Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
2.1. FTIR
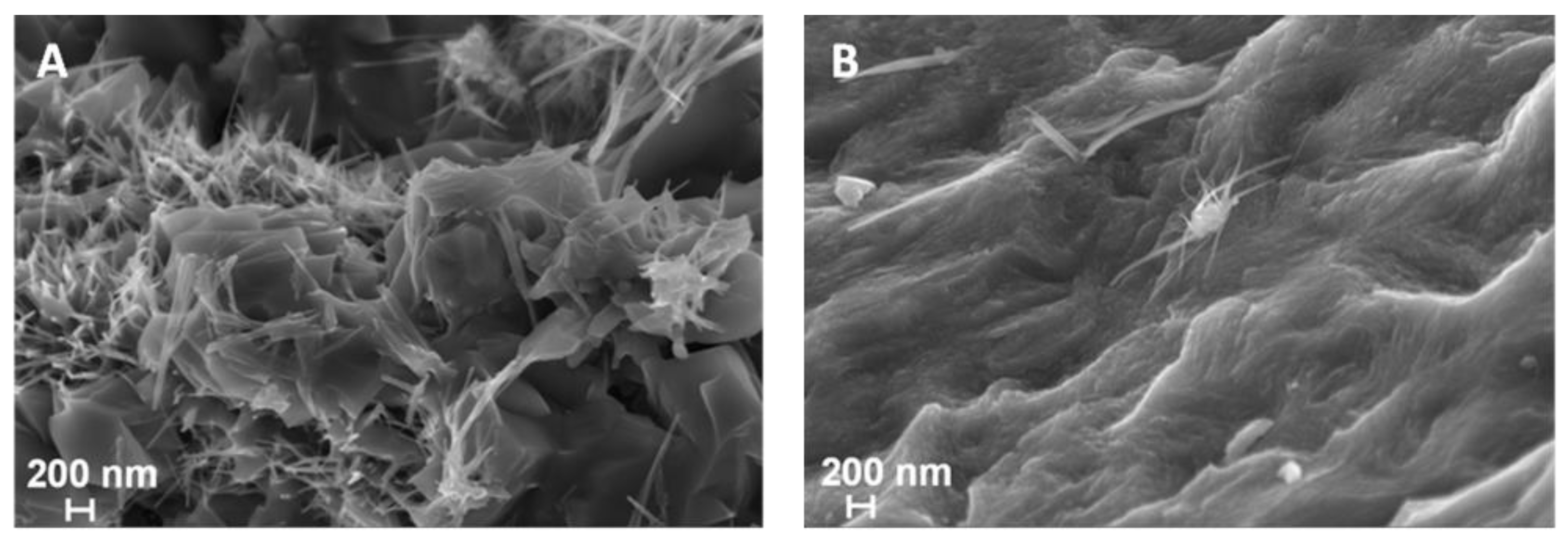
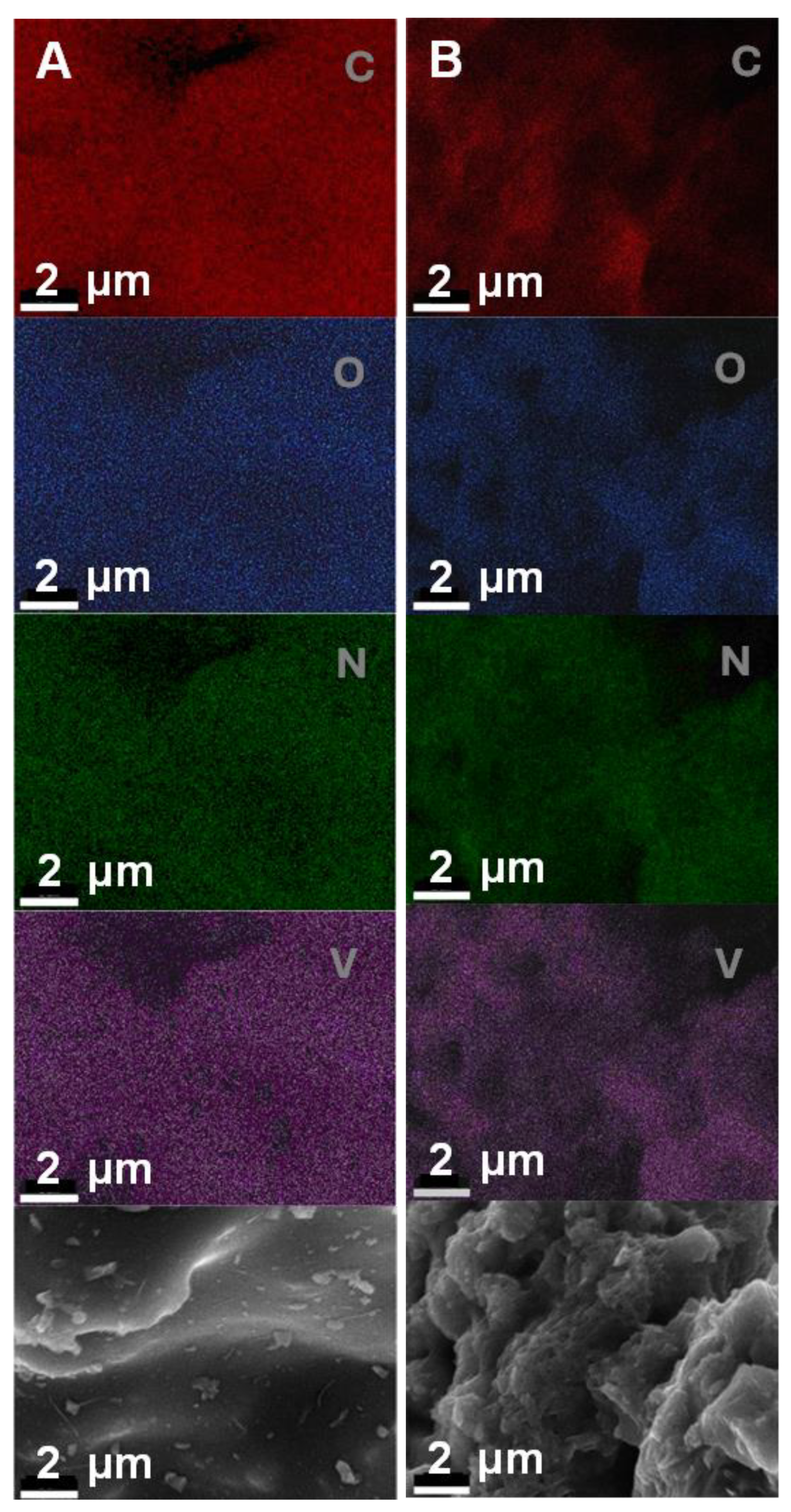
2.2. SEM/EDS
2.3. TEM
2.4. XRD
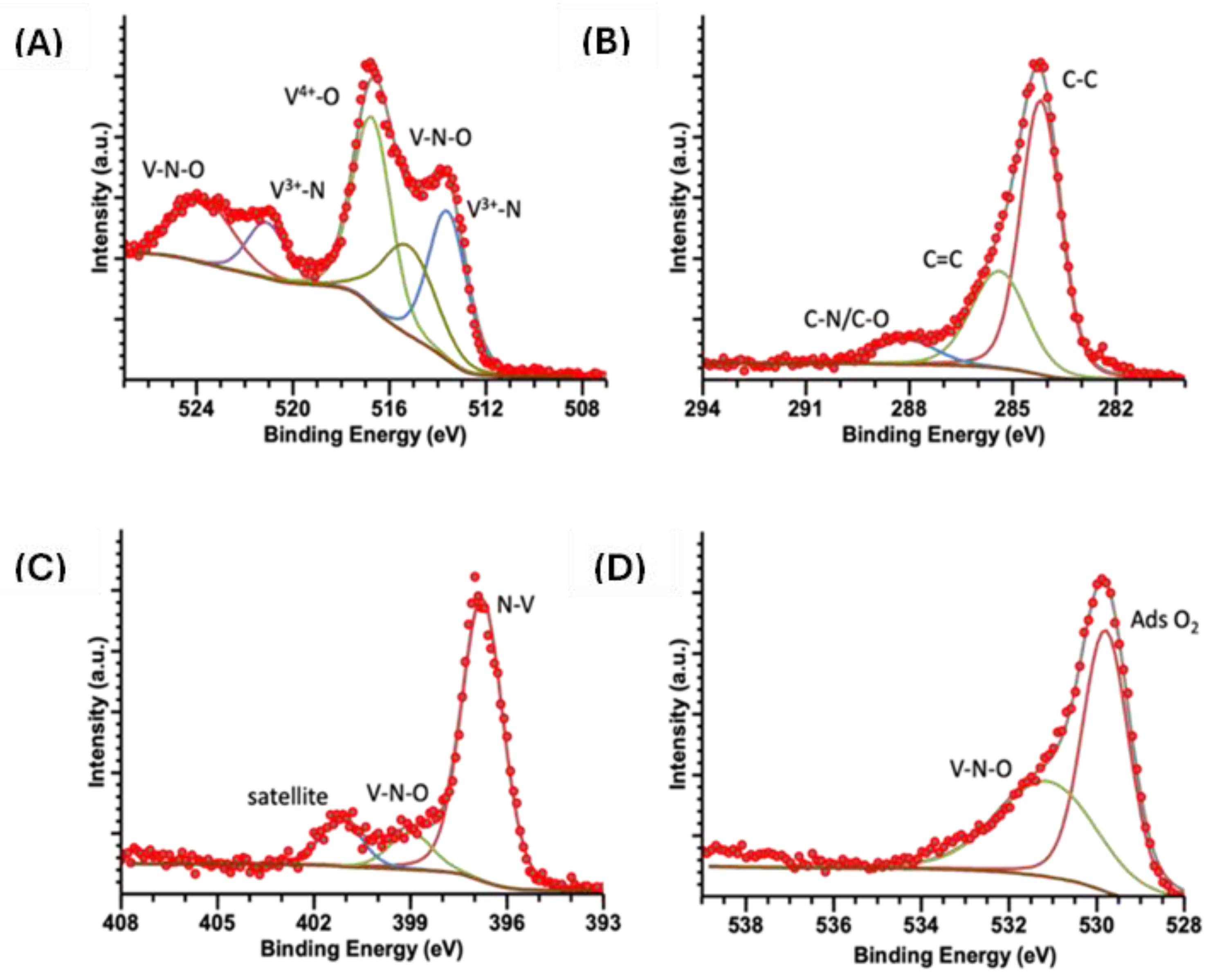
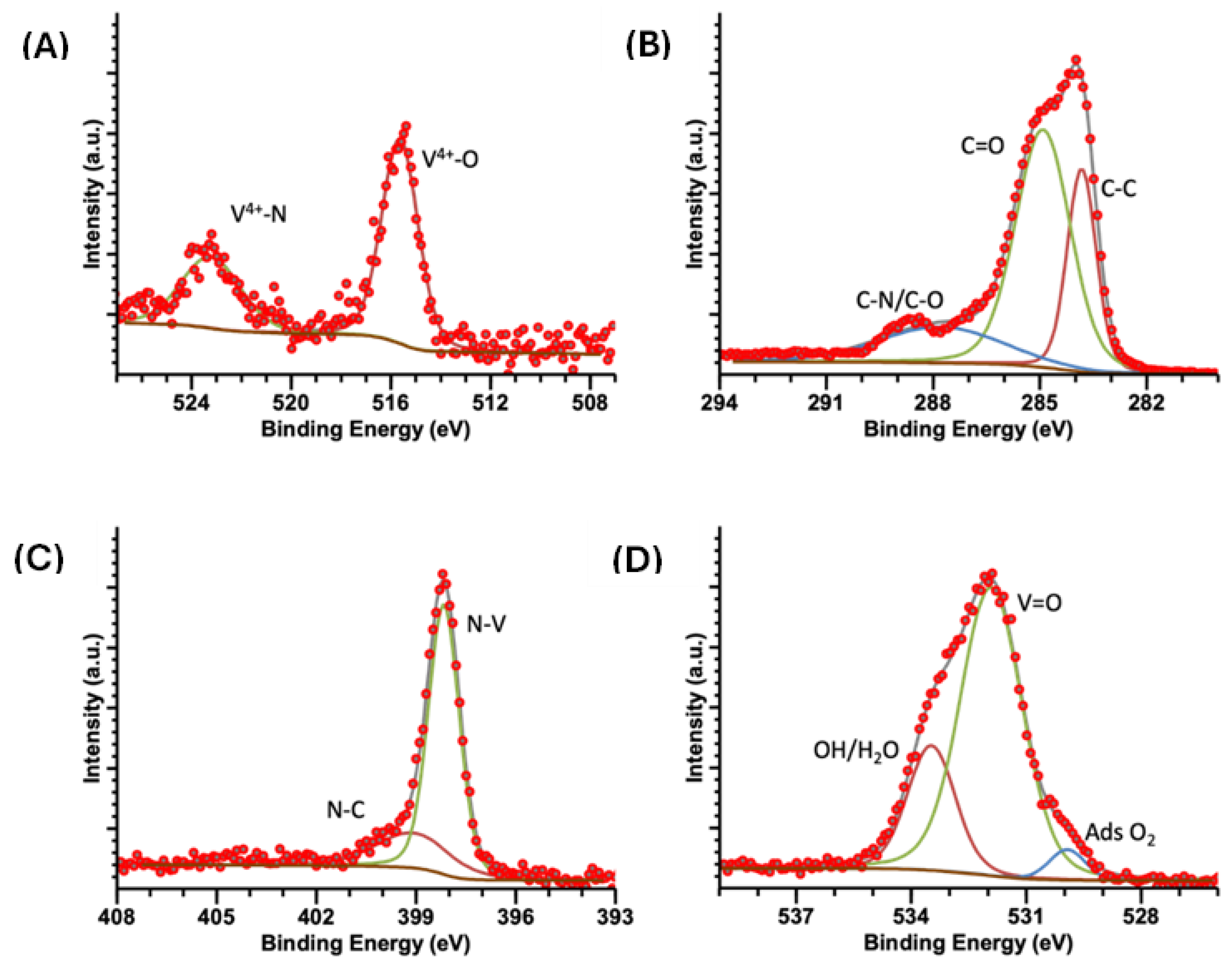
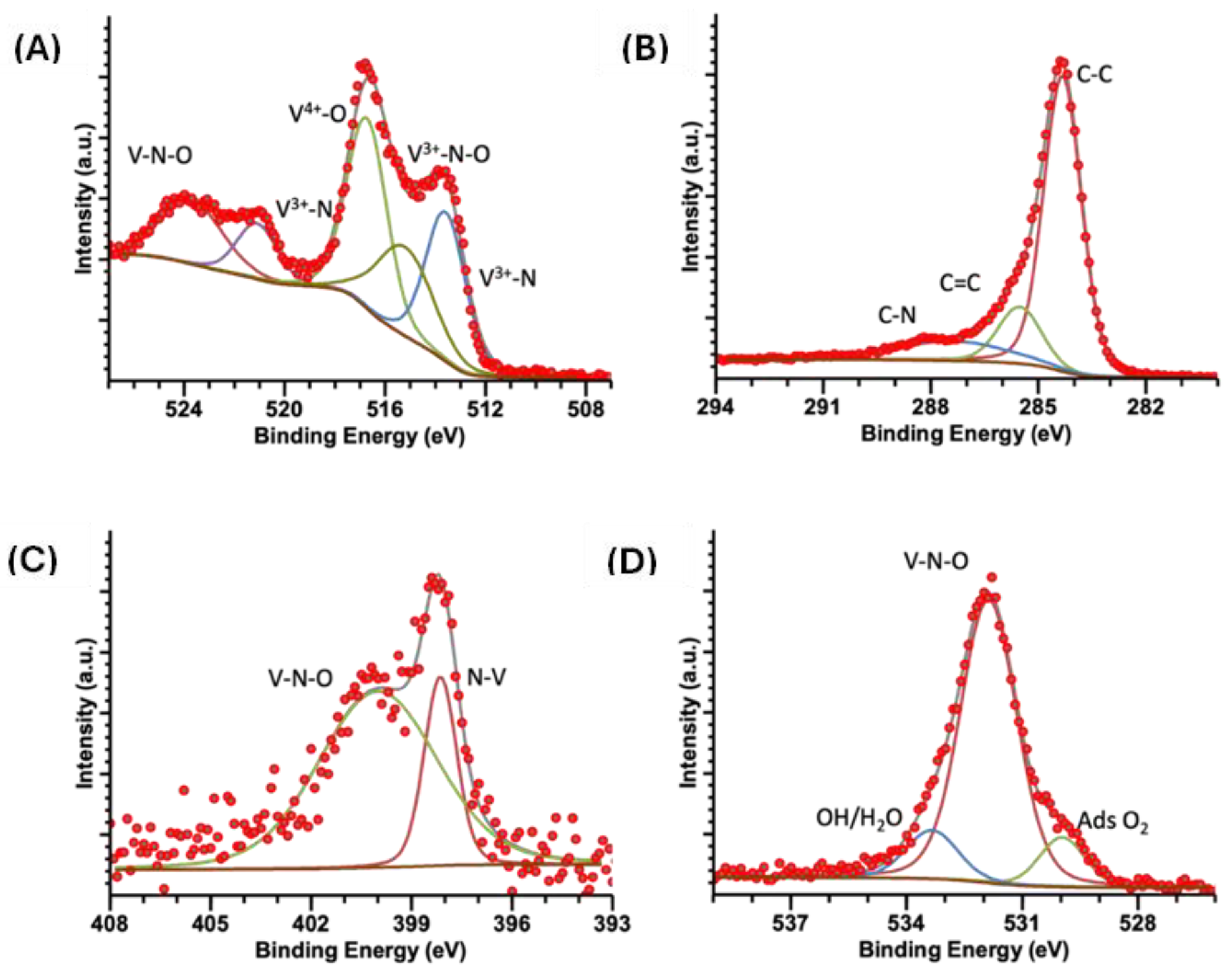
2.5. XPS
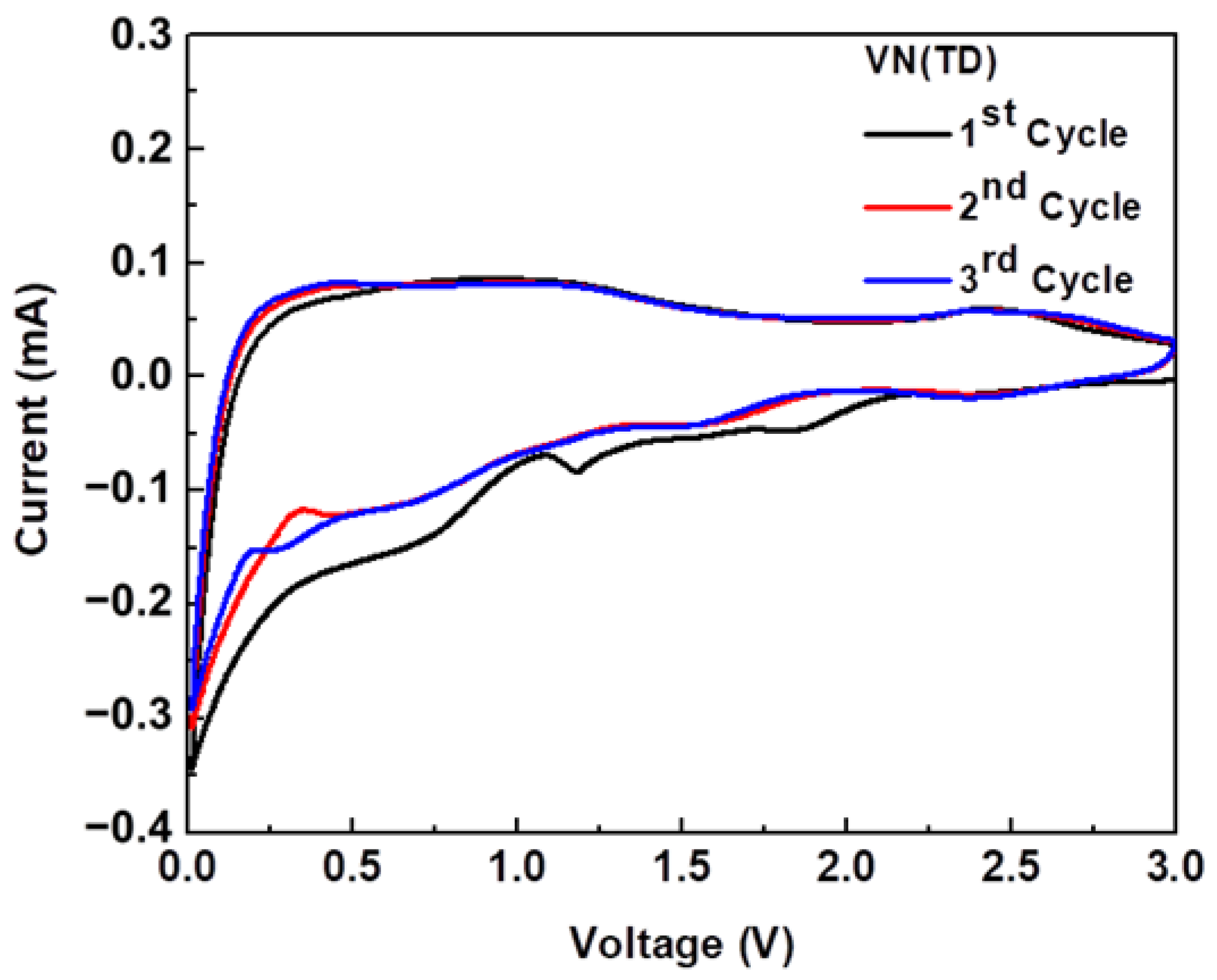
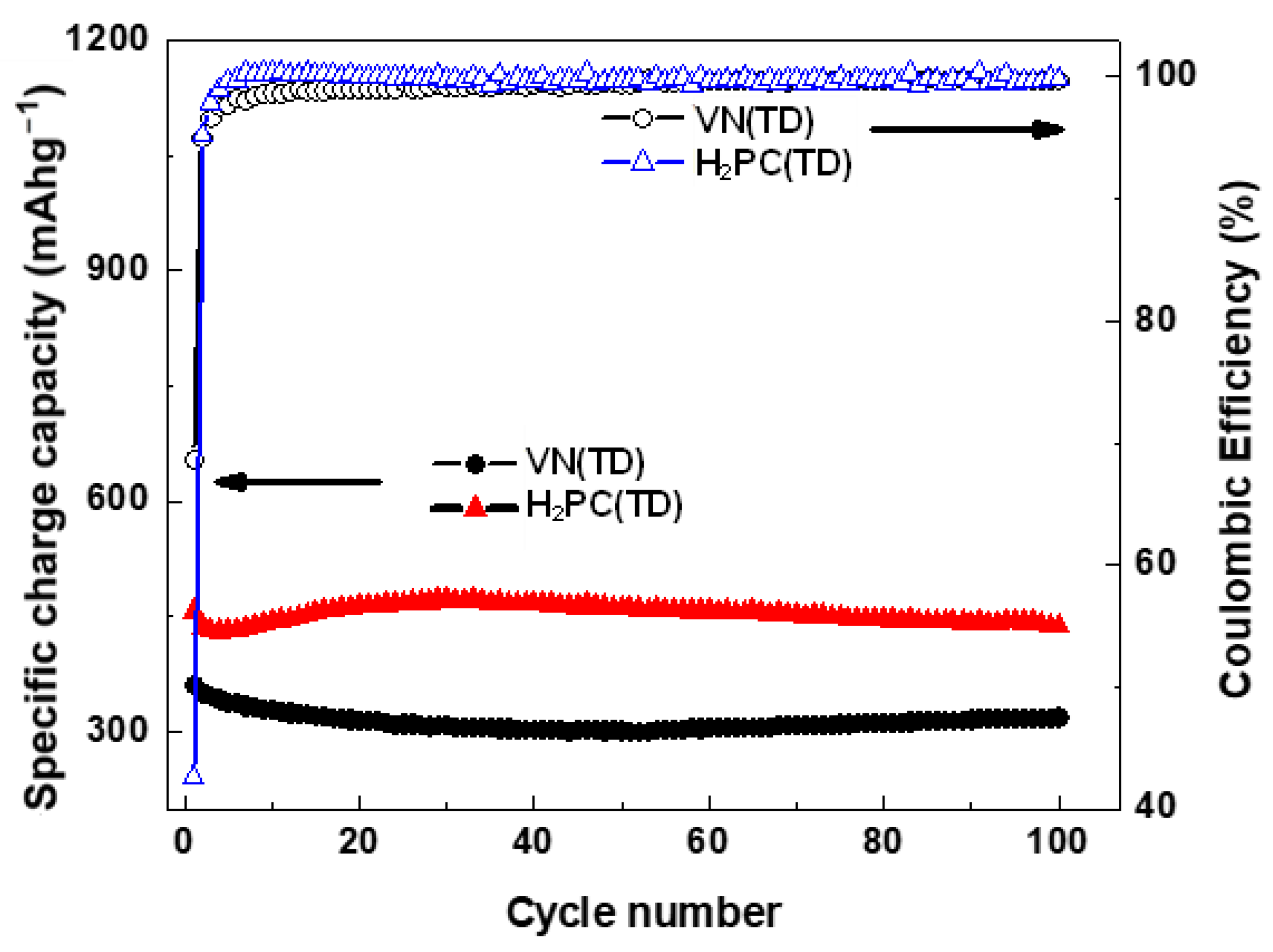
2.6. Cyclic Voltammetry
3. Materials and Methods
3.1. Materials Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balogun, M.-S.; Yu, M.; Li, C.; Zhai, T.; Liu, T.; Lu, X.; Tong, Y. Facile synthesis of titanium nitride nanowires on carbon fabric for flexible and high-rate lithium ion batteries. J. Mater. Chem. A 2014, 2, 10825–10829. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Ma, Y.; Lijun, X.; Yao, L.; Wenqiang, Z.; Haihong, Z.; Yahui, Y.; Liqiu, M.; Lishan, Y. Advanced Inorganic Nitride Nanomaterials for Renewable Energy: A Mini Review of Synthesis Methods. Front. Chem. 2021, 9, 638216. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized Surface Plasmon Resonance in Semiconductor Nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef] [PubMed]
- Sliwak, A.; Moyseowicz, A.; Gryglewicz, G. Hydrothermal-assisted synthesis of an iron nitride–carbon composite as a novel electrode material for supercapacitors. J. Mater. Chem. A 2017, 5, 5680–5684. [Google Scholar] [CrossRef]
