Combined Application of Cold Physical Plasma and Chemotherapeutics against Chondrosarcoma Cells
Abstract
:1. Introduction
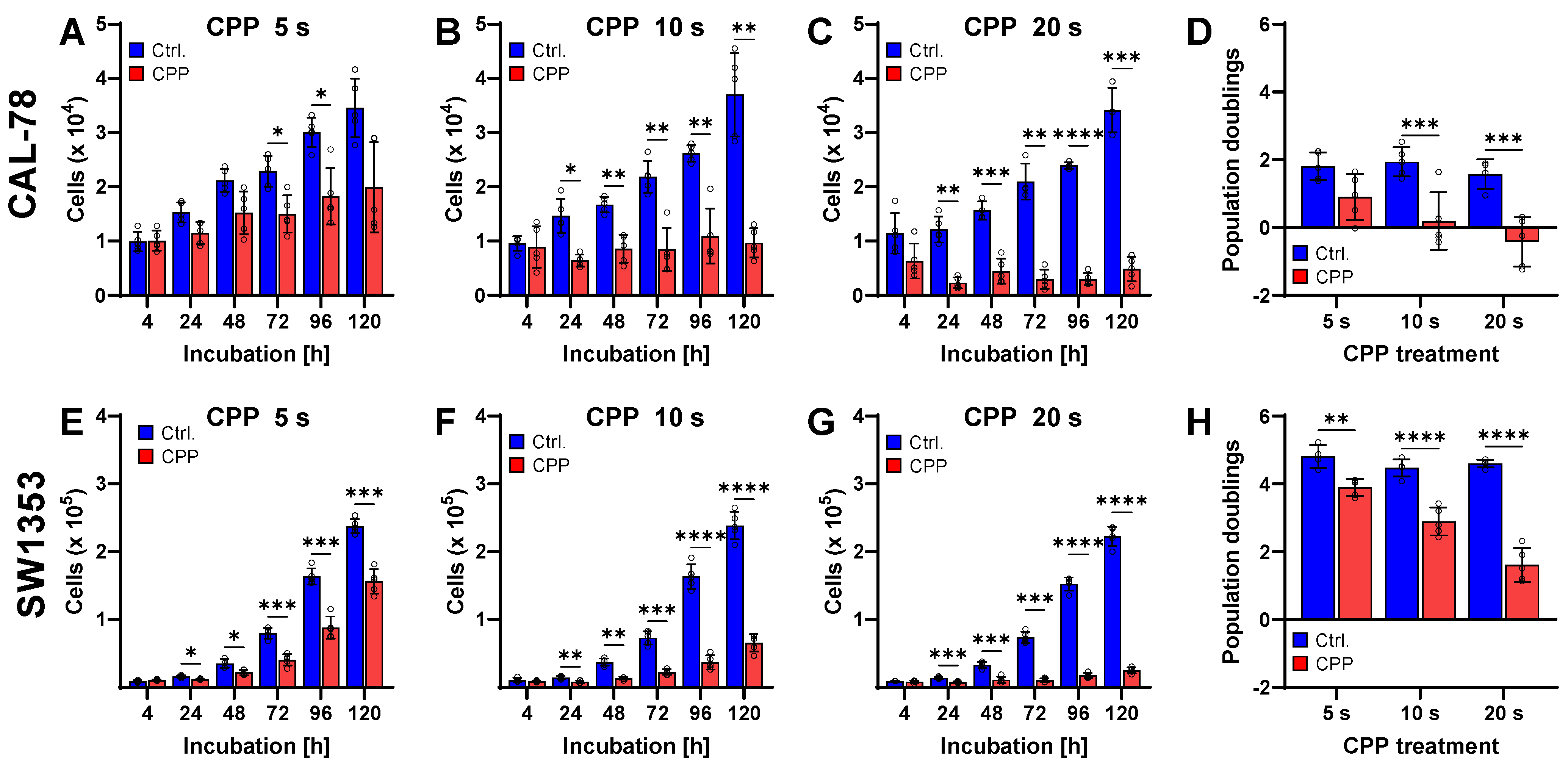
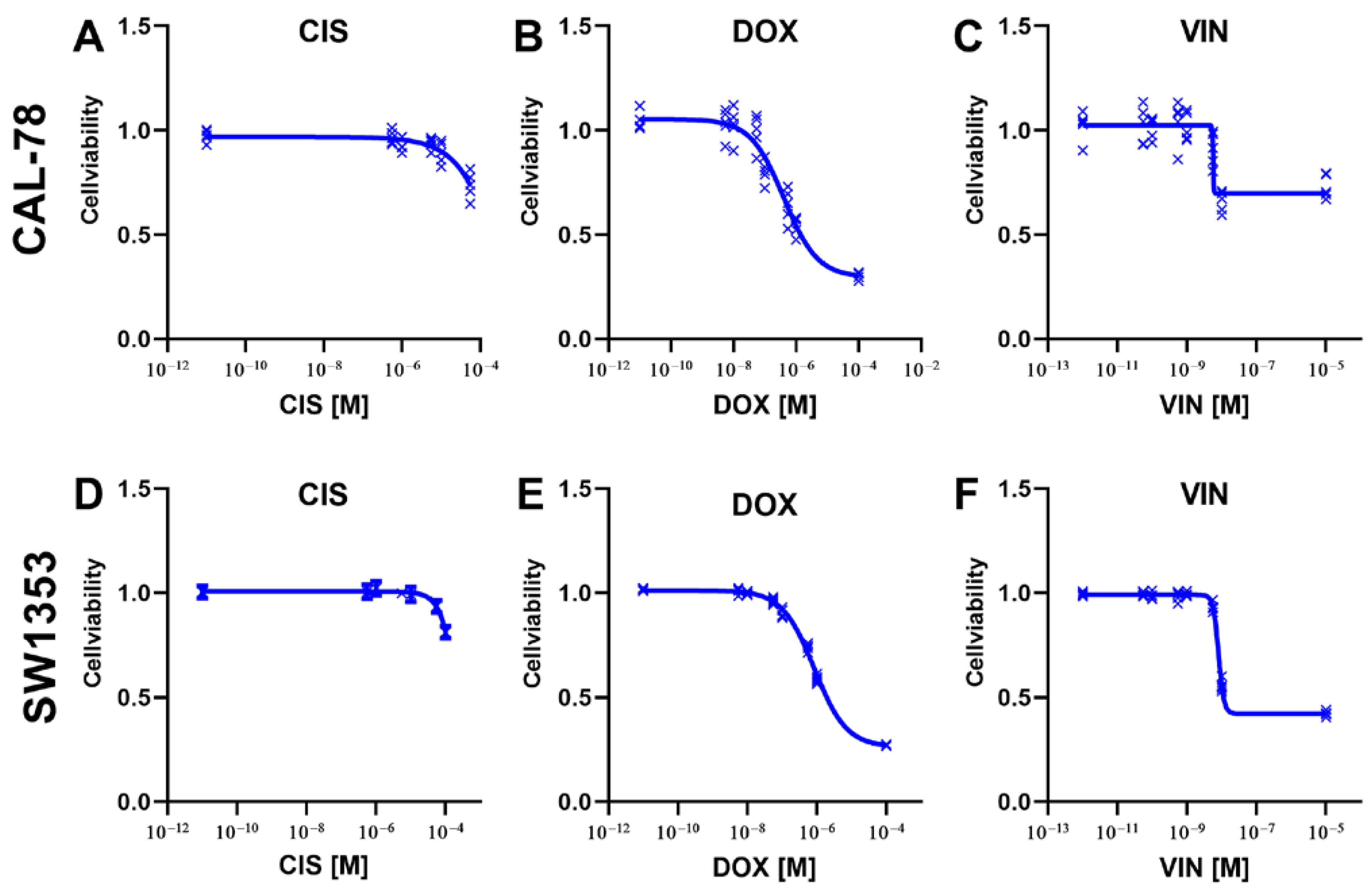
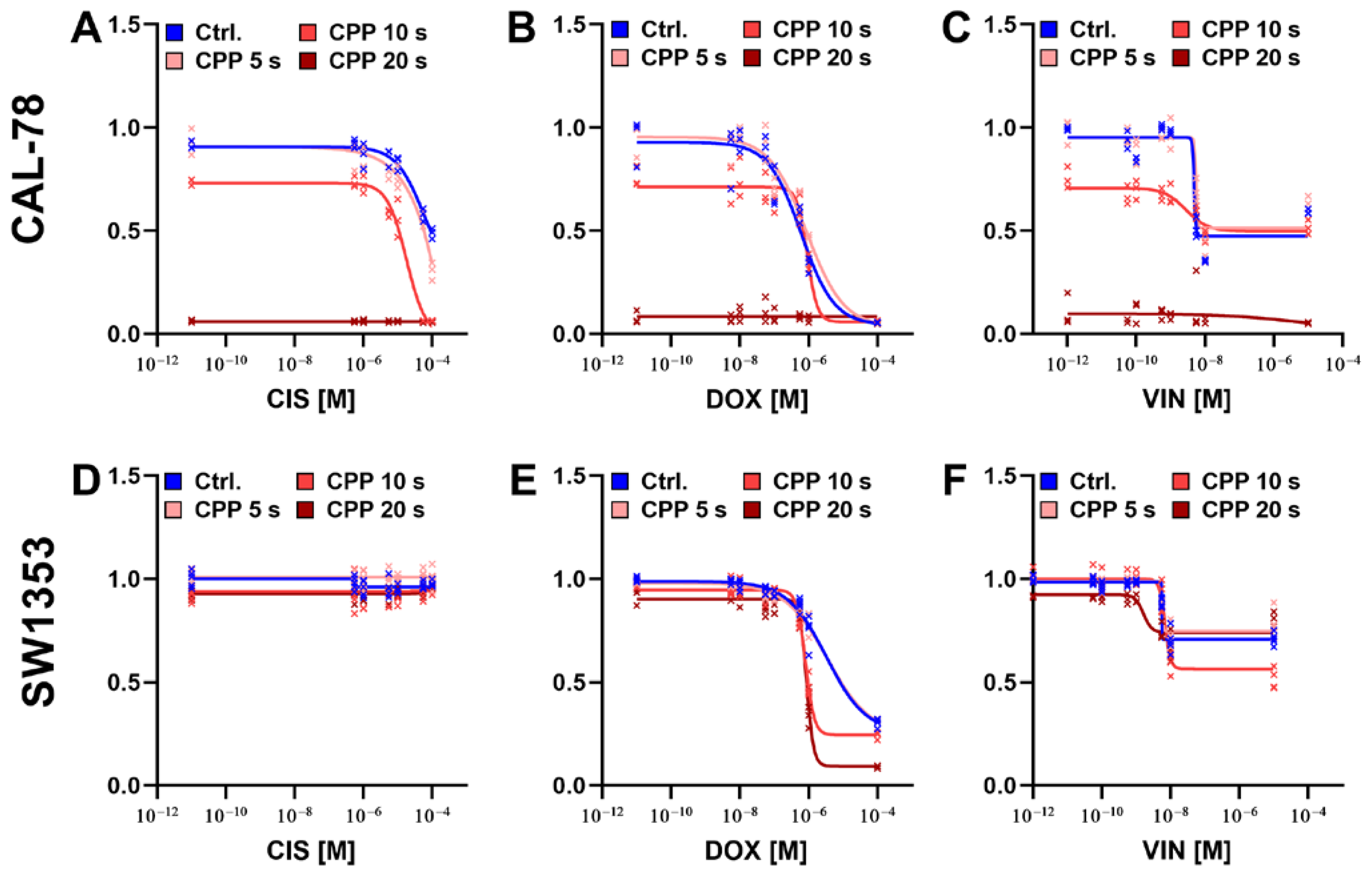
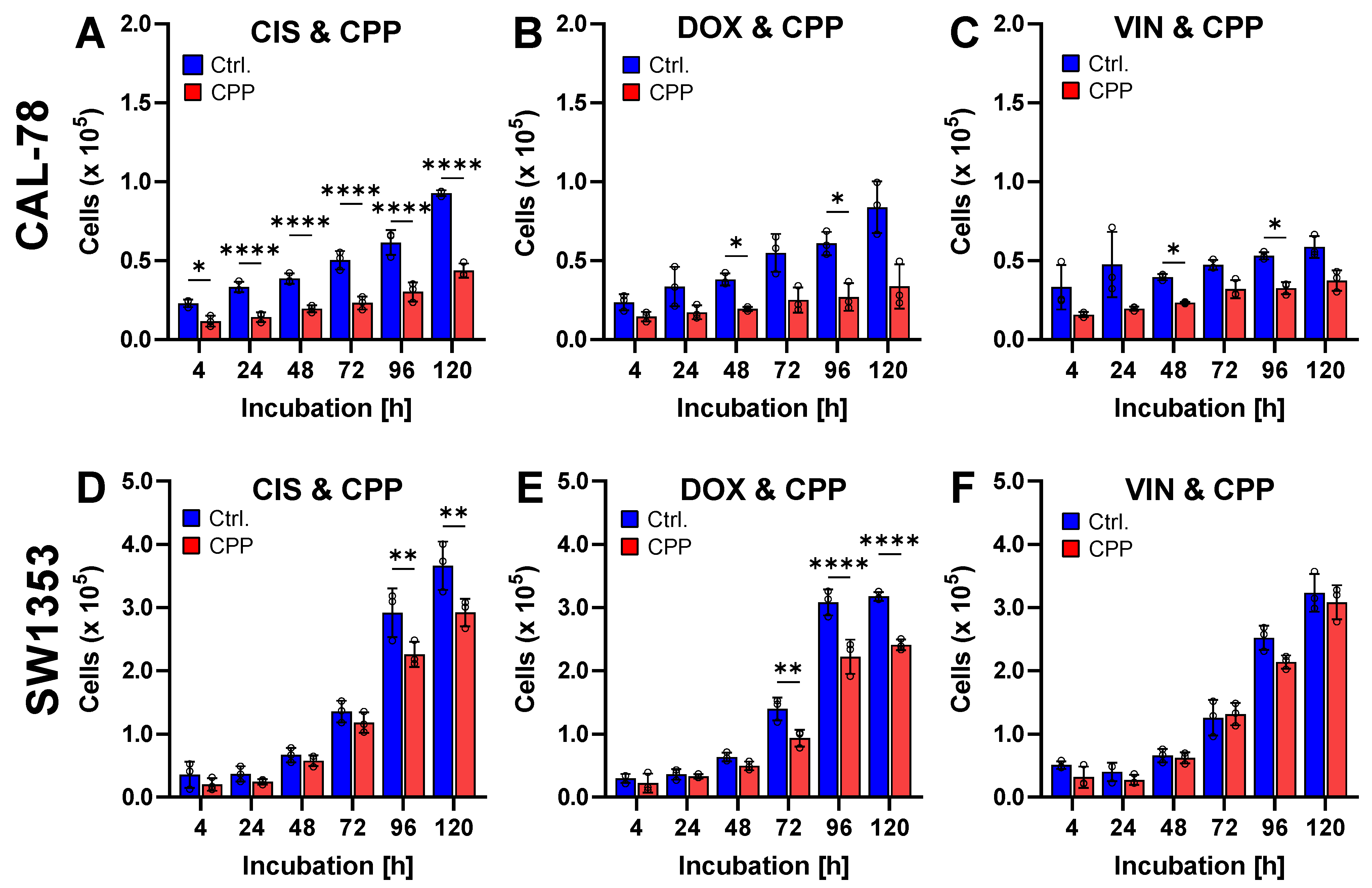
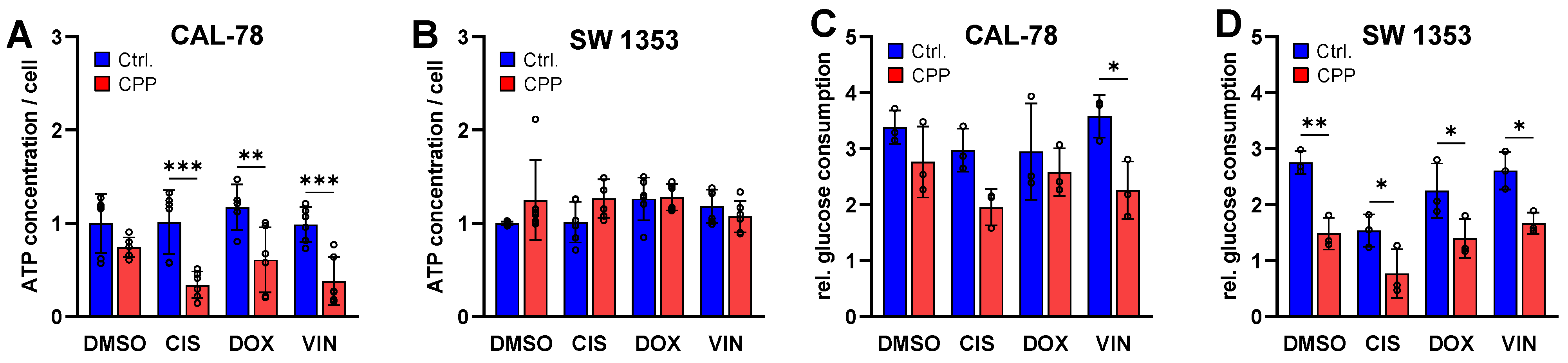
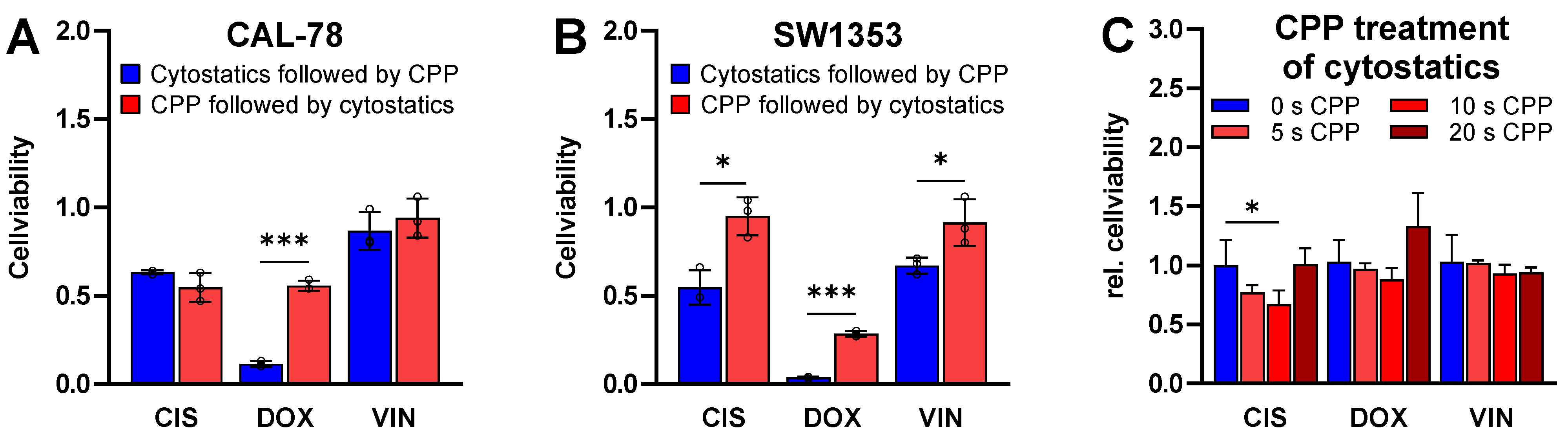
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Proliferation Assay after CPP Exposure
4.3. Chemotherapeutics
4.4. Cell Viability Assay after Cytostatic Exposure
4.5. Cell Viability Assay after Cytostatic and CPP Exposure
4.6. Proliferation Assay after Cytostatic and CPP Exposure
4.7. Detection of Caspase 3/7 Activity
4.8. TUNEL Assay
4.9. Cytotoxicity Assay
4.10. Cell Metabolism Assay
4.11. Glucose Consumption Assay
4.12. Sequential Cytostatic and CPP Treatment
4.13. CPP Exposure of Cytostatics
4.14. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limaiem, F.; Davis, D.D.; Sticco, K.L. Chondrosarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Thorkildsen, J.; Myklebust, T.Å. The National Incidence of Chondrosarcoma of Bone; a Review. Acta Oncol. 2023, 62, 110–117. [Google Scholar] [CrossRef]
- Cosci, I.; Del Fiore, P.; Mocellin, S.; Ferlin, A. Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review. Cancers 2024, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Weinschenk, R.C.; Wang, W.-L.; Lewis, V.O. Chondrosarcoma. JAAOS—J. Am. Acad. Orthop. Surg. 2021, 29, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, Chondrosarcoma, and Ewing’s Sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res.® 2007, 459, 40. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Hameed, M.; Kransdorf, M. The 2020 World Health Organization Classification of Bone Tumors: What Radiologists Should Know. Skelet. Radiol. 2023, 52, 329–348. [Google Scholar] [CrossRef]
