Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility
Abstract
:1. Introduction
2. General Toxicity of Pesticides
2.1. Overall Toxicity
2.2. Neurotoxicity of Pesticides
2.3. Cardiotoxicity of Pesticides
2.4. Hepatotoxicity of Pesticides
2.5. Pulmonary Toxicity of Pesticides
| Organ | Pesticide, Dose | Effect | Species | Reference |
|---|---|---|---|---|
| Pulmonary toxicity | Cypermethrin (0.5%) in a time-dependent manner | An increase in the number of alveolar cells was observed following 10 d of cypermethrin exposure. | Albino mice | [84] |
| Alpha-cypermethrin, 14.5 mg/kg; fipronil, 40 mg/kg-BW | Inflammation of the lungs, leading to pulmonary edema, alveolitis, and pulmonary fibrosis, as well as an increase in lung weight across all treatment groups. | Albino rats, Wistar rats | [83,93] | |
| Methoxychlor and piperonyl butoxide, 30 or 300 mg/kg/day; parathion, 0.15 or 1.5 mg/kg/day | Caused allergic airway inflammation | NC/Nga mice | [91] | |
| Hepatotoxicity | Deltamethrin, 300 mg/kg diet, deltamethrin, 1.28 mg/kg-BW | Deltamethrin induced oxidative stress, leading to histopathological, biochemical, and physiological alterations in the kidney and liver. In addition, it congested and widened portal blood vessels, inflammatory cells between hepatic cords, and thickened the walls of hepatic blood vessels. | Cobb broiler chicks, Sprague Dawley rats | [79,94] |
| Chlorpyrifos, 5.4 mg/kg-BW | Elevated levels of serum enzymes, including ALP, ALT, AST, and LDH, following exposure. | Wistar rats | [23] | |
| Triflumuron, 350 mg/kg-BW | Induced oxidative stress and reactive oxygen species (ROS) generation in liver tissues. | BalbC mice | [76] | |
| DDT, 500 ppm | Hepatocellular adenomas and carcinomas | F344 rats | [78] | |
| Neurotoxicity | Deltamethrin, 7.2 mg/kg-BW | Decreased AChE activity, leading to brain dysfunction. | Wistar rats | [56] |
| Cyhalothrin and deltamethrin, 60 mg/kg | Cyhalothrin and deltamethrin decreased acetylcholine levels in the hippocampus of rats, whereas allethrin increased acetylcholine levels in the hippocampus. | Sprague Dawley rats | [59] | |
| Bifenthrin, 0.6 and 2.1 mg/kg-BW | Bifenthrin treatment significantly reduced Na+/K+-ATPase and Mg2+-ATPase activities in the hippocampus, decreased mRNA expression and protein levels of Nurr-1, and lowered AChE and BuChE activities. | Wistar Rats | [95] | |
| Allethrin, (10, 25, 50, 100, 200) μM | Allethrin reduced cell viability in human dopaminergic neuroblastoma SH-SY5Y cells, and elevated ROS levels. | Human | [96] | |
| Cardiac toxicity | Organophosphate, unclear dose | QT prolongation, resulting in torsade de pointes (TdP) and myocardial damage. | Human | [67,68] |
| Dichlorvos, 170 μM | Dichlorvos caused necrotic cell death in H9C2 cells by significantly increasing the levels of intracellular and mitochondrial ROS, thus triggering oxidative stress in cardiac cells. | Wistar rats | [71] | |
| Kidney | Deltamethrin, 300 mg/kg diet; deltamethrin 1.28 mg/kg-BW | Deltamethrin increased ALT, AST, urea, and creatinine in the serum of treated birds. It also led to severe kidney damage, evidenced by glomerular hyperplasia, necrosis, tubular dilation, epithelial cell sloughing, and the infiltration of lymphocytes. | Cobb broiler chicks, Sprague Dawley rats | [79,94] |
| Triflumuron, 500 mg/kg-BW | Increment in lipid peroxidation and antioxidant enzyme activity, as well as the deterioration of proteins. | Balb/C mice | [76] |
3. Effect of Pesticides on Reproductive Functions
3.1. General Reproductive Toxicity
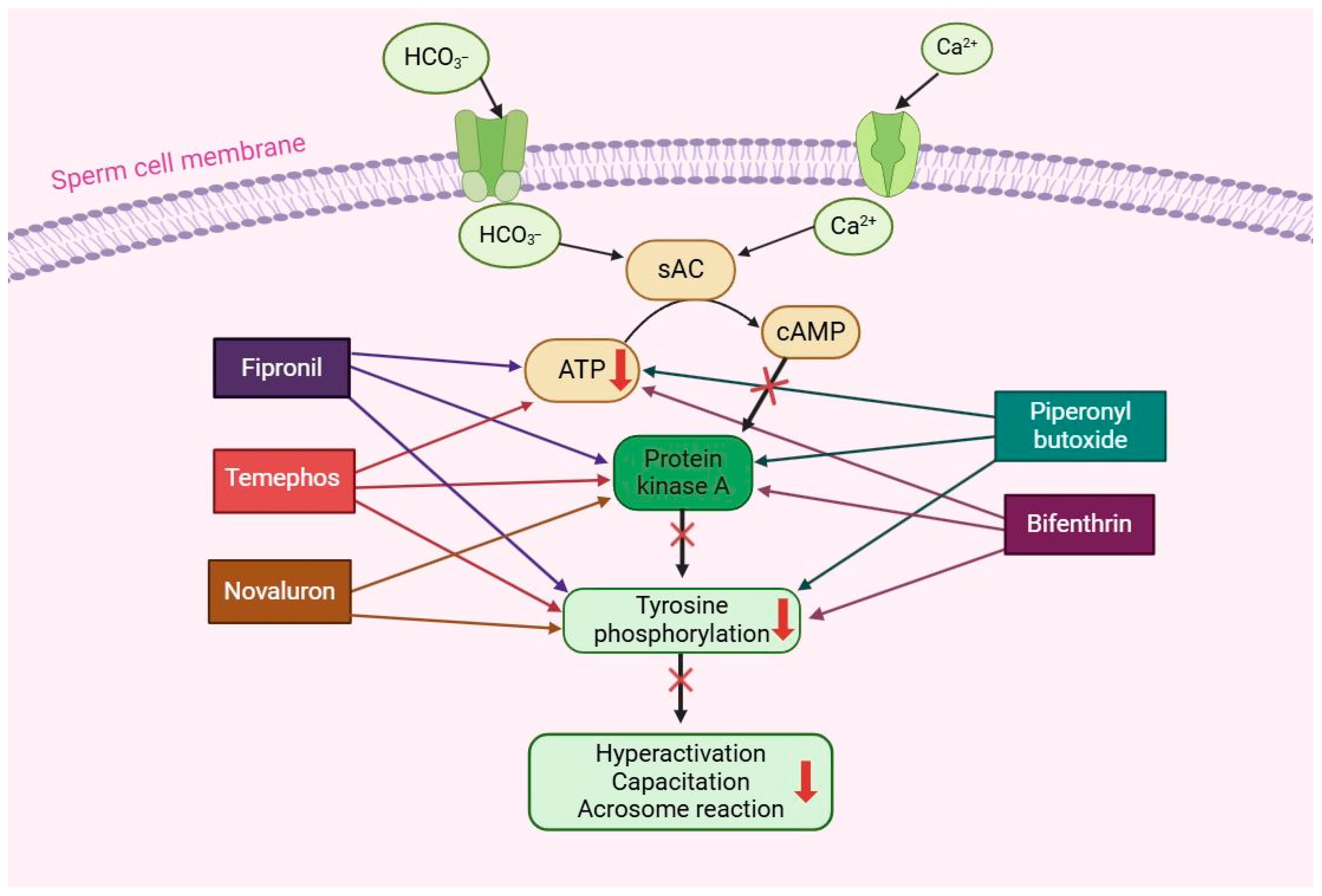
3.2. Effect of Pesticides on Male Fertility
4. Summary and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef] [PubMed]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. J. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Kumar, A.; Chery, L.; Biswas, C.; Dubhashi, N.; Dutta, P.; Dua, V.K.; Kacchap, M.; Kakati, S.; Khandeparkar, A.; Kour, D. Malaria in South Asia: Prevalence and control. Acta Trop. 2012, 121, 246–255. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, A.M.; Hill, J.; Noor, A.M.; Snow, R.W.; ter Kuile, F.O. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2015, 3, e617–e628. [Google Scholar] [CrossRef]