- Qin, M.; Wu, H.; Cao, Z.; Zhang, D.; Jia, B.; Qu, X. A novel method to synthesize vanadium nitride nanopowders by ammonia reduction from combustion precursors. J. Alloys Compd. 2019, 772, 808–813. [Google Scholar] [CrossRef]
- Giordano, C.; Erpen, C.; Yao, W.; Milke, B.; Antonietti, M. Metal Nitride Metal Carbide Nanoparticles by a Soft Urea Pathway. Chem. Mater. 2009, 21, 5136–5144. [Google Scholar] [CrossRef]
- Glaser, A.; Surnev, S.; Netzer, F.P.; Fateh, N.; Fontalvo, G.A.; Mitterer, C. Oxidation of vanadium nitride and titanium nitride coatings. Surf. Sci. 2007, 601, 1153–1159. [Google Scholar] [CrossRef]
- Roldan, M.A.; López-Flores, V.; Alcala, M.D.; Ortega, A.C. Mechanochemical synthesis of vanadium nitride. J. Eur. Ceram. Soc. 2010, 30, 2099–2107. [Google Scholar] [CrossRef]
- Huang, J.W.; Peng, H.; Xia, G.B. Microwave synthesis of vanadium nitride for industrial applications. Ironmak. Steelmak. 2009, 36, 110–114. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Shi, L.; Yang, Z.; Ma, J.; Qian, Y. A room-temperature synthesis of nanocrystalline vanadium nitride. Solid State Commun. 2004, 132, 343–346. [Google Scholar] [CrossRef]
- Choi, J.-G.; Ha, J.; Hong, J.-W. Synthesis and catalytic properties of vanadium interstitial compounds. Appl. Catal. A Gen. 1998, 168, 47–56. [Google Scholar] [CrossRef]
