Abstract
Drought stress is one of the significant abiotic stresses that limit soybean (Glycine max [L.] Merr.) growth and production. Ankyrin repeat (ANK) proteins, being highly conserved, occupy a pivotal role in diverse biological processes. ANK genes were classified into nine subfamilies according to conserved domains in the soybean genome. However, the function of ANK-TM subfamily proteins (Ankyrin repeat proteins with a transmembrane domain) in the abiotic-stress response to soybean remains poorly understood. In this study, we first demonstrated the subcellular localization of GmANKTM21 in the cell membrane and nucleus. Drought stress-induced mRNA levels of GmANKTM21, which encodes proteins belonging to the ANK-TM subfamily, Transgenic 35S:GmANKTM21 soybean improved drought tolerance at the germination and seedling stages, with higher stomatal closure in soybean, lower water loss, lower malondialdehyde (MDA) content, and less reactive oxygen species (ROS) production compared with the wild-type soybean (Dongnong50). RNA-sequencing (RNA-seq) and RT-qPCR analysis of differentially expressed transcripts in overexpression of GmANKTM21 further identified potential downstream genes, including GmSPK2, GmSPK4, and GmCYP707A1, which showed higher expression in transgenic soybean, than those in wild-type soybean and KEGG enrichment analysis showed that MAPK signaling pathways were mostly enriched in GmANKTM21 overexpressing soybean plants under drought stress conditions. Therefore, we demonstrate that GmANKTM21 plays an important role in tolerance to drought stress in soybeans.
1. Introduction
Soybeans [Glycine max (L.) Merr.] are an essential source of protein, oil, carbohydrates, and micronutrients both in the human diet and in animal feed. However, soybeans are highly vulnerable to abiotic stresses, including drought, high temperatures, hail, and other extreme weather events [1,2,3]. As a fundamental crop on a global scale, soybeans are profoundly vulnerable to water scarcity, leading to considerable reductions in productivity worldwide. Drought conditions, acknowledged as a predominant factor impeding soybean growth and maturation, present a considerable challenge to its cultivation [4]. Therefore, improving drought tolerance in soybeans is necessary.
The ankyrin repeat (ANK) domain, comprised of multiple units each typically containing 33 amino acid residues, plays a crucial role in plant growth and development [5,6]. To analyze the function and domain distribution of ANK genes, ANK genes were classified into different subfamilies based on their domain composition: ANK-U (unique ANK domain), ANK-TM (transmembrane domain), ANK-PK (containing the protein kinase domain), ANK-ZnF (zinc-finger), ANK-BPA (the BAR, PH and ArfGap domains), ANK-ACBP (Acyl-CoA-binding protein), ANK-GPCR (GPCR-chapero-1), ANK-IQ (calmodulin-binding domain) and ANK-O (containing different domains). Among these genes, the transmembrane domain (ANK-TM) was the second most abundant subfamily [7,8].
ANK genes are essential for various biological processes, as evidenced by the AKR code containing a protein encoding five anchor protein repeats. Loss of AKR function leads to embryonic defects, along with severe damage to chloroplasts and mesophyll cells in homozygous akr/akr plant lines [9]. Specifically, the ANK6 ankyrin repeat protein is indispensable for the fertilization process in Arabidopsis thaliana, as its dysfunction results in embryo demise. Heterozygous +/ank6 plants exhibit significantly smaller unfertilized atrophous ovules compared to wild-type ovules, leading to a marked decrease in the fruiting rate [10].
ANK family genes also play a crucial role in plant stress responses. Studies have shown that LR14A, a representative of the ANK-TM protein family, is involved in disease resistance in wheat. Similarly, the wheat stripe rust resistance gene YrU1 encodes an NLR protein with an integrated ANK structural domain. The ANK structural domain of YrU1 is derived from the ANK-TM protein, suggesting that ANK-TM proteins are involved in plant disease resistance [11]. Ankyrin repeat (ANK) C3HC4-type genes encode XB3-like proteins. Proteins from the XB3-like gene family are found in different plant species and are involved in a variety of developmental processes. XBAT35 belongs to a subgroup of Arabidopsis ankyrin repeat-containing E3 ligases, and XBAT35 transcript levels are modulated by temperature and high salinity stress [12]. Furthermore, research indicates that the ankyrin repeat sequence CaKR1, when overexpressed in tomatoes, can enhance the salt stress tolerance of transgenic tomato plants [13].
Genome-scale analyses of ANK genes from some plant species, including Arabidopsis and rice, have shown that ANK-TM accounts for 40% or more of the total number of identified ANK genes. Despite the large number of genes in the ANK-TM subfamily, it is poorly understood [6,14,15], making it particularly important to study the soybean ANK-TM subfamily genes.