- van Praag (Veroniek), V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; van der Geest, I.C.; Bramer, J.A.; Schaap, G.R.; Jutte, P.C.; Schreuder, H.B.; Ploegmakers, J.J.W.; et al. Incidence, Outcomes and Prognostic Factors during 25 Years of Treatment of Chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.L.; Ayala, A.G.; Romsdahl, M.M. Prognostic Factors in Chondrosarcoma of Bone. A Clinicopathologic Analysis with Emphasis on Histologic Grading. Cancer 1977, 40, 818–831. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kamoda, H.; Hagiwara, Y.; Kinoshita, S.; Ohtori, S.; Yonemoto, T. Prognostic Factors for Survival in Patients with High-Grade Chondrosarcoma. Cancer Diagn. Progn. 2022, 2, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, F.; Abudu, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Ayoub, K.; Mangham, D.C.; Davies, A.M. Risk Factors for Survival and Local Control in Chondrosarcoma of Bone. J. Bone Jt. Surgery. Br. Vol. 2002, 84-B, 93–99. [Google Scholar] [CrossRef]
- Stevenson, J.D.; Laitinen, M.K.; Parry, M.C.; Sumathi, V.; Grimer, R.J.; Jeys, L.M. The Role of Surgical Margins in Chondrosarcoma. Eur. J. Surg. Oncol. 2018, 44, 1412–1418. [Google Scholar] [CrossRef]
- Zając, A.E.; Kopeć, S.; Szostakowski, B.; Spałek, M.J.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.M.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef]
- Mery, B.; Espenel, S.; Guy, J.-B.; Rancoule, C.; Vallard, A.; Aloy, M.-T.; Rodriguez-Lafrasse, C.; Magné, N. Biological Aspects of Chondrosarcoma: Leaps and Hurdles. Crit. Rev. Oncol./Hematol. 2018, 126, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Moussavi-Harami, F.; Mollano, A.; Martin, J.A.; Ayoob, A.; Domann, F.E.; Gitelis, S.; Buckwalter, J.A. Intrinsic Radiation Resistance in Human Chondrosarcoma Cells. Biochem. Biophys. Res. Commun. 2006, 346, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Terek, R.M.; Schwartz, G.K.; Devaney, K.; Glantz, L.; Mak, S.; Healey, J.H.; Albino, A.P. Chemotherapy and P-glycoprotein Expression in Chondrosarcoma. J. Orthop. Res. 1998, 16, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.