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and Reversible Surface Redox Reaction in Nanocrystalline Vanadium Nitride Supercapacitors. Adv. Mater. 2006, 18, 1178–1182. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N.; Hu, P. Preparation of Vanadium Nitride Using a Thermally Processed Precursor with Coating Structure. Metals 2017, 7, 360. [Google Scholar] [CrossRef]
- Wade, T.; Ross, C.B.; Crooks, R.M. Electrochemical Synthesis of Ceramic Materials. 5. An Electrochemical Method Suitable for the Preparation of Nine Metal Nitrides. Chem. Mater. 1997, 9, 248–254. [Google Scholar] [CrossRef]
- Fix, R.; Gordon, R.G.; Hoffman, D.M. Chemical vapor deposition of vanadium, niobium, and tantalum nitride thin films. Chem. Mater. 1993, 5, 614–619. [Google Scholar] [CrossRef]
- Gajbhiye, N.S.; Ningthoujam, R.S. Low temperature synthesis, crystal structure and thermal stability studies of nanocrystalline VN particles. Mater. Res. Bull. 2006, 41, 1612–1621. [Google Scholar] [CrossRef]
- Gonzalez, G.; Sanchez, D.; Ramirez, D.; Myers, J.C.; Lodge, T.P.; Parsons, J.; Alcoutlabi, M. Preparation of SnO2/TiO2/C composite fibres and their use as binder-free anodes for lithium-ion batteries. Bull. Mater. Sci. 2023, 46, 58. [Google Scholar] [CrossRef]
