Meconium Proteins Involved in Iron Metabolism
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Meconium Collection and Homogenate Preparation
4.3. Laboratory Methods
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelić, M.; Ganz, T.; Nemeth, E. Effects of maternal iron status on placental and fetal iron homeostasis. J. Clin. Investig. 2020, 130, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lou, J.; Rao, R.; Georgieff, M.K.; Kaciroti, N.; Felt, B.T.; Zhao, Z.-Y.; Lozoff, B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutr. 2012, 142, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. S1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.K.; Dutta, P.; Parimi, S.; Das, N.K. The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites 2022, 12, 609. [Google Scholar] [CrossRef]
- Holbein, B.E.; Lehmann, C. Dysregulated Iron Homeostasis as Common Disease Etiology and Promising Therapeutic Target. Antioxidants 2023, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, A.M.; Rao, R.; Long, J.D.; Widness, J.A.; Georgieff, M.K. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology 2007, 92, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Skarżyńska, E.; Mularczyk, K.; Issat, T.; Jakimiuk, A.; Lisowska-Myjak, B. Meconium Transferrin and Ferritin as Markers of Homeostasis in the Developing Fetus. Int. J. Mol. Sci. 2023, 24, 15937. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Wilczyńska, P.; Bartoszewicz, Z.; Jakimiuk, A.; Skarżyńska, E. Can aminopeptidase N determined in the meconium be a candidate for biomarker of fetal intrauterine environment? Exp. Mol. Pathol. 2020, 115, 104446. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Skarżyńska, E.; Wojdan, K.; Nasierowska-Guttmejer, A. Protein and peptide profiles in neonatal meconium. J. Obstet. Gynaecol. Res. 2019, 45, 556–564. [Google Scholar] [CrossRef]
- DePalma, R.G.; Hayes, V.W.; O’Leary, T.J. Optimal serum ferritin level range: Iron status measure and inflammatory biomarker. Metallomics 2021, 13, mfab030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Petoukhov, M.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B.; Bartunik, H.; Svergun, D.I. Ceruloplasmin: Macromolecular assemblies with iron-containing acute phase proteins. PLoS ONE 2013, 8, e67145. [Google Scholar] [CrossRef] [PubMed]
- Fagoonee, S.; Gburek, J.; Hirsch, E.; Marro, S.; Moestrup, S.K.; Laurberg, J.M.; Christensen, E.I.; Silengo, L.; Altruda, F.; Tolosano, E. Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 2005, 166, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Bert, S.; Ward, E.J.; Nadkarni, S. Neutrophils in pregnancy: New insights into innate and adaptive immune regulation. Immunology 2021, 164, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Molina, B.; Muller, I.; Kropf, P.; Sykes, L. The Role of Neutrophils in Pregnancy, Term and Preterm Labour. Life 2022, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Zakahrova, E.T.; Kostevich, V.A.; Samygina, V.R.; Vasilyev, V.B. Lactoferrin, myeloperoxidase, and ceruloplasmin: Complementary gearwheels cranking physiological and pathological processes. BioMetals 2014, 27, 815–828, Erratum in BioMetals 2014, 27, 829. Zakahrova, Elena T [corrected to Zakharova, Elena T]. [Google Scholar] [CrossRef] [PubMed]
- Otnaess, A.K.; Meberg, A.; Sande, H.A. Plasma lactoferrin measured by an enzyme-linked immunosorbent assay (ELISA). Measurements on adult and infant plasma. Scand. J. Haematol. 1983, 31, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 5, 1018336. [Google Scholar] [CrossRef]
- Rocha-Penha, L.; Caldeira-Dias, M.; Tanus-Santos, J.E.; Cavalli, R.D.C.; Sandrim, V.C. Myeloperoxidase in Hypertensive Disorders of Pregnancy and Its Relation with Nitric Oxide. Hypertension 2017, 69, 1173–1180. [Google Scholar] [CrossRef]
- Mayyas, F.A.; Al-Jarrah, M.I.; Ibrahim, K.S.; Alzoubi, K.H. Level and significance of plasma myeloperoxidase and the neutrophil to lymphocyte ratio in patients with coronary artery disease. Exp. Ther. Med. 2014, 8, 1951–1957. [Google Scholar] [CrossRef][Green Version]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Yin, X.; Huo, Y.; Liu, L.; Pan, Y.; Liu, S.; Wang, R. Serum Levels and Placental Expression of NGAL in Gestational Diabetes Mellitus. Int. J. Endocrinol. 2020, 2020, 8760563. [Google Scholar] [CrossRef] [PubMed]
- Jarlborg, M.; Courvoisier, D.S.; Lamacchia, C.; Prat, L.M.; Mahler, M.; Bentow, C.; Finckh, A.; Gabay, C.; Nissen, M.J. Serum calprotectin: A promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res. Ther. 2020, 22, 105. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Zhang, B.; Krebs, C.; Nolan, E.M. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015, 11, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Skarżyńska, E.; Zborowska, H.; Jakimiuk, A.J.; Karlińska, M.; Lisowska-Myjak, B. Variations in serum concentrations of C-reactive protein, ceruloplasmin, lactoferrin and myeloperoxidase and their interactions during normal human pregnancy and postpartum period. J. Trace Elem. Med. Biol. 2018, 46, 83–87. [Google Scholar] [CrossRef]
- Jamka, M.; Krzyżanowska-Jankowska, P.; Mądry, E.; Lisowska, A.; Bogdański, P.; Walkowiak, J. No Difference in Lactoferrin Levels between Metabolically Healthy and Unhealthy Obese Women. Nutrients 2019, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Hoy, A.; Trégouët, D.; Leininger-Muller, B.; Poirier, O.; Maurice, M.; Sass, C.; Siest, G.; Tiret, L.; Visvikis, S. Serum myeloperoxidase concentration in a healthy population: Biological variations, familial resemblance and new genetic polymorphisms. Eur. J. Hum. Genet. 2001, 9, 780–786. [Google Scholar] [CrossRef][Green Version]