-O.; Shin, M.J.; Ha, J.H.; Seo, S.W.; Yang, J.; Lee, F.Y. siRNA-Based Targeting of Antiapoptotic Genes Can Reverse Chemoresistance in P-Glycoprotein Expressing Chondrosarcoma Cells. Mol. Cancer 2009, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of Using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.W.; Kim, H.; Kim, H.W.; Yun, S.H.; Park, J.E.; Choi, E.H.; Kim, S.J. Genome-Wide Comparison of the Target Genes of the Reactive Oxygen Species and Non-Reactive Oxygen Species Constituents of Cold Atmospheric Plasma in Cancer Cells. Cancers 2020, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, M.; Tahmasebi, G.; Eslami, E.; Seydi, E.; Pourahmad, J. Cold Atmospheric Plasma versus Cisplatin against Oral Squamous Cell Carcinoma: A Mitochondrial Targeting Study. Iran. J. Pharm. Res. 2022, 21, e124106. [Google Scholar] [CrossRef]
- Silva-Teixeira, R.; Laranjo, M.; Lopes, B.; Almeida-Ferreira, C.; Gonçalves, A.C.; Rodrigues, T.; Matafome, P.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F. Plasma Activated Media and Direct Exposition Can Selectively Ablate Retinoblastoma Cells. Free Radic. Biol. Med. 2021, 171, 302–313. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells In Vitro and In Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Nitsch, A.; Stope, M.B. The Future Therapy of Renal Cell Carcinoma? Non-Invasive Physical Plasma as an Innovative Oncological Therapy Modality. J. Cancer Ther. 2021, 12, 602–610. [Google Scholar] [CrossRef]
- Dai, X.; Wu, J.; Lu, L.; Chen, Y. Current Status and Future Trends of Cold Atmospheric Plasma as an Oncotherapy. Biomol. Ther. 2023, 31, 496–514. [Google Scholar] [CrossRef]
- Nitsch, A.; Strakeljahn, S.; Jacoby, J.M.; Sieb, K.F.; Mustea, A.; Bekeschus, S.; Ekkernkamp, A.; Stope, M.B.; Haralambiev, L. New Approach against Chondrosoma Cells—Cold Plasma Treatment Inhibits Cell Motility and Metabolism, and Leads to Apoptosis. Biomedicines 2022, 10, 688. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Jacoby, J.M.; Strakeljahn, S.; Bekeschus, S.; Mustea, A.; Ekkernkamp, A.; Stope, M.B. Cold Atmospheric Plasma Treatment of Chondrosarcoma Cells Affects Proliferation and Cell Membrane Permeability. Int. J. Mol. Sci. 2020, 21, 2291. [Google Scholar] [CrossRef] [PubMed]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.