- Gonzalez, G.; Hasan, M.D.T.; Ramirez, D.; Parsons, J.; Alcoutlabi, M. Synthesis of SnO2/TiO2 micro belt fibers from polymer composite precursors and their applications in Li-ion batteries. Polym. Eng. Sci. 2022, 62, 360–372. [Google Scholar] [CrossRef]
- Ayala, J.; Ramirez, D.; Myers, J.C.; Lodge, T.P.; Parsons, J.; Alcoutlabi, M. Performance and morphology of centrifugally spun Co3O4/C composite fibers for anode materials in lithium-ion batteries. J. Mater. Sci. 2021, 56, 16010–16027. [Google Scholar] [CrossRef]
- Ravikant, A.; Meenakshi, S.; Siddharth, S.; Ashwani, K.; Gaurav, M.; Rabah, B.; Ramesh, C. Metal nitrides as efficient electrode material for supercapacitors: A review. J. Energy Storage 2022, 56, 105912. [Google Scholar]
- Fischer, A.; Antonietti, M.; Thomas, A. Growth Confined by the Nitrogen Source: Synthesis of Pure Metal Nitride Nanoparticles in Mesoporous Graphitic Carbon Nitride. Adv. Mater. 2007, 19, 264–267. [Google Scholar] [CrossRef]
- Cui, G.; Gu, L.; Thomas, A.; Fu, L.; Van Aken, P.A.; Antonietti, M.; Maier, J. A Carbon/Titanium Vanadium Nitride Composite for Lithium Storage. Chemphyschem 2010, 11, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, J.; Yin, L.; Hu, G.; Fang, R.; Cheng, H.-M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries. Nat. Commun. 2017, 8, 14627. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, L.; Qi, S.; Ge, W.; Ma, W.; Zhao, Y.; Huang, R.; Xu, L.; Qian, Y. Ultrahigh-Areal-Capacity Battery Anodes Enabled by Free-Standing Vanadium Nitride@N-Doped Carbon/Graphene Architecture. ACS Appl. Mater. Interfaces 2020, 12, 49607–49616. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Roy, X.; Shopsowitz, K.E.; Hui, J.K.-H.; MacLachlan, M.J. Liquid-Crystal Templating in Ammonia: A Facile Route to Micro- and Mesoporous Metal Nitride/Carbon Composites. Angew. Chem. Int. Ed. 2010, 49, 9740–9743. [Google Scholar] [CrossRef] [PubMed]
- Mehek, R.; Iqbal, N.; Noor, T.; Amjad, M.Z.B.; Ali, G.; Vignarooban, K.; Khan, M.A. Metal–organic framework based electrode materials for lithium-ion batteries: A review. RSC Adv. 2021, 11, 29247–29266. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Mathieu, S.; Ghanbaja, J.; Celzard, A.; Fierro, V. Structure and electrochemical properties of carbon nanostructures derived from nickel(II) and iron(II) phthalocyanines. J. Adv. Res. 2020, 22, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Mack, H.G.; Harbeck, S. Experimental and Theoretical Investigations on the IR and Raman Spectra for CuPc and TiOPC; Universitat Tubingen: Tübingen, Germany, 2013; pp. 1–19. Available online: http://hdl.handle.net/10900/49961 (accessed on 17 January 2024).