- Jahaj, E.; Vassiliou, A.G.; Pratikaki, M.; Gallos, P.; Mastora, Z.; Dimopoulou, I.; Orfanos, S.E.; Orfanos, P.; Lagiou, P.; Kotanidou, A. Serum Neutrophil Gelatinase-Associated Lipocalin (NGAL) Could Provide Better Accuracy Than Creatinine in Predicting Acute Kidney Injury Development in Critically Ill Patients. J. Clin. Med. 2021, 10, 5379. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Devarajan, P.; Ma, Q.; Harmon, K.; Monaco, M.; Cvijanovich, N.; Wong, H.R. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit. Care Med. 2008, 36, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Azramezani Kopi, T.; Shahrokh, S.; Mirzaei, S.; Asadzadeh Aghdaei, H.; Amini Kadijani, A. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: A review study. Gastroenterol. Hepatol. Bed Bench 2019, 12, 183–189. [Google Scholar] [PubMed] [PubMed Central]
- Kämmerer, L.; Mohammad, G.; Wolna, M.; Robbins, P.A.; Lakhal-Littleton, S. Fetal liver hepcidin secures iron stores in utero. Blood 2020, 136, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Sandnes, M.; Ulvik, R.J.; Vorland, M.; Reikvam, H. Hyperferritinemia—A Clinical Overview. J. Clin. Med. 2021, 10, 2008. [Google Scholar] [CrossRef]
- Skikne, B.S.; Whittaker, P.; Cooke, A.; Cook, J.D. Ferritin excretion and iron balance in humans. Br. J. Haematol. 1995, 90, 681–687. [Google Scholar] [CrossRef]
| Protein (µg/g) | Mean ± SD | Median | Range | CV (%) |
|---|---|---|---|---|
| Serum-derived Proteins in Meconium | ||||
| Ferritin | 78.6 ± 49.6 | 66.2 | 13.0–286.3 | 63.1 |
| Transferrin | 55.9 ± 109.1 | 12.6 | 1.1–655.7 | 194.9 |
| Haptoglobin | 1.2 ± 1.4 | 0.7 | 0.56–12.6 | 111.9 |
| Ceruloplasmin | 38.7 ± 38.4 | 24.7 | 0.9–218.5 | 99.4 |
| Neutrophil-derived Proteins in Meconium | ||||
| Lactoferrin | 34.1 ± 61.4 | 13.4 | 0.9–322.1 | 179.8 |
| Myeloperoxidase | 2.3 ± 3.6 | 1.4 | 0.04–22.0 | 154.6 |
| Neutrophil gelatinase-associated lipocalin | 2.5 ± 2.3 | 1.7 | 0.6–13.6 | 89.6 |
| Calprotectin | 239.6 ± 203.4 | 196.7 | 12.4–1284.7 | 84.9 |
| Ratios | Meconium | Serum | |
|---|---|---|---|
| Median | Range | ||
| Ferritin to transferrin | 4.79 | 0.05–150.31 | 5.4 × 10−5 |
| Ferritin to haptoglobin | 72.65 | 5.03–394.51 | 1.3 × 10−4 |
| Ferritin to ceruloplasmin | 2.46 | 0.15–133.58 | 5.8 × 10−4 |
| Transferrin to ferritin | 0.21 | 0.01–20.53 | 18,625.0 |
| Transferrin to haptoglobin | 17.81 | 1.53–222.31 | 2.5 |
| Transferrin to ceruloplasmin | 0.62 | 0.01–91.20 | 10.8 |
| Haptoglobin to ferritin | 0.014 | 0.003–0.20 | 7500.0 |
| Haptoglobin to transferrin | 0.056 | 0.005–0.65 | 0.4 |
| Haptoglobin to ceruloplasmin | 0.035 | 0.003–1.18 | 4.4 |
| Ceruloplasmin to ferritin | 0.41 | 0.01–6.63 | 1718.8 |
| Ceruloplasmin to transferrin | 1.61 | 0.01–86.38 | 0.1 |
| Ceruloplasmin to haptoglobin | 28.35 | 0.85–313.14 | 0.2 |
| Ratios | Meconium | Serum | |