- Omotoso, G.; Onanuga, I.; Ibrahim, R. Histological effects of permethrin insecticide on the testis of adult wistar rats. Ibnosina J. Med. Biomed. Sci. 2014, 6, 125–129. [Google Scholar] [CrossRef]
- Wandiga, S.O. Use and distribution of organochlorine pesticides. The future in Africa. Pure Appl. Chem. 2001, 73, 1147–1155. [Google Scholar] [CrossRef]
- Almeida, V.E.S.d.; Friedrich, K.; Tygel, A.F.; Melgarejo, L.; Carneiro, F.F. Use of genetically modified crops and pesticides in Brazil: Growing hazards. Cienc. Saude Coletiva 2017, 22, 3333–3339. [Google Scholar] [CrossRef]
- Candiotto, L.Z.P.; De Souza, L.C.; Victorino, V.J.; Panis, C. Regulation and monitoring of pesticide residues in water and food in brazil. In Food Toxicology; Apple Academic Press: Williston, VT, USA, 2017; pp. 391–432. [Google Scholar]
- Stoytcheva, M. Pesticides in the Modern World: Pesticides Use and Management; BoD–Books on Demand: Norderstedt, Germany, 2011. [Google Scholar]
- Lozowicka, B.; Kaczynski, P.; Paritova, A.; Kuzembekova, G.; Abzhalieva, A.; Sarsembayeva, N.; Alihan, K. Pesticide residues in grain from Kazakhstan and potential health risks associated with exposure to detected pesticides. Food Chem. Toxicol. 2014, 64, 238–248. [Google Scholar] [CrossRef]
- Lamichhane, R.; Lama, N.; Subedi, S.; Singh, S.; Sah, R.; Yadav, B. Use of pesticides and health risk among farmers in Sunsari District, Nepal. J. Nepal Health Res. Counc. 2019, 17, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shanker, U. Highly efficient removal of endocrine disrupting pesticides by metal ferrites loaded Guar gum based green nanomaterials. J. Mol. Liq. 2023, 387, 122611. [Google Scholar]
- Zaki, N.I. Evaluation of profenofos intoxication in white rats. Nat. Sci. 2012, 10, 67–77. [Google Scholar]
- El-Nahhal, Y.; El-Nahhal, I. Cardiotoxicity of some pesticides and their amelioration. Environ. Sci. Pollut. Res. 2021, 28, 44726–44754. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, P.B. Host specificity and biological pest control. BioScience 1996, 46, 401–405. [Google Scholar] [CrossRef]
- Georgiadis, N.; Tsarouhas, K.; Tsitsimpikou, C.; Vardavas, A.; Rezaee, R.; Germanakis, I.; Tsatsakis, A.; Stagos, D.; Kouretas, D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018, 353, 1–14. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y. Acute Poisoning among Farmers by Chlorpyrifos: Case Report from Gaza Strip. Occup. Dis. Environ. Med. 2017, 5, 47–57. [Google Scholar] [CrossRef]
- Keifer, M.C.; Firestone, J. Neurotoxicity of pesticides. J. Agromed. 2007, 12, 17–25. [Google Scholar] [CrossRef]
- Vardavas, A.I.; Stivaktakis, P.D.; Tzatzarakis, M.N.; Fragkiadaki, P.; Vasilaki, F.; Tzardi, M.; Datseri, G.; Tsiaoussis, J.; Alegakis, A.K.; Tsitsimpikou, C. Long-term exposure to cypermethrin and piperonyl butoxide cause liver and kidney inflammation and induce genotoxicity in New Zealand white male rabbits. Food Chem. Toxicol. 2016, 94, 250–259. [Google Scholar] [CrossRef]
- Uzun, F.G.; Kalender, Y. Chlorpyrifos induced hepatotoxic and hematologic changes in rats: The role of quercetin and catechin. Food Chem. Toxicol. 2013, 55, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeed, F.A.; Naz, S.; Saeed, M.H.; Hussain, R.; Iqbal, S.; Mustafa Chatha, A.M.; Ghaffar, A.; Akram, R. Oxidative Stress, Antioxidant Enzymes, Genotoxicity and Histopathological Profile in Oreochromis niloticus Exposed to Lufenuron. Pak. Vet. J. 2023, 43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-M.; Bae, J.-W.; Jung, E.-J.; Lee, W.-J.; Kwon, W.-S. Novaluron has detrimental effects on sperm functions. Int. J. Environ. Res. Public Health 2021, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-W.; Kwon, W.-S. Investigating the effects of fipronil on male fertility: Insight into the mechanism of capacitation. Reprod. Toxicol. 2020, 94, 1–7. [Google Scholar] [CrossRef]
