Photosealed Neurorrhaphy Using Autologous Tissue
Abstract
1. Introduction
2. Results
2.1. Functional Recovery
2.2. Gastrocnemius Muscle Mass Retention
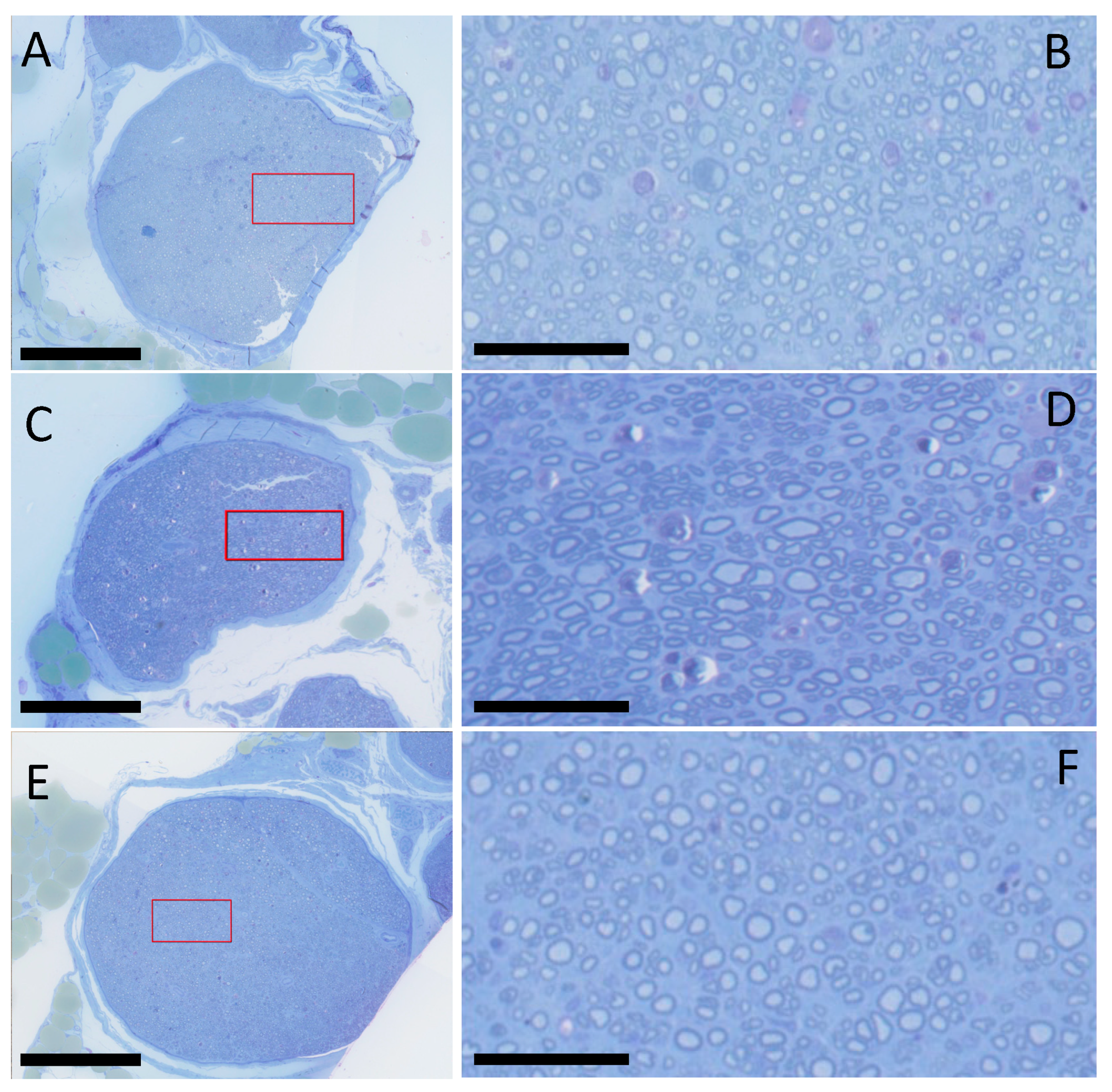
2.3. Histology and Histomorphometry
3. Discussion
4. Materials and Methods
4.1. Preparation of Crosslinked Human Amnion (xHAM)
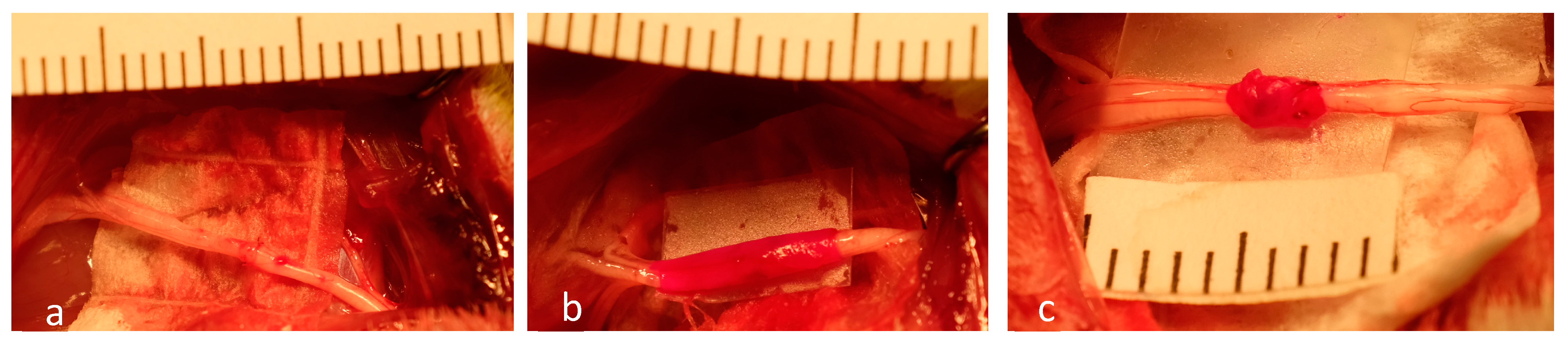
4.2. Surgical Procedures
- Group 1: Standard Neurorraphy (n = 12)
- Group 2: Photosealed xHAM Wrap (n = 12).
- Group 3: Photosealed Autologous vein Wrap (n = 12).
4.3. Functional Recovery
4.4. Gastrocnemius Muscle Mass Retention
4.5. Histology and Histomorphometry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sridharan, R.; Reilly, R.B.; Buckley, C.T. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J. Mech. Behav. Biomed. Mater. 2015, 41, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, F.A.; Boriani, F.; Bolognesi, F.; Fazio, N.; Marchetti, C.; Baldini, N. Cell-Enhanced Acellular Nerve Allografts for Peripheral Nerve Reconstruction: A Systematic Review and a Meta-Analysis of the Literature. Neurosurgery 2019, 85, 575–604. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.E.; Torres, R.Y. Epidemiology of Traumatic Peripheral Nerve Injuries Evaluated with Electrodiagnostic Studies in a Tertiary Care Hospital Clinic. Puerto Rico Health Sci. J. 2016, 35, 76–80. [Google Scholar]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef] [PubMed]
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Thorsén, F.; Rosberg, H.E.; Steen Carlsson, K.; Dahlin, L.B. Digital nerve injuries: Epidemiology, results, costs, and impact on daily life. J. Plast. Surg. Hand Surg. 2012, 46, 184–190. [Google Scholar] [CrossRef]
- Novak, C.B.; Anastakis, D.J.; Beaton, D.E.; Mackinnon, S.E.; Katz, J. Relationships among pain disability, pain intensity, illness intrusiveness, and upper extremity disability in patients with traumatic peripheral nerve injury. J. Hand Surg. Am. 2010, 35, 1633–1639. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Lisboa, B.C.; Frattini, F.; Almeida, F.M.; Tomaz, M.A.; Matsumoto, P.K.; Langone, F.; Lora, S.; Melo, P.A.; Borojevic, R.; et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011, 37, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, M.; Holzbach, T.; Matiasek, K.; Schlegel, J.; Giunta, R.E. Vascular endothelial growth factor gene therapy improves nerve regeneration in a model of obstetric brachial plexus palsy. Neurol. Res. 2015, 37, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Dvali, L.; Mackinnon, S. The role of microsurgery in nerve repair and nerve grafting. Hand Clin. 2007, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mackinnon, S.E.; Wood, M.D. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve 2020, 61, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Koulaxouzidis, G.; Reim, G.; Witzel, C. Fibrin glue repair leads to enhanced axonal elongation during early peripheral nerve regeneration in an in vivo mouse model. Neural Regen. Res. 2015, 10, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Maragh, H.; Meyer, B.S.; Davenport, D.; Gould, J.D.; Terzis, J.K. Morphofunctional evaluation of fibrin glue versus microsuture nerve repairs. J. Reconstr. Microsurg. 1990, 6, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Masgutov, R.; Masgutova, G.; Mullakhmetova, A.; Zhuravleva, M.; Shulman, A.; Rogozhin, A.; Syromiatnikova, V.; Andreeva, D.; Zeinalova, A.; Idrisova, K.; et al. Adipose-Derived Mesenchymal Stem Cells Applied in Fibrin Glue Stimulate Peripheral Nerve Regeneration. Front. Med. 2019, 6, 68. [Google Scholar] [CrossRef]
- Silva, D.N.; Silva, A.C.; Aydos, R.D.; Viterbo, F.; Pontes, E.R.; Odashiro, D.N.; Castro, R.J.; Augusto, D.G. Nerve growth factor with fibrin glue in end-to-side nerve repair in rats. Acta Cir. Bras. 2012, 27, 325–332. [Google Scholar] [CrossRef]
- Fairbairn, N.G.; Ng-Glazier, J.; Meppelink, A.M.; Randolph, M.A.; Valerio, I.L.; Fleming, M.E.; Winograd, J.M.; Redmond, R.W. Light-Activated Sealing of Nerve Graft Coaptation Sites Improves Outcome following Large Gap Peripheral Nerve Injury. Plast. Reconstr. Surg. 2015, 136, 739–750. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Medical Applications of Rose Bengal- and Riboflavin-Photosensitized Protein Crosslinking. Photochem. Photobiol. 2019, 95, 1097–1115. [Google Scholar] [CrossRef]
- Fairbairn, N.G.; Randolph, M.A.; Redmond, R.W. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 662–675. [Google Scholar] [CrossRef]
- Scott, B.B.; Wu, R.C.; Nietlispach, V.; Randolph, M.A.; Redmond, R.W. A Photosealed Cap Prevents Disorganized Axonal Regeneration and Neuroma following Nerve Transection in Rats. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4168. [Google Scholar] [CrossRef]
- Nicolas, C.F.; Corvi, J.J.; Zheng, Y.; Park, K.H.; Akelina, Y.; Engemann, A.; Strauch, R.J. Resorbable Nerve Wraps: Can They Be Overtightened? J. Reconstr. Microsurg. 2022, 38, 694–702. [Google Scholar] [CrossRef]