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Ahmad, A.; Collins, R.A. FTIR characterization of triclinic lead phthalocyanine. J. Phys. D Appl. Phys. 1991, 24, 1894. [Google Scholar] [CrossRef]
- Ross, A.; Soares, D.C.; Covelli, D.; Pannecouque, C.; Budd, L.; Collins, A.; Robertson, N.; Parsons, S.; De Clercq, E.; Kennepohl, P.; et al. Oxovanadium(IV) cyclam and bicyclam complexes: Potential CXCR4 receptor. Inorg. Chem. 2010, 49, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, I.M.; Veenstra, F.L.P.; Jungbacker, P.; Rothenberg, G. A simple synthesis of symmetric phthalocyanines and their respective perfluoro and transition-metal complexes. Appl. Organomet. Chem. 2019, 33, 4872. [Google Scholar] [CrossRef]
- Neamtu, M.; Nadejde, C.; Brinza, L.; Dragos, O.; Gherghel, D.; Paul, A. Iron phthalocyanine-sensitized magnetic catalysts for BPA photodegradation. Sci. Rep. 2020, 10, 5376. [Google Scholar] [CrossRef] [PubMed]
- Botez, C.E.; Morris, J.L.; Encerrado Manriquez, A.J.E.; Anchondo, A. Heating induced structural and chemical behavior of KD2PO4 in the 25 °C–215 °C temperature range. Mater. Charact. 2013, 83, 74–78. [Google Scholar] [CrossRef]
- Liu, J.; Hull, S.; Ahmed, I.; Skinner, S.J. Application of combined neutron diffraction and impedance spectroscopy for in-situ structure and conductivity studies of La2Mo2O9. Nucl. Instrum. Methods Phys. Res. Sect. B 2011, 269, 539–543. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Li, C.; Han, H.; Hu, X.; Ma, Y.; Yang, Y. One-Step Preparation of Metal-Free Phthalocyanine with Controllable Crystal Form. Green Process. Synth. 2021, 10, 95–100. [Google Scholar] [CrossRef]
- Ziolo, R.F.; Griffiths, C.H.; Troup, J.M. Crystal Structure of Vanadyl Phthalocyanine, phase II. J. Chem. Soc. Dalton Trans. 1980, 11, 2300–2301. [Google Scholar] [CrossRef]
- Brauer, G.; Schnell, W.D. Zur kenntnis des Systems Vanadium-Stickstoff und des reinen vanadiums. J. Less Common Met. 1964, 6, 326–332. [Google Scholar] [CrossRef]
- Li, N.; Xu, Z.; Wang, P.; Zhang, Z.; Hong, B.; Li, J.; Lai, Y. High-rate lithium-sulfur batteries enabled via vanadium nitride nanoparticle/3D porous graphene through regulating the polysulfides transformation. Chem. Eng. J. 2020, 398, 125432. [Google Scholar] [CrossRef]
- Farahmand, S.; Ghiaci, M.; Asghari, S. Oxo-vanadium (IV) phthalocyanine implanted onto the modified SBA-15 as a catalyst for direct hydroxylation of benzene to phenol in acetonitrile-water medium: A kinetic study. Chem. Eng. Sci. 2021, 232, 116331. [Google Scholar] [CrossRef]
- Cheng, H.; Garcia-Araez, N.; Hector, A.L.; Soulé, S. Synthesis of Hard Carbon-TiN/TiC Composites by Reacting Cellulose with TiCl4 Followed by Carbothermal Nitridation/Reduction. Inorg. Chem. 2019, 58, 5776–5786. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.T.; Nuzzo, R.G. Determining hybridization differences for amorphous carbon from the XPS C 1s envelope. Appl. Surf. Sci. 1995, 90, 195–203. [Google Scholar] [CrossRef]
- Zhang, L.; Holt, C.M.B.; Luber, E.J.; Olsen, B.C.; Wang, H.; Danaie, M.; Cui, X.; Tan, X.; Lui, V.W.; Kalisvaart, W.P.; et al. High Rate Electrochemical Capacitors from Three-Dimensional Arrays of Vanadium Nitride Functionalized Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 24381–24393. [Google Scholar] [CrossRef]