- Bae, J.-W.; Kwon, W.-S. The deleterious toxic effects of bifenthrin on male fertility. Reprod. Toxicol. 2021, 101, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Bae, J.-W.; Kim, D.-H.; Jeong, D.-J.; Ha, J.J.; Yi, J.K.; Kwon, W.-S. Detrimental effects of temephos on male fertility: An in vitro study on a mouse model. Reprod. Toxicol. 2020, 96, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ali Abd El-Rahman, H.; Omar, A.R. Ameliorative effect of avocado oil against lufenuron induced testicular damage and infertility in male rats. Andrologia 2022, 54, e14580. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-W.; Im, H.; Hwang, J.-M.; Kim, S.-H.; Ma, L.; Kwon, H.J.; Kim, E.; Kim, M.O.; Kwon, W.-S. Vanadium adversely affects sperm motility and capacitation status via protein kinase A activity and tyrosine phosphorylation. Reprod. Toxicol. 2020, 96, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-W.; Kwon, W.-S. Piperonyl butoxide, a synergist of pesticides can elicit male-mediated reproductive toxicity. Reprod. Toxicol. 2021, 100, 120–125. [Google Scholar] [CrossRef]
- Jalouli, M.; Mofti, A.; Elnakady, Y.A.; Nahdi, S.; Feriani, A.; Alrezaki, A.; Sebei, K.; Bizzarri, M.; Alwasel, S.; Harrath, A.H. Allethrin promotes apoptosis and autophagy associated with the oxidative stress-related PI3K/AKT/mTOR signaling pathway in developing rat ovaries. Int. J. Mol. Sci. 2022, 23, 6397. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; Song, G. Flufenoxuron suppresses the proliferation of testicular cells by targeting mitochondria in mice. Pestic. Biochem. Physiol. 2021, 173, 104773. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; You, S.; Lim, W.; Song, G. Flufenoxuron disturbs early pregnancy in pigs via induction of cell death with ER-mitochondrial dysfunction. J. Hazard. Mater. 2021, 401, 122996. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The pesticides case. Comptes Rendus-Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, L.; Li, J.; Liu, W.; Wang, Z.; Wu, Q.; Wang, C. Synthesis of amine-functionalized magnetic porous organic polymers for effective extraction of phenolic endocrine disrupting chemicals. J. Chromatogr. A 2023, 1706, 464271. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.; Plant, J.; Bell, J.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Zago, A.M.; Faria, N.M.; Favero, J.L.; Meucci, R.D.; Woskie, S.; Fassa, A.G. Pesticide exposure and risk of cardiovascular disease: A systematic review. Glob. Public Health 2022, 17, 3944–3966. [Google Scholar] [CrossRef] [PubMed]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current research on the safety of pyrethroids used as insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Maitra, S. Reproductive toxicity of organophosphate pesticides. Ann. Clin. Toxicol. 2018, 1, 1004. [Google Scholar]
- Anand, S.; Singh, S.; Nahar Saikia, U.; Bhalla, A.; Paul Sharma, Y.; Singh, D. Cardiac abnormalities in acute organophosphate poisoning. Clin. Toxicol. 2009, 47, 230–235. [Google Scholar] [CrossRef]
- Mortensen, A.S.; Arukwe, A. The persistent DDT metabolite, 1, 1-dichloro-2, 2-bis (p-chlorophenyl) ethylene, alters thyroid hormone-dependent genes, hepatic cytochrome P4503A, and pregnane× receptor gene expressions in atlantic salmon (Salmo salar) parr. Environ. Toxicol. Chem. Int. J. 2006, 25, 1607–1615. [Google Scholar] [CrossRef]
- Bretveld, R.W.; Thomas, C.M.; Scheepers, P.T.; Zielhuis, G.A.; Roeleveld, N. Pesticide exposure: The hormonal function of the female reproductive system disrupted? Reprod. Biol. Endocrinol. 2006, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Rathore, M.; Bhatnagar, P.; Mathur, D.; Saxena, G. Burden of organochlorine pesticides in blood and its effect on thyroid hormones in women. Sci. Total Environ. 