth muscle cell migration by inducing FAK phosphorylation and ILK dephosphorylation. Biochem. Biophys. Res. Commun. 2007, 356, 13–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, L.; Kim, H.; Jeong, J.; Han, S.H.; Yu, Y.-B.; Song, H. Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor. Int. J. Mol. Sci. 2024, 25, 6899. https://doi.org/10.3390/ijms25136899
Lim L, Kim H, Jeong J, Han SH, Yu Y-B, Song H. Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor. International Journal of Molecular Sciences. 2024; 25(13):6899. https://doi.org/10.3390/ijms25136899
Chicago/Turabian StyleLim, Leejin, Hyeonhwa Kim, Jihye Jeong, Sung Hee Han, Young-Bob Yu, and Heesang Song. 2024. "Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor" International Journal of Molecular Sciences 25, no. 13: 6899. https://doi.org/10.3390/ijms25136899
APA StyleLim, L., Kim, H., Jeong, J., Han, S. H., Yu, Y.-B., & Song, H. (2024). Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor. International Journal of Molecular Sciences, 25(13), 6899. https://doi.org/10.3390/ijms25136899