|---|---|---|---|
| Median | Range | ||
| Lactoferrin to MPO | 12.65 | 0.90–111.93 | 32.27 |
| Lactoferrin to NGAL | 7.77 | 0.38–134.28 | 19.67 |
| Lactoferrin to calprotectin | 0.09 | 0.002–1.24 | 1.92 |
| MPO to lactoferrin | 0.08 | 0.01–1.11 | 0.03 |
| MPO to NGAL | 0.69 | 0.02–8.74 | 0.61 |
| MPO to calprotectin | 0.01 | 0.0002–0.10 | 0.06 |
| NGAL to lactoferrin | 0.13 | 0.01–2.66 | 0.05 |
| NGAL to MPO | 1.45 | 0.11–51.15 | 1.64 |
| NGAL to calprotectin | 0.01 | 0.002–0.64 | 01.0 |
| Calprotectin to lactoferrin | 11.75 | 0.81–559.52 | 0.52 |
| Calprotectin to MPO | 142.56 | 10.42–4559.29 | 16.79 |
| Calprotectin to NGAL | 93.88 | 1.55–604.32 | 10.24 |
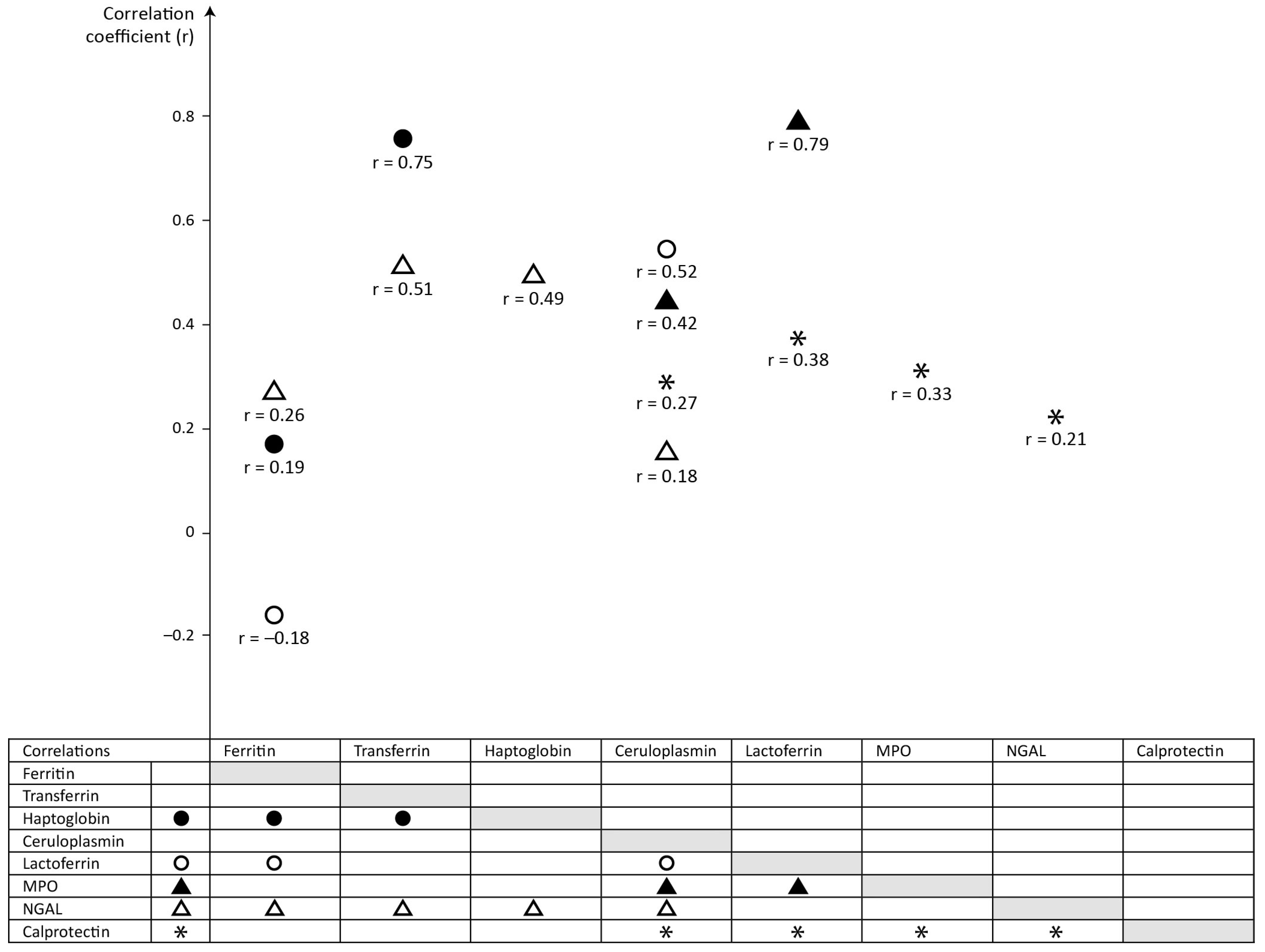
| Meconium Protein | Significant Correlations (p < 0.05) | |
|---|---|---|
| Serum-Derived Proteins | Neutrophil-Derived Proteins | |
| Ferritin | vs haptoglobin, r = 0.19, p = 0.038 | vs lactoferrin, r = −0.18, p = 0.042 vs NGAL, r = 0.26, p = 0.004 |
| Transferrin | vs haptoglobin, r = 0.75, p < 0.001 | vs NGAL, r = 0.51, p < 0.001 |
| Haptoglobin | vs ferritin, r = 0.19, p = 0.038 vs transferrin, r = 0.75, p < 0.001 | vs NGAL, r = 0.49, p < 0.001 |
| Ceruloplasmin | vs MPO, r = 0.42, p < 0.001 vs NGAL, r = 0.18, p = 0.047 vs lactoferrin, r = 0.52, p < 0.001 vs calprotectin, r = 0.27, p = 0.003 | |
| Lactoferrin | vs ferritin, r = −0.18, p = 0.042 vs ceruloplasmin, r = 0.52, p < 0.001 | vs MPO, r = 0.79, p < 0.001 vs calprotectin, r = 0.38, p < 0.001 |
| MPO | vs ceruloplasmin, r = 0.42, p < 0.001 | vs lactoferrin, r = 0.79, p < 0.001 vs calprotectin, r = 0.33, p < 0.001 |
| NGAL | vs ferritin, r = 0.26, p = 0.004 vs transferrin, r = 0.51, p < 0.001 vs haptoglobin, r = 0.49, p < 0.001 vs ceruloplasmin, r = 0.18, p = 0.047 | vs calprotectin, r = 0.21, p = 0.020 |
| Calprotectin | vs ceruloplasmin, r = 0.27, p = 0.003 | vs lactoferrin, r = 0.38, p < 0.001 vs MPO, r = 0.33, p < 0.001 vs NGAL, r = 0.21, p = 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarżyńska, E.; Jakimiuk, A.; Issat, T.; Lisowska-Myjak, B. Meconium Proteins Involved in Iron Metabolism. Int. J. Mol. Sci. 2024, 25, 6948. https://doi.org/10.3390/ijms25136948
Skarżyńska E, Jakimiuk A, Issat T, Lisowska-Myjak B. Meconium Proteins Involved in Iron Metabolism. International Journal of Molecular Sciences. 2024; 25(13):6948. https://doi.org/10.3390/ijms25136948
Chicago/Turabian StyleSkarżyńska, Ewa, Artur Jakimiuk, Tadeusz Issat, and Barbara Lisowska-Myjak. 2024. "Meconium Proteins Involved in Iron Metabolism" International Journal of Molecular Sciences 25, no. 13: 6948. https://doi.org/10.3390/ijms25136948
APA StyleSkarżyńska, E., Jakimiuk, A., Issat, T., & Lisowska-Myjak, B. (2024). Meconium Proteins Involved in Iron Metabolism. International Journal of Molecular Sciences, 25(13), 6948. https://doi.org/10.3390/ijms25136948