-P.; Tornín, J.; Canal, C. Cold Atmospheric Plasma Enhances Doxorubicin Selectivity in Metastasic Bone Cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic Effect of Cold Atmospheric Pressure Plasma and Free or Liposomal Doxorubicin on Melanoma Cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Tamoxifen Sensitivity in Resistant MCF-7 Breast Cancer Cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2016, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, Q.; Malyavko, A.; Zolotukhin, D.B.; Adhikari, M.; Sherman, J.H.; Keidar, M. The Anti-Glioblastoma Effect of Cold Atmospheric Plasma Treatment: Physical Pathway vs. Chemical Pathway. Sci. Rep. 2020, 10, 11788. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Moritz, J.; Helfrich, I.; Boeckmann, L.; Weltmann, K.-D.; Emmert, S.; Metelmann, H.-R.; Stoffels, I.; von Woedtke, T. Ex Vivo Exposure of Human Melanoma Tissue to Cold Physical Plasma Elicits Apoptosis and Modulates Inflammation. Appl. Sci. 2020, 10, 1971. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma Apoptotic and Oxidative Effects on MCF7 and HCC1806 Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Rowe, W.; Ly, L.; Shashurin, A.; Zhuang, T.; Wigh, S.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. Treatment of Triple-Negative Breast Cancer Cells with the Canady Cold Plasma Conversion System: Preliminary Results. Plasma 2018, 1, 218–228. [Google Scholar] [CrossRef]
- Nitsch, A.; Sieb, K.F.; Qarqash, S.; Schoon, J.; Ekkernkamp, A.; Wassilew, G.I.; Niethard, M.; Haralambiev, L. Selective Effects of Cold Atmospheric Plasma on Bone Sarcoma Cells and Human Osteoblasts. Biomedicines 2023, 11, 601. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Einenkel, R.; Muzzio, D.O.; Gelbrich, N.; Burchardt, M.; Zygmunt, M.; Ekkernkamp, A.; Stope, M.B.; Gümbel, D. The Effect of Cold Atmospheric Plasma on the Membrane Permeability of Human Osteosarcoma Cells. Anticancer Res. 2020, 40, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of Sensitivity in Chemo—Resistant Glioma Cells by Cold Atmospheric Plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination Therapy of Cold Atmospheric Plasma (CAP) with Temozolomide in the Treatment of U87MG Glioblastoma Cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Lin, L.; Sherman, J.H.; Peters, K.B.; Keir, S.T.; Keidar, M. Cold Plasma Discharge Tube Enhances Antitumoral Efficacy of Temozolomide. ACS Appl. Bio Mater. 2022, 5, 1610–1623. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of Chemotherapy and Physical Plasma Elicits Melanoma Cell Death via Upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Golpour, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold Atmospheric Plasma Is a Potent Tool to Improve Chemotherapy in Melanoma In Vitro and In Vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Dezhpour, A.; Ghafouri, H.; Jafari, S.; Nilkar, M. Effects of Cold Atmospheric-Pressure Plasma in Combination with Doxorubicin Drug against Breast Cancer Cells in Vitro and in Vivo. Free Radic. Biol. Med. 2023, 209, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Zahedian, S.; Hekmat, A.; Tackallou, S.H.; Ghoranneviss, M. The Impacts of Prepared Plasma-Activated Medium (PAM) Combined with Doxorubicin on the Viability of MCF-7 Breast Cancer Cells: A New Cancer Treatment Strategy. Rep. Biochem. Mol. Biol. 2022, 10, 640–652. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Paclitaxel Sensitivity to Paclitaxel-Resistant Breast Cancer Cells by Reversing Expression of Resistance-Related Genes. Cancers 2019, 11, 2011. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold Physical Plasma Selectively Elicits Apoptosis in Murine Pancreatic Cancer Cells In Vitro and In Ovo. Anticancer Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.-D.; Hackbarth, C.; Heidecke, C.