- Mayrhofer-Schmid, M.; Klemm, T.T.; Aman, M.; Kneser, U.; Eberlin, K.R.; Harhaus, L.; Boecker, A.H. Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. J. Pers. Med. 2023, 13, 1431. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Randolph, M.A.; Bujold, K.E.; Kochevar, I.E.; Redmond, R.W.; Winograd, J.M. Photochemical sealing improves outcome following peripheral neurorrhaphy. J. Surg. Res. 2009, 151, 33–39. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Randolph, M.A.; Bujold, K.E.; Kochevar, I.E.; Redmond, R.W.; Winograd, J.M. Preparation and integration of human amnion nerve conduits using a light-activated technique. Plast. Reconstr. Surg. 2009, 124, 428–437. [Google Scholar] [CrossRef]
- Fairbairn, N.G.; Ng-Glazier, J.; Meppelink, A.M.; Randolph, M.A.; Valerio, I.L.; Fleming, M.E.; Kochevar, I.E.; Winograd, J.M.; Redmond, R.W. Light-Activated Sealing of Acellular Nerve Allografts following Nerve Gap Injury. J. Reconstr. Microsurg. 2016, 32, 421–430. [Google Scholar] [CrossRef]
- Fairbairn, N.G.; Ng-Glazier, J.; Meppelink, A.M.; Randolph, M.A.; Winograd, J.M.; Redmond, R.W. Improving Outcomes in Immediate and Delayed Nerve Grafting of Peripheral Nerve Gaps Using Light-Activated Sealing of Neurorrhaphy Sites with Human Amnion Wraps. Plast. Reconstr. Surg. 2016, 137, 887–895. [Google Scholar] [CrossRef]
- Henry, F.P.; Goyal, N.A.; David, W.S.; Wes, D.; Bujold, K.E.; Randolph, M.A.; Winograd, J.M.; Kochevar, I.E.; Redmond, R.W. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery 2009, 145, 313–321. [Google Scholar] [CrossRef]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S.; McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.Z.; Baggish, M.S.; ElBakry, M.M.; Baltoyannis, P. Evaluation of tissue healing and adhesion formation after an intraabdominal amniotic membrane graft in the rat. J. Reprod. Med. 1989, 34, 198–202. [Google Scholar] [PubMed]
- Brandt, F.T.; Albuquerque, C.D.; Lorenzato, F.R. Female urethral reconstruction with amnion grafts. Int. J. Surg. Investig. 2000, 1, 409–414. [Google Scholar] [PubMed]
- Erdener, A.; Ulman, I.; Ilhan, H.; Soydan, S. Amniotic membrane wrapping: An alternative method to the splenorrhaphy in the injured spleen. Eur. J. Pediatr. Surg. 1992, 2, 26–28. [Google Scholar] [CrossRef]
- Ghanbari, Z.; Dahaghin, M.; Borna, S. Long-term outcomes of vaginal reconstruction with and without amnion grafts. Int. J. Gynaecol. Obstet. 2006, 92, 163–164. [Google Scholar] [CrossRef]
- Gruss, J.S.; Jirsch, D.W. Human amniotic membrane: A versatile wound dressing. Can. Med. Assoc. J. 1978, 118, 1237–1246. [Google Scholar] [PubMed]
- Nemec, H.M.; Atalah, H.; Kling, M.; Nichols, L.; Powers, B.; Montgomery, A.; Ashley, D.W. Does Human Amnion Membrane Prevent Postoperative Abdominal Adhesions? Am. Surg. 2020, 86, 1038–1042. [Google Scholar] [CrossRef]