- Eguchi, K.; Nakagawa, T.; Takagi, Y.; Yokoyama, T. Direct Synthesis of Vanadium Phthalocyanine and Its Electronic and Magnetic States in Monolayers and Multilayers on Ag (111). J. Phys. Chem. C 2015, 119, 9805–9815. [Google Scholar] [CrossRef]
- Osonkie, A.; Lee, V.; Chukwunenye, P.; Cundari, T.; Kelber, J. Plasma modification of vanadium oxynitride surfaces: Characterization by in situ XPS experiments and DFT calculations. J. Chem. Phys. 2020, 153, 144709. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.E.; Rocha, T.C.R.; Knop-Gericke, A.; Stampfl, C.; Schlögl, R.; Piccinin, S. Thermodynamic and spectroscopic properties of oxygen on silver under an oxygen atmosphere. Phys. Chem. Chem. Phys. 2015, 17, 9288–9312. [Google Scholar] [CrossRef] [PubMed]
- Klofta, T.J.; Danziger, J.; Lee, P.; Pankow, J.; Nebesny, K.W.; Armstrong, N.R. Photoelectrochemical and spectroscopic characterization of thin films of titanyl phthalocyanine: Comparisons with vanadyl phthalocyanine. J. Phys. Chem. 1987, 91, 5646–5651. [Google Scholar] [CrossRef]
- Morales, H.M.; Vieyra, H.; Sanchez, D.A.; Fletes, E.M.; Odlyzko, M.; Lodge, T.P.; Padilla-Gainza, V.; Alcoutlabi, M.; Parsons, J.G. Synthesis and Characterization of Titanium Nitride–Carbon Composites and Their Use in Lithium-Ion Batteries. Nanomaterials 2024, 14, 624. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, W.; Wang, L.; Hu, L.; Jin, W.; Gao, A.; Zhang, X.; Huo, K.; Chu, P.K. Lithiation Kinetics in High-Performance Porous Vanadium Nitride Nanosheet Anode. Electrochim. Acta 2016, 214, 201–207. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Zhang, P.; Lin, Z.; Yan, X. Fast and Large Lithium Storage in 3D Porous VN Nanowires–Graphene Composite as a Superior Anode Toward High-Performance Hybrid Supercapacitors. Adv. Funct. Mater. 2015, 25, 2270–2278. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Wang, W.; Fang, P.; Lu, X.; Tong, Y. Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J. Mater. Chem. A 2015, 3, 1364–1387. [Google Scholar] [CrossRef]
- Goedken, V.L.; Dessy, G.; Ercolani, C.; Fares, V. Synthesis, reactivity, and x-ray crystal structure of dichloro (phthalocyaninato) titanium (IV). Inorg. Chem. 1985, 24, 991–995. [Google Scholar] [CrossRef]
- Christie, R.M. Colour and constitution relationships in organic pigments. Part 5: The influence of solvents, the central metal atom and substituents on the electronic spectra of phthalocyanines. Dyes Pigment. 1995, 27, 35–43. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
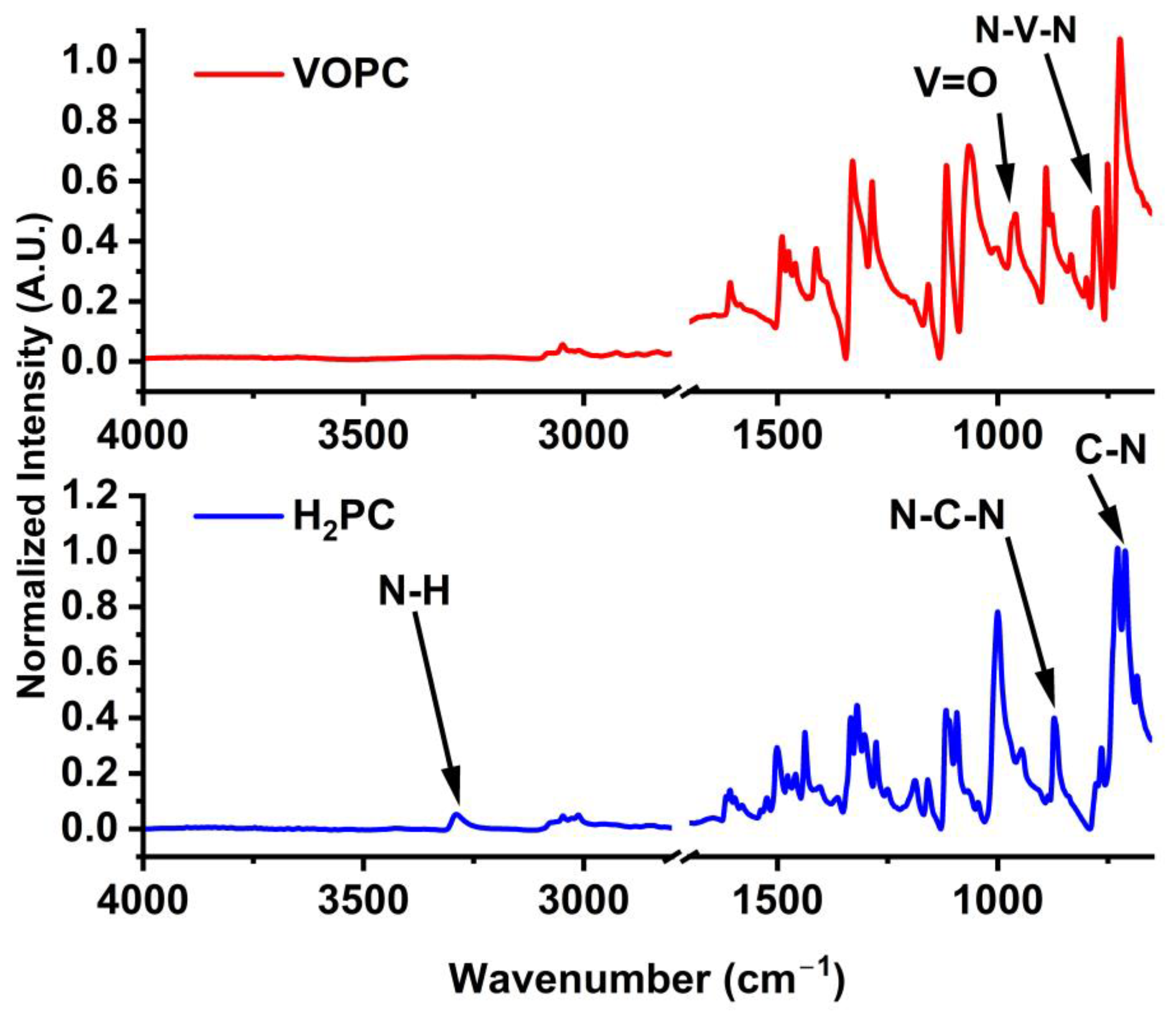


| H2PC Peak Position (cm−1) | VOPC Peak Position (cm−1) | Assignment |
|---|---|---|
| 710 | C-N [29] | |
| 729 | 724 | C-H out of plane deformation [30,31] |
| 762 | 751 | Macrocycle Ring Stretching [29] |
| 778 | 775 | C-N stretching [30] |
| 801 | isoindole stretching coupling N-V-N [29] | |
| 839 | C-N-C Ring Breathing | |
| 872 | 876 | N-H stretching coupling with isoindole deformation [29] |
| 898 | Isoindole deformation with coupling aza stretching [29] | |
| 944 | ||
| 953 | V=O [32] | |
| 998 | 1000 | Benzene ring and C=C [29] |
| 1064 | 1064 | C–N stretching in pyrrole vibration [30] |
| 1075 | 1075 | |
| 1091 | ||
| 1116 | 1118 | C–H in-plane deformation [29,30,31] |
| 1157 | 1159 | C–N in-plane and C–H in-plane [30,33] |
| 1187 | 1192 | isoindole stretching [30] |
| 1275 | ||
| 1299 | 1284 | C–N in isoindol stretching [30,34] |
| 1324 | ||
| 1336 | 1332 | C–C in isoindole [30] |
| 1417 | 1418 | isoindole stretching [29] |
| 1437 | ||