-D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic Inhibition of Tumor Cell Proliferation by Cold Plasma and Gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Nitsch, A.; Qarqash, S.; Römer, S.; Schoon, J.; Ekkernkamp, A.; Niethard, M.; Reichert, J.C.; Wassilew, G.I.; Tzvetkov, M.V.; Haralambiev, L. Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. Int. J. Mol. Sci. 2023, 24, 8669. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of Cell Growth Arrest by Atmospheric Non-Thermal Plasma in Colorectal Cancer Cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef]
- Koensgen, D.; Besic, I.; Guembel, D.; Kaul, A.; Weiss, M.; Diesing, K.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. Cold Atmospheric Plasma (CAP) and CAP-Stimulated Cell Culture Media Suppress Ovarian Cancer Cell Growth—A Putative Treatment Option in Ovarian Cancer Therapy. Anticancer Res. 2017, 37, 6739–6744. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, A.; Popovic, S.; Parasu, N.; Ghert, M. Chondrosarcoma: A Clinical Review. J. Clin. Med. 2023, 12, 2506. [Google Scholar] [CrossRef]
- Bovée, J.V.; Cleton-Jansen, A.-M.; Taminiau, A.H.; Hogendoorn, P.C. Emerging Pathways in the Development of Chondrosarcoma of Bone and Implications for Targeted Treatment. Lancet Oncol. 2005, 6, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.E.; et al. Advanced Chondrosarcomas: Role of Chemotherapy and Survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- van Oosterwijk, J.G.; Herpers, B.; Meijer, D.; Briaire-de Bruijn, I.H.; Cleton-Jansen, A.M.; Gelderblom, H.; van de Water, B.; Bovée, J.V.M.G. Restoration of Chemosensitivity for Doxorubicin and Cisplatin in Chondrosarcoma in Vitro: BCL-2 Family Members Cause Chemoresistance. Ann. Oncol. 2012, 23, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yao, W. Chemotherapeutic Drugs for Soft Tissue Sarcomas: A Review. Front. Pharmacol. 2023, 14, 1199292. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-Cell-Autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865.e6. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Pomicter, A.D.; Li, F.; Bhatt, S.; Chen, C.; Li, W.; Qi, M.; Huang, C.; Deininger, M.W.; Kong, M.G.; et al. Trident Cold Atmospheric Plasma Blocks Three Cancer Survival Pathways to Overcome Therapy Resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107220118. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, H.; Farahmand, L.; Yaserian, K.; Jalili, N.; Majidzadeh-A, K. The Antiproliferative Effects of Cold Atmospheric Plasma-Activated Media on Different Cancer Cell Lines, the Implication of Ozone as a Possible Underlying Mechanism. J. Cell. Physiol. 2019, 234, 6778–6782. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S. Chemistry and Biochemistry of Cold Physical Plasma Derived Reactive Species in Liquids. Biol. Chem. 2019, 400, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Wenske, S.; Lackmann, J.-W.; Busch, L.M.; Bekeschus, S.; von Woedtke, T.; Wende, K. Reactive Species Driven Oxidative Modifications of Peptides—Tracing Physical Plasma Liquid Chemistry. J. Appl. Phys. 2021, 129, 193305. [Google Scholar] [CrossRef]
- Bauer, G.; Graves, D.B. Mechanisms of Selective Antitumor Action of Cold Atmospheric Plasma-Derived Reactive Oxygen and Nitrogen Species. Plasma Process. Polym. 2016, 13, 1157–1178. [Google Scholar] [CrossRef]
- Yan, X.; Zou, F.; Zhao, S.; Lu, X.; He, G.; Xiong, Z.; Xiong, Q.; Zhao, Q.; Deng, P.; Huang, J.; et al. On the Mechanism of Plasma Inducing Cell Apoptosis. IEEE Trans. Plasma Sci. 2010, 38, 2451–2457. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Anderson, H.; Gonzales, X.F. Induction of Apoptosis in Human Myeloid Leukemia Cells by Remote Exposure of Resistive Barrier Cold Plasma. Biotech. Bioeng. 2014, 111, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Z.; Pasandi, M.S.; Golpour, M.; Eslami, M.; Rafiei, A. Effect of Cold Atmospheric Plasma on Changing of Biomolecular Structures Involved in Apoptosis Pathways of Melanoma Cancer. Ski. Res. Technol. 2024, 30, e13544. [Google Scholar] [CrossRef] [PubMed]
- Sersenová, D.; Machala, Z.; Repiská, V.; Gbelcová, H. Selective Apoptotic Effect of Plasma Activated Liquids on Human Cancer Cell Lines. Molecules 2021, 26, 4254. [Google Scholar] [CrossRef]