2002, 295, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Garg, D.; Deb, R.; Samtani, R. Toxicological profile of organochlorines aldrin and dieldrin: An Indian perspective. Rev. Environ. Health 2017, 32, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Katz, F.S.; Pecic, S.; Tran, T.H.; Trakht, I.; Schneider, L.; Zhu, Z.; Ton-That, L.; Luzac, M.; Zlatanic, V.; Damera, S. Discovery of new classes of compounds that reactivate acetylcholinesterase inhibited by organophosphates. ChemBioChem 2015, 16, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- Sirin, G.S.; Zhang, Y. How is acetylcholinesterase phosphonylated by soman? An ab initio QM/MM molecular dynamics study. J. Phys. Chem. A 2014, 118, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Chauhan, A.; Jindal, T. In-silico and in-vitro evaluation of human acetylcholinesterase inhibition by organophosphates. Environ. Toxicol. Pharmacol. 2018, 57, 131–140. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; Furtado, M.D.A.; Braga, M.F. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef]
- Figueiredo, T.H.; Apland, J.P.; Braga, M.F.; Marini, A.M. Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia 2018, 59, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A. Experimental and clinical toxicology of anticholinesterase agents. Toxicol. Lett. 1998, 102, 509–513. [Google Scholar] [CrossRef]
- Amajad Iqbal, K. Neuroprotective Mechanisms Induced in Acute Organophosphate Poisoning. Ph.D. Dissertation, The Tamilnadu Dr. MGR Medical University, Chennai, India, 2010. [Google Scholar]
- Zaidi, N.; Soltani, N. Chronic toxicity of flucycloxuron in the mosquitofish, Gambusia affinis: Acetylcholinesterase and catalase activities and pattern of recovery. Ann. Biol. Res. 2010, 1, 210–217. [Google Scholar]
- Chen, J.; Ye, X.; Wang, J.; Xia, B.; Xin, T. Transcriptome analysis of Tetranychus cinnabarinus responses to exposure of an insecticide (diflubenzuron). Syst. Appl. Acarol. 2020, 25, 1329–1342. [Google Scholar]
- Saoudi, M.; Badraoui, R.; Bouhajja, H.; Ncir, M.; Rahmouni, F.; Grati, M.; Jamoussi, K.; El Feki, A. Deltamethrin induced oxidative stress in kidney and brain of rats: Protective effect of Artemisia campestris essential oil. Biomed. Pharmacother. 2017, 94, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Hess, L.; Tardivo, A.; Lajmanovich, R.; Attademo, A.; Poletta, G.; Simoniello, M.F.; Yodice, A.; Lavarello, S.; Chialvo, D. Neurologic dysfunction and genotoxicity induced by low levels of chlorpyrifos. Neurotoxicology 2014, 45, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Howard, A.; Bruun, D.; Ajua-Alemanj, M.; Pickart, C.; Lein, P.J. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol. Appl. Pharmacol. 2008, 228, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Suzuki, T.; Sato, I.; Takewaki, T.; Suzuki, K.; Kobayashi, H. The modulatory effect of pyrethroids on acetylcholine release in the hippocampus of freely moving rats. Neurotoxicology 2004, 25, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Suzuki, T.; Sato, I.; Takewaki, T.; Suzuki, K.; Kobayashi, H. Neuromechanical effects of pyrethroids, allethrin, cyhalothrin and deltamethrin on the cholinergic processes in rat brain. Life Sci. 2005, 77, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Nath Tiwari, M.; Prakash, O.; Pratap Singh, M. A current review of cypermethrin-induced neurotoxicity and nigrostriatal dopaminergic neurodegeneration. Curr. Neuropharmacol. 2012, 10, 64–71. [Google Scholar] [CrossRef]
- Yu-Tao, T.; Zhao-Wei, L.; Yang, Y.; Zhuo, Y.; Tao, Z. Effect of alpha-cypermethrin and theta-cypermethrin on delayed rectifier potassium currents in rat hippocampal neurons. Neurotoxicology 2009, 30, 269–273. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.; Li, X.; Sun, J.; Yang, H. Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats. Toxics 2023, 11, 999. [Google Scholar] [CrossRef]
- Magalhães, C.A.; Ferreira, C.N.; Loures, C.M.; Fraga, V.G.; Chaves, A.C.; Oliveira, A.C.R.; de Souza, L.C.; de PF Resende, E.; Carmona, K.C.; Guimarães, H.C. Leptin, hsCRP, TNF-α and IL-6 levels from normal aging to dementia: Relationship with cognitive and functional status. J. Clin. Neurosci. 2018, 56, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Wieggel, W.A.; Chen, J.; Bacchi, A.; Rogers, S.W.; Gahring, L.C. Inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α impart neuroprotection to an excitotoxin through distinct pathways. J. Immunol. 1999, 163, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Liu, W.; Wang, J.-L.; Zhang, Y.; Zhao, D.-J.; Wang, T.-J.; Li, Y.-Y. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 905. [Google Scholar] [PubMed]