| 1461 | 1461 | C–H in-plane bending [30] |
| 1477 | 1475 | C=N pyrrole [29] |
| 1501 | 1497 | C–H bending in aryl [30] |
| 1523 | 1521 | C–H aryl [30] |
| 1576 | ||
| 1595 | 1587 | benzene C-C stretching [34] |
| 1610 | 1607 | C–C stretching vibration in pyrrole [30] |
| 2923 | 2923 | C–H stretching [30] |
| 3004 | 3004 | C-H stretching [30] |
| 3050 | 3050 | C–H stretching vibration in the ring [30] |
| 3282 | N-H [30] |
| Sample | Element | Atomic Percentage (%) |
|---|---|---|
| VN(SG) | V | 33.7 |
| N | 19.2 | |
| O | 35.3 | |
| C | 11.8 | |
| VO(PC) | V | 1.5 |
| N | 23.1 | |
| O | 0.9 | |
| C | 74.5 | |
| VN(TD) | V | 9.1 |
| N | 10.0 | |
| O | 5.9 | |
| C | 75.1 |
| Compound | Space Group | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | χ2 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| α-H2PC | C2/n | 25.76 | 3.77 | 23.40 | 90.00 | 93.11 | 90.00 | 2.07 | This work |
| α-H2PC | C2/n | 26.12 | 3.80 | 23.88 | 90.00 | 94.16 | 90.00 | [37] | |
| VOPC | P-1 | 12.06 | 12.56 | 8.71 | 96.20 | 94.94 | 68.20 | 1.67 | This work |
| VOPC | P-1 | 12.03 | 12.57 | 8.69 | 96.04 | 94.80 | 68.20 | [38] | |
| VN(TD) | FM-3M | 4.13 | 4.13 | 4.13 | 90.00 | 90.00 | 90.00 | 1.02 | This work |
| VN(SG) | FM-3M | 4.13 | 4.13 | 4.13 | 90.00 | 90.00 | 90.00 | 1.11 | This work |
| VN | FM-3M | 4.13 | 4.13 | 4.13 | 90.00 | 90.00 | 90.00 | [39] |
| Sample | Energy (eV) | V 2p3/2 | Energy (eV) | V 2p½ | Energy (eV) | C 1s | Energy (eV) | N 1s | Energy (eV) | O 1s |
|---|---|---|---|---|---|---|---|---|---|---|
| VN(SG) | 513.6 | V3+-N | 521.1 | V3+-N | 284.2 | C-C | 396.8 | N-V3+ | 529.8 | O2ads |
| 515.3 | VN-O | 523.7 | VNO | 285.4 | C=C | 399.0 | V-N-O | 531.2 | V-N-O | |
| 516.7 | V-O | 288.1 | C-N/C-O | 401.2 | Satellite | |||||
| VOPC | 515.6 | V4+-N | 283.8 | C-C | 398.3 | N-V4+ | 529.9 | O2ads | ||
| 523.4 | V4+-O | 284.9 | C=C | 398.9 | N-C | 531.9 | V=O | |||
| 533.5 | OH/H2O | |||||||||
| VN(TD) | 513.2 | V3+-N | 521.0 | V3+-N | 287.9 | C-N/C-O | 398.1 | N-V3+ | 529.9 | O2ads |
| 515.0 | VN-O | 523.4 | VN-O | 284.3 | C-C | 400.0 | V-N-O | 531.9 | V-N-O | |
| 516.4 | V-O | 285.5 | C=C | 533.3 | OH/H2O | |||||
| 287.4 | C-N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, H.M.; Vieyra, H.; Sanchez, D.A.; Fletes, E.M.; Odlyzko, M.; Lodge, T.P.; Padilla-Gainza, V.; Alcoutlabi, M.; Parsons, J.G. Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites. Int. J. Mol. Sci. 2024, 25, 6952. https://doi.org/10.3390/ijms25136952
Morales HM, Vieyra H, Sanchez DA, Fletes EM, Odlyzko M, Lodge TP, Padilla-Gainza V, Alcoutlabi M, Parsons JG. Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites. International Journal of Molecular Sciences. 2024; 25(13):6952. https://doi.org/10.3390/ijms25136952
Chicago/Turabian StyleMorales, Helia Magali, Horacio Vieyra, David A. Sanchez, Elizabeth M. Fletes, Michael Odlyzko, Timothy P. Lodge, Victoria Padilla-Gainza, Mataz Alcoutlabi, and Jason G. Parsons. 2024. "Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites" International Journal of Molecular Sciences 25, no. 13: 6952. https://doi.org/10.3390/ijms25136952
APA StyleMorales, H. M., Vieyra, H., Sanchez, D. A., Fletes, E. M., Odlyzko, M., Lodge, T. P., Padilla-Gainza, V., Alcoutlabi, M., & Parsons, J. G. (2024). Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites. International Journal of Molecular Sciences, 25(13), 6952. https://doi.org/10.3390/ijms25136952