- Salinas, H.M.; Khan, S.I.; McCormack, M.C.; Fernandes, J.R.; Gfrerer, L.; Watkins, M.T.; Redmond, R.W.; Austen, W.G., Jr. Prevention of vein graft intimal hyperplasia with photochemical tissue passivation. J. Vasc. Surg. 2017, 65, 190–196. [Google Scholar] [CrossRef]
- Goldstone, R.N.; McCormack, M.C.; Goldstein, R.L.; Mallidi, S.; Randolph, M.A.; Watkins, M.T.; Redmond, R.W.; Austen, W.G., Jr. Photochemical Tissue Passivation Attenuates AV Fistula Intimal Hyperplasia. Ann. Surg. 2018, 267, 183–188. [Google Scholar] [CrossRef]
- Scott, B.B.; Randolph, M.A.; Guastaldi, F.P.S.; Wu, R.C.; Redmond, R.W. Light-Activated Vascular Anastomosis. Surg. Innov. 2023, 30, 143–149. [Google Scholar] [CrossRef]
- Rechthand, E.; Rapoport, S.I. Regulation of the microenvironment of peripheral nerve: Role of the blood-nerve barrier. Prog. Neurobiol. 1987, 28, 303–343. [Google Scholar] [CrossRef]
- Seitz, R.J.; Reiners, K.; Himmelmann, F.; Heininger, K.; Hartung, H.P.; Toyka, K.V. The blood-nerve barrier in Wallerian degeneration: A sequential long-term study. Muscle Nerve 1989, 12, 627–635. [Google Scholar] [CrossRef]
- Pagnanelli, D.M.; Pait, T.G.; Rizzoli, H.V.; Kobrine, A.I. Scanning electron micrographic study of vascular lesions caused by microvascular needles and suture. J. Neurosurg. 1980, 53, 32–36. [Google Scholar] [CrossRef]
- Padmashree, S.; Ramprakash, C.H.; Jayalekshmy, R. Foreign body induced neuralgia: A diagnostic challenge. Case Rep. Dent. 2013, 2013, 352671. [Google Scholar] [CrossRef]
- Daeschler, S.C.; So, K.J.W.; Feinberg, K.; Manoraj, M.; Cheung, J.; Zhang, J.; Mirmoeini, K.; Santerre, J.P.; Gordon, T.; Borschel, G.H. A functional tacrolimus-releasing nerve wrap for enhancing nerve regeneration following surgical nerve repair. Neural Regen. Res. 2025, 20, 291–304. [Google Scholar] [CrossRef]
- Fernandes, J.R.; Salinas, H.M.; Broelsch, G.F.; McCormack, M.C.; Meppelink, A.M.; Randolph, M.A.; Redmond, R.W.; Austen, W.G., Jr. Prevention of capsular contracture with photochemical tissue passivation. Plast. Reconstr. Surg. 2014, 133, 571–577. [Google Scholar] [CrossRef]
- Chrząszcz, P.; Derbisz, K.; Suszyński, K.; Miodoński, J.; Trybulski, R.; Lewin-Kowalik, J.; Marcol, W. Application of peripheral nerve conduits in clinical practice: A literature review. Neurol. Neurochir. Pol. 2018, 52, 427–435. [Google Scholar] [CrossRef]
- Schlosshauer, B.; Dreesmann, L.; Schaller, H.E.; Sinis, N. Synthetic nerve guide implants in humans: A comprehensive survey. Neurosurgery 2006, 59, 740–747, discussion 747–748. [Google Scholar] [CrossRef]