- Karki, P.; Ansari, J.; Bhandary, S.; Koirala, S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singap. Med. J. 2004, 45, 385–389. [Google Scholar]
- Laudari, S.; Patowary, B.S.; Sharma, S.K.; Dhungel, S.; Subedi, K.; Bhattacharya, R.; Guru-Prasad, S.; Gangapatnam, S. Cardiovascular effects of acute organophosphate poisoning. Asia Pac. J. Med. Toxicol. 2014, 3, 64–67. [Google Scholar]
- Howard, M.D.; Mirajkar, N.; Karanth, S.; Pope, C.N. Comparative effects of oral chlorpyrifos exposure on cholinesterase activity and muscarinic receptor binding in neonatal and adult rat heart. Toxicology 2007, 238, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zafiropoulos, A.; Tsarouhas, K.; Tsitsimpikou, C.; Fragkiadaki, P.; Germanakis, I.; Tsardi, M.; Maravgakis, G.; Goutzourelas, N.; Vasilaki, F.; Kouretas, D. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum. Exp. Toxicol. 2014, 33, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.B.; Boussabbeh, M.; Da Silva, J.P.; Saidi, N.E.; Abid-Essefi, S.; Lemaire, C. Effects of Dichlorvos on cardiac cells: Toxicity and molecular mechanism of action. Chemosphere 2023, 330, 138714. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Sethi, S.; Benard, L.; Moshier, E.; Haraldsson, B.; Buettner, C. Perinatal DDT exposure induces hypertension and cardiac hypertrophy in adult mice. Environ. Health Perspect. 2016, 124, 1722–1727. [Google Scholar] [CrossRef]
- Vadhana, M.D.; Arumugam, S.S.; Carloni, M.; Nasuti, C.; Gabbianelli, R. Early life permethrin treatment leads to long-term cardiotoxicity. Chemosphere 2013, 93, 1029–1034. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Zama, D.; Meraihi, Z.; Tebibel, S.; Benayssa, W.; Benayache, F.; Benayache, S.; Vlietinck, A. Chlorpyrifos-induced oxidative stress and tissue damage in the liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J. Pharmacol. 2007, 39, 145. [Google Scholar] [CrossRef]
- Timoumi, R.; Amara, I.; Neffati, F.; Najjar, M.F.; El Golli-Bennour, E.; Bacha, H.; Abid-Essefi, S. Acute triflumuron exposure induces oxidative stress responses in liver and kidney of Balb/C mice. Environ. Sci. Pollut. Res. 2019, 26, 3723–3730. [Google Scholar] [CrossRef]
- Jellali, R.; Gilard, F.; Pandolfi, V.; Legendre, A.; Fleury, M.J.; Paullier, P.; Legallais, C.; Leclerc, E. Metabolomics-on-a-chip approach to study hepatotoxicity of DDT, permethrin and their mixtures. J. Appl. Toxicol. 2018, 38, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Takeda, M.; Kojima, S.; Tomiyama, N. Toxicity and carcinogenicity of dichlorodiphenyltrichloroethane (DDT). Toxicol. Res. 2016, 32, 21–33. [Google Scholar] [CrossRef]
- Allam, A.; Abdeen, A.; Devkota, H.P.; Ibrahim, S.S.; Youssef, G.; Soliman, A.; Abdel-Daim, M.M.; Alzahrani, K.J.; Shoghy, K.; Ibrahim, S.F. N-acetylcysteine alleviated the deltamethrin-induced oxidative cascade and apoptosis in liver and kidney tissues. Int. J. Environ. Res. Public Health 2022, 19, 638. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, N.K.; Soni, I.; Mathur, P. Hepatotoxicity of cyfluthrin after acute exposure in Swiss albino mice. Bull. Environ. Pharmacol. Life Sci. 2015, 4, 128–134. [Google Scholar]
- Jebur, A.B.; Nasr, H.M.; El-Demerdash, F.M. Selenium modulates β-cyfluthrin-induced liver oxidative toxicity in rats. Environ. Toxicol. 2014, 29, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.; Dosemeci, M.; Samanic, C.; Lubin, J.; Lynch, C.F.; Knott, C.; Barker, J.; Hoppin, J.A.; Sandler, D.P.; Coble, J. Pesticides and lung cancer risk in the agricultural health study cohort. Am. J. Epidemiol. 2004, 160, 876–885. [Google Scholar] [CrossRef]