- Lee, C.-M.; Jeong, Y.-I.; Kook, M.-S.; Kim, B.-H. Combinatorial Effect of Cold Atmosphere Plasma (CAP) and the Anticancer Drug Cisplatin on Oral Squamous Cell Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk Assessment of a Cold Argon Plasma Jet in Respect to Its Mutagenicity. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Müller, K.; Dombrowski, F.; von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side Effects by Oral Application of Atmospheric Pressure Plasma on the Mucosa in Mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Daeschlein, G.; von Woedtke, T.; Smeets, R.; Gosau, M.; Metelmann, H.-R. Long-Term Risk Assessment for Medical Application of Cold Atmospheric Pressure Plasma. Diagnostics 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauer, G.; Metelmann, P.; Seebauer, C. Side Effects in Cold Plasma Treatment of Advanced Oral Cancer—Clinical Data and Biological Interpretation. Clin. Plasma Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsch, A.; Qarqash, S.; Schulze, F.; Nonnenmacher, L.; Bekeschus, S.; Tzvetkov, M.V.; Wassilew, G.I.; Haralambiev, L. Combined Application of Cold Physical Plasma and Chemotherapeutics against Chondrosarcoma Cells. Int. J. Mol. Sci. 2024, 25, 6955. https://doi.org/10.3390/ijms25136955
Nitsch A, Qarqash S, Schulze F, Nonnenmacher L, Bekeschus S, Tzvetkov MV, Wassilew GI, Haralambiev L. Combined Application of Cold Physical Plasma and Chemotherapeutics against Chondrosarcoma Cells. International Journal of Molecular Sciences. 2024; 25(13):6955. https://doi.org/10.3390/ijms25136955
Chicago/Turabian StyleNitsch, Andreas, Sara Qarqash, Frank Schulze, Lars Nonnenmacher, Sander Bekeschus, Mladen V. Tzvetkov, Georgi I. Wassilew, and Lyubomir Haralambiev. 2024. "Combined Application of Cold Physical Plasma and Chemotherapeutics against Chondrosarcoma Cells" International Journal of Molecular Sciences 25, no. 13: 6955. https://doi.org/10.3390/ijms25136955