- Lee, S.Y.; Thow, S.Y.; Abdullah, S.; Ng, M.H.; Mohamed Haflah, N.H. Advancement of Electrospun Nerve Conduit for Peripheral Nerve Regeneration: A Systematic Review (2016–2021). Int. J. Nanomed. 2022, 17, 6723–6758. [Google Scholar] [CrossRef]
- Thomson, S.E.; Ng, N.Y.; Riehle, M.O.; Kingham, P.J.; Dahlin, L.B.; Wiberg, M.; Hart, A.M. Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb. Cochrane Database Syst. Rev. 2022, 12, Cd012574. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Sarikcioglu, L.; Demirel, B.M.; Utuk, A. Walking track analysis: An assessment method for functional recovery after sciatic nerve injury in the rat. Folia Morphol. 2009, 68, 1–7. [Google Scholar]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–381. [Google Scholar] [CrossRef] [PubMed]

| Group | SFI (120 Days) | % Muscle Mass | Intrafascicular Area Ratio (D/P) | Axon Diameter Ratio (D/P) |
|---|---|---|---|---|
| Microsurgery | −58.4 +/− 10.9 | 69 +/− 7 | 0.84 +/− 0.22 | 0.77 +/− 0.17 |
| PTB/xHAM | −57.9 +/− 8.7 | 70 +/− 7 | 0.98 +/− 0.19 | 0.78 +/− 0.20 |
| PTB/vein | −52.4 +/− 17.1 | 70 +/− 7 | 0.95 +/− 0.19 | 0.80 +/− 0.26 |
| Group | Preop | 30 Days | 60 Days | 90 Days | 120 Days |
|---|---|---|---|---|---|
| Microsurgery | 19.4 +/− 1.8 | 7.9 +/− 2.2 | 8.5 +/− 2.7 | 12.8 +/− 2.3 | 11.7 +/− 1.9 |
| PTB/xHAM | 19.9 +/− 0.9 | 8.5 +/− 1.6 | 10.0 +/− 3.5 | 12.0 +/− 1.6 | 12.1 +/− 1.3 |
| PTB/vein | 19.2 +/− 0.7 | 8.7 +/− 1.9 | 11.2 +/− 1.8 | 12.7 +/− 2.1 | 12.9 +/− 2/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, N.; Bejar-Chapa, M.; Giorgino, R.; Scott, B.B.; Kostyra, D.M.; Peretti, G.M.; Randolph, M.A.; Redmond, R.W. Photosealed Neurorrhaphy Using Autologous Tissue. Int. J. Mol. Sci. 2024, 25, 6958. https://doi.org/10.3390/ijms25136958
Rossi N, Bejar-Chapa M, Giorgino R, Scott BB, Kostyra DM, Peretti GM, Randolph MA, Redmond RW. Photosealed Neurorrhaphy Using Autologous Tissue. International Journal of Molecular Sciences. 2024; 25(13):6958. https://doi.org/10.3390/ijms25136958
Chicago/Turabian StyleRossi, Nicolò, Maria Bejar-Chapa, Riccardo Giorgino, Benjamin B. Scott, David M. Kostyra, Giuseppe M. Peretti, Mark A. Randolph, and Robert W. Redmond. 2024. "Photosealed Neurorrhaphy Using Autologous Tissue" International Journal of Molecular Sciences 25, no. 13: 6958. https://doi.org/10.3390/ijms25136958
APA StyleRossi, N., Bejar-Chapa, M., Giorgino, R., Scott, B. B., Kostyra, D. M., Peretti, G. M., Randolph, M. A., & Redmond, R. W. (2024). Photosealed Neurorrhaphy Using Autologous Tissue. International Journal of Molecular Sciences, 25(13), 6958. https://doi.org/10.3390/ijms25136958