- Arafa, M.H.; Mohamed, D.A.; Atteia, H.H. Ameliorative effect of N-acetyl cysteine on alpha-cypermethrin-induced pulmonary toxicity in male rats. Environ. Toxicol. 2015, 30, 26–43. [Google Scholar] [CrossRef]
- SHEIKH, N.; JAVED, S.; Asmatullah, A.; AHMAD, K.R.; ABBAS, T.; IQBAL, J. Histological changes in the lung and liver tissues in mice exposed to pyrethroid inhalation. Walailak J. Sci. Technol. (WJST) 2014, 11, 843–849. [Google Scholar]
- Angelini, D.J.; Moyer, R.A.; Cole, S.; Willis, K.L.; Oyler, J.; Dorsey, R.M.; Salem, H. The pesticide metabolites paraoxon and malaoxon induce cellular death by different mechanisms in cultured human pulmonary cells. Int. J. Toxicol. 2015, 34, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Diesel, B.; Zarbock, R.; Breinig, T.; Monz, D.; Koch, M.; Meyerhans, A.; Gortner, L.; Lehr, C.-M.; Huwer, H. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Ben, D.-F.; Yu, X.-Y.; Ji, G.-Y.; Zheng, D.-Y.; Lv, K.-Y.; Ma, B.; Xia, Z.-F. TLR4 mediates lung injury and inflammation in intestinal ischemia-reperfusion. J. Surg. Res. 2012, 174, 326–333. [Google Scholar] [CrossRef]
- Merkowsky, K.; Sethi, R.S.; Gill, J.P.; Singh, B. Fipronil induces lung inflammation in vivo and cell death in vitro. J. Occup. Med. Toxicol. 2016, 11, 10. [Google Scholar] [CrossRef]
- Pandit, A.A.; Gandham, R.K.; Mukhopadhyay, C.; Verma, R.; Sethi, R. Transcriptome analysis reveals the role of the PCP pathway in fipronil and endotoxin-induced lung damage. Respir. Res. 2019, 20, 24. [Google Scholar] [CrossRef]
- Schaale, K.; Neumann, J.; Schneider, D.; Ehlers, S.; Reiling, N. Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur. J. Cell Biol. 2011, 90, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Nishino, R.; Fukuyama, T.; Tajima, Y.; Miyashita, L.; Watanabe, Y.; Ueda, H.; Kosaka, T. Prior oral exposure to environmental immunosuppressive chemicals methoxychlor, parathion, or piperonyl butoxide aggravates allergic airway inflammation in NC/Nga mice. Toxicology 2013, 309, 1–8. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Walker, J.K.; Berman, K.G.; Foster, W.M.; Schwartz, D.A. Allergen-induced airway disease is mouse strain dependent. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 285, L32–L42. [Google Scholar] [CrossRef]
- Kingsley, I.A.; Sunday, I.P.; Blessing, A.A. Histological assessment of the Effects of PyrethroidsinsecticideMorteinon the Lungs of Adult WistarRats. IOSR J. Dent. Med. Sci. 2015, 14, 77–80. [Google Scholar]
- Küçükler, S.; Kandemir, F.M.; Özdemir, S.; Çomaklı, S.; Caglayan, C. Protective effects of rutin against deltamethrin-induced hepatotoxicity and nephrotoxicity in rats via regulation of oxidative stress, inflammation, and apoptosis. Environ. Sci. Pollut. Res. 2021, 28, 62975–62990. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Bouchard, M.; Saliba, S.W.; Fetoui, H.; Fiebich, B.L. Repeated bifenthrin exposure alters hippocampal Nurr-1/AChE and induces depression-like behavior in adult rats. Behav. Brain Res. 2019, 370, 111898. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Barrios-Arpi, L.; Ramos-Gonzalez, M.; Vidal, P.; Gonzales-Irribarren, A.; Ramos-Cevallos, N.; Rodríguez, J.-L. Neurotoxicity associated with oxidative stress and inflammasome gene expression induced by allethrin in SH-SY5Y cells. Toxicol. Ind. Health 2022, 38, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kwack, S.J.; Kim, H.S.; Lee, B.-M. Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of actions on putative human diseases. J. Toxicol. Environ. Health Part B 2014, 17, 127–174. [Google Scholar] [CrossRef]
- Pascotto, V.M.; Guerra, M.T.; Franci, J.A.A.; de Camargo, J.L.V.; Kempinas, W.G.; Franchi, C.A. Effects of a mixture of pesticides on the adult female reproductive system of Sprague-Dawley, Wistar, and Lewis rats. J. Toxicol. Environ. Health Part A 2015, 78, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.A.; Rizvi, F.; Khan, M.Z.; Khan, A. Toxic effects of cypermethrin and methamidophos on bovine corpus luteal cells and progesterone production. Exp. Toxicol. Pathol. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Ohi, M.; Dalsenter, P.; Andrade, A.; Nascimento, A. Reproductive adverse effects of fipronil in Wistar rats. Toxicol. Lett. 2004, 146, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ryan, L.; Barr, D.B.; Hauser, R. Exposure to nonpersistent insecticides and male reproductive hormones. Epidemiology 2006, 17, 61–68. [Google Scholar] [CrossRef]
- Bagherpour, H.; Malekshah, A.K.; Amiri, F.T.; Azadbakht, M. Protective effect of green tea extract on the deltamethrin-induced toxicity in mice testis: An experimental study. Int. J. Reprod. BioMed. 2019, 17, 337. [Google Scholar] [CrossRef]
- De Barros, A.L.; Bae, J.H.; Borges, C.S.; Rosa, J.L.; Cavariani, M.M.; Silva, P.V.; Pinheiro, P.F.; Anselmo-Franci, J.A.; Arena, A.C. Perinatal exposure to insecticide fipronil: Effects on the reproductive system in male rats. Reprod. Fertil. Dev. 2017, 29, 1130–1143. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.-Y.; Zhang, R.; Hong, J.-W.; Li, Z.; Ding, Z.; Wang, H.-X.; Zhang, J.-P.; Zhang, M.-R.; Xu, L.-C. Effects and mechanisms of pyrethroids on male reproductive system. Toxicology 2020, 438, 152460. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; Yeh, S.; Tzeng, C.-R.; Chang, C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009, 30, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Shen, O.; Sun, H.; Fei, J.; Lu, C.; Song, L.; Xia, Y.; Wang, S.; Wang, X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-L.; Ding, Z.; Ge, X.; Shi, Q.-M.; Wang, H.-X.; Chen, G.; Li, M.-X.; Wang, H.; Ju, Q.; Wang, Q. Cypermethrin inhibits interleukin-6-induced androgen receptor transactivation through signal transducer and activator of transcription 3. Toxicol. Mech. Methods 2017, 27, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of environmental toxin exposure on male fertility potential. Transl. Androl. Urol. 2020, 9, 2797. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Pereira, S.C.; Seco-Rovira, V.; Oliveira, P.F.; Alves, M.G.; Pereira, M.d.L. Pesticides and male fertility: A dangerous crosstalk. Metabolites 2021, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; El Bohy, K.M.; El Sharkawy, N.I.; Imam, T.S.; El-Metwally, A.E.; Hamed Arisha, A.; Mohammed, H.A.; Abd-Elhakim, Y.M. Iprodione and chlorpyrifos induce testicular damage, oxidative stress, apoptosis and suppression of steroidogenic-and spermatogenic-related genes in immature male albino rats. Andrologia 2021, 53, e13978. [Google Scholar] [CrossRef]
- Sardar, A.; David, M.; Jahan, S.; Afsar, T.; Ahmad, A.; Ullah, A.; Almajwal, A.; Shafique, H.; Razak, S. Determination of biochemical and histopathological changes on testicular and epididymis tissues induced by exposure to insecticide Imidacloprid during postnatal development in rats. BMC Pharmacol. Toxicol. 2023, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Kobir, M.A.; Akter, L.; Sultana, N.; Pervin, M.; Awal, M.A.; Karim, M.R. Effects of imidacloprid-contaminated feed exposure on spermatogenic cells and Leydig cells in testes of adult male rabbits (Oryctolagus cuniculus). Saudi J. Biol. Sci. 2023, 30, 103541. [Google Scholar]
- Abarikwu, S.O.; Adesiyan, A.C.; Oyeloja, T.O.; Oyeyemi, M.O.; Farombi, E.O. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 2010, 58, 874–882. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Wang, Y.; Zhou, X.; Wang, S.; Wang, Z. Meta-analysis and experimental validation identified atrazine as a toxicant in the male reproductive system. Environ. Sci. Pollut. Res. 2021, 28, 37482–37497. [Google Scholar] [CrossRef] [PubMed]
- Erthal, R.P.; Staurengo-Ferrari, L.; Fattori, V.; Luiz, K.G.; Cunha, F.Q.; Pescim, R.R.; Cecchini, R.; Verri Jr, W.A.; Guarnier, F.A.; Fernandes, G.S.A. Exposure to low doses of malathion during juvenile and peripubertal periods impairs testicular and sperm parameters in rats: Role of oxidative stress and testosterone. Reprod. Toxicol. 2020, 96, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Zou, C.; Zhu, Q.; Wang, Y.; Chen, H.; Yang, W.; Tu, Y.; Yan, H.; Li, X. Cypermethrin inhibits Leydig cell development and function in pubertal rats. Environ. Toxicol. 2022, 37, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Nassar, A.M.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.; Hassan, S.M.; Saati, A.A.; Hetta, H.F. Chemo-protective potential of cerium oxide nanoparticles against fipronil-induced oxidative stress, apoptosis, inflammation and reproductive dysfunction in male white albino rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Mirzaiinajmabadi, K.; Roudsari, R.L.; Rakhshkhorshid, M. The factors affecting male infertility: A systematic review. Int. J. Reprod. Biomed. 2021, 19, 681. [Google Scholar] [CrossRef]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ewen, K.A.; Koopman, P. Mouse germ cell development: From specification to sex determination. Mol. Cell. Endocrinol. 2010, 323, 76–93. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reprod.-Camb. 2001, 121, 347–354. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, C.; Gao, S.-Q.; Kong, T.-T.; Chen, L.; Li, X.-F.; Song, L.; Wang, Y.-B. Effects of permethrin, cypermethrin and 3-phenoxybenzoic acid on rat sperm motility in vitro evaluated with computer-assisted sperm analysis. Toxicol. Vitr. 2010, 24, 382–386. [Google Scholar] [CrossRef]
- Chakraborty, S.; Saha, S. Understanding sperm motility mechanisms and the implication of sperm surface molecules in promoting motility. Middle East Fertil. Soc. J. 2022, 27, 4. [Google Scholar] [CrossRef]
- Kwon, W.-S.; Park, Y.-J.; Kim, Y.-H.; You, Y.-A.; Kim, I.C.; Pang, M.-G. Vasopressin effectively suppresses male fertility. PLoS ONE 2013, 8, e54192. [Google Scholar] [CrossRef] [PubMed]
- Sagare-Patil, V.; Vernekar, M.; Galvankar, M.; Modi, D. Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol. Cell. Endocrinol. 2013, 374, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lim, W.; You, S.; Song, G. Butylated hydroxyanisole induces testicular dysfunction in mouse testis cells by dysregulating calcium homeostasis and stimulating endoplasmic reticulum stress. Sci. Total Environ. 2020, 702, 134775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Li, W.; Yue, L.; Zhang, T.; Tang, Q.; Zhang, N.; Lan, X.; Pan, C. Chlorpyrifos induces male infertility in pigs through ROS and PI3K-AKT pathway. Iscience 2023, 26, 106558. [Google Scholar] [CrossRef] [PubMed]
| Pesticide, Dose | Effect | Species | Reference |
|---|---|---|---|
| Chlorpyrifos, 7.45 mg/kg-BW |
| Albino rats | [110] |
| Iprodione, 200 mg/kg-BW and their mixtures | |||
| Imidacloprid, 5 and 10 mg/kg |
| Dawley rats | [111] |
| Imidacloprid, (0.5 mL (100 mg)/L was sprayed on green grass in the field) | Rabbit | [112] | |
| Atrazine, 200 mg/kg-BW |
| Wistar rats | [113] |
| Atrazine, 120 mg/kg-BW | Sprague Dawley rats | [114] | |
| Malathion, 10 or 50 mg/kg-BW |
| Wistar rats | [115] |
| Mixture of dichlorvos (2.3), dicofol (2.1), dieldrin (0.05), endosulfan (3.8), and permethrin (25) (mg/kg) |
| Sprague Dawley, Wistar, and Lewis rats | [98] |
| Cypermethrin (10 ppm) and methamidophos (10 ppm) |
| Bovine | [99] |
| Cypermethrin, 50 mg/kg-BW/day and 200 μM | Sprague Dawley rats | [116] | |
| Fipronil, 5 mg/kg-BW |
| Albino rats | [117] |
| Deltamethrin, 0.6 mg/kg-BW |
| Mice | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwamahoro, C.; Jo, J.-H.; Jang, S.-I.; Jung, E.-J.; Lee, W.-J.; Bae, J.-W.; Kwon, W.-S. Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility. Int. J. Mol. Sci. 2024, 25, 6945. https://doi.org/10.3390/ijms25136945
Uwamahoro C, Jo J-H, Jang S-I, Jung E-J, Lee W-J, Bae J-W, Kwon W-S. Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility. International Journal of Molecular Sciences. 2024; 25(13):6945. https://doi.org/10.3390/ijms25136945
Chicago/Turabian StyleUwamahoro, Claudine, Jae-Hwan Jo, Seung-Ik Jang, Eun-Ju Jung, Woo-Jin Lee, Jeong-Won Bae, and Woo-Sung Kwon. 2024. "Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility" International Journal of Molecular Sciences 25, no. 13: 6945. https://doi.org/10.3390/ijms25136945