2. Results
2.1. Subcellular Localization
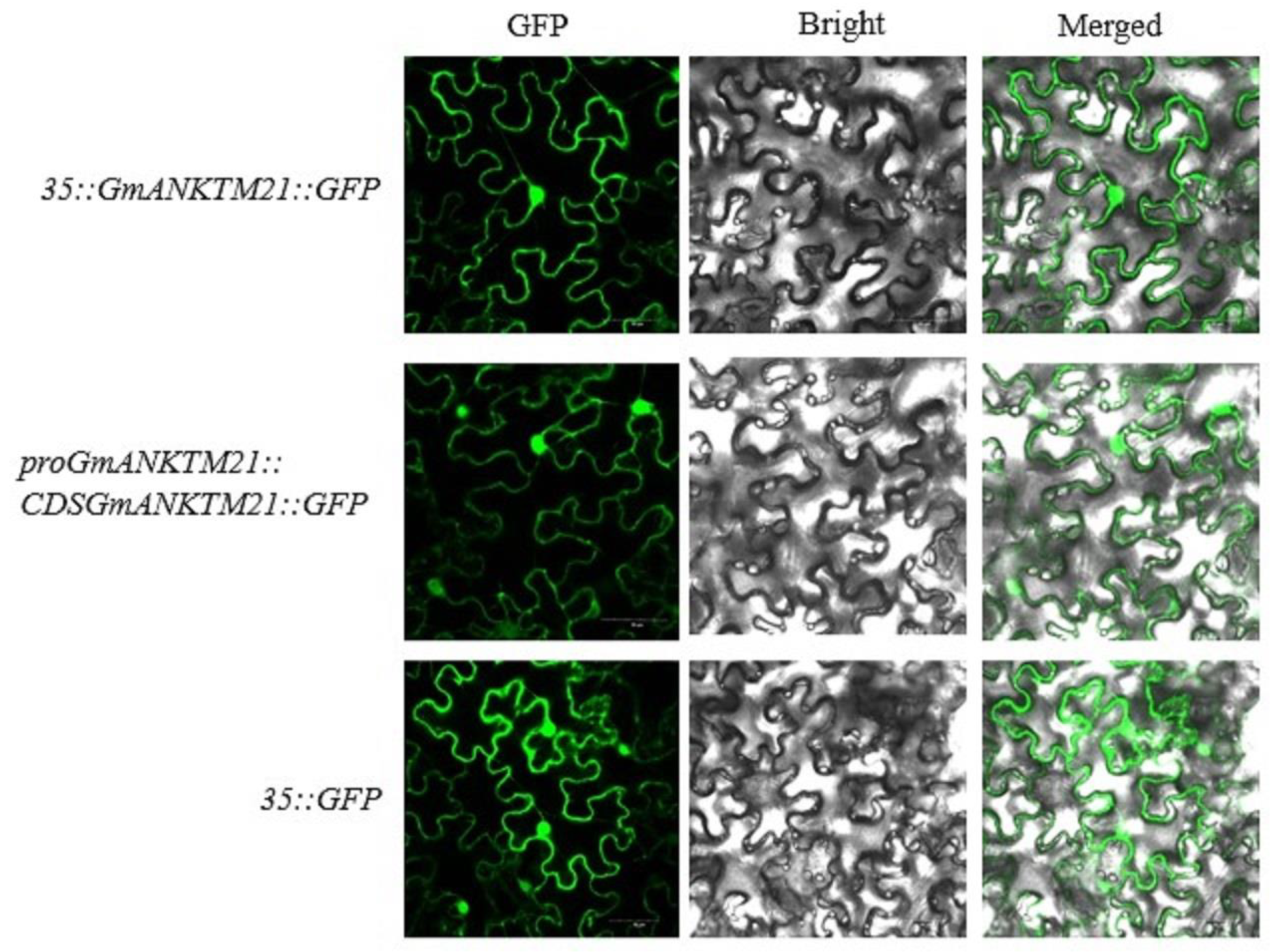
To determine the subcellular localization of GmANKTM21, a 35S-controlled GmANKTM21-green fluorescent protein chimera (CaMV35S::GmANKTM21-GFP), a GmANKTM21 promoter-controlled GmANKTM21-green fluorescent protein chimera (proGmANKTM21::GmANKTM21-GFP), and a control vector (CaMV35S::GFP) were transformed into Agrobacterium tumefaciens strain GV3101 and infiltrated into tobacco, and it was found that CaMV35S::GmANKTM21-GFP and proGmANKTM21::GmANKTM21-GFP fusion proteins were both localized in the cell membrane and nucleus (Figure 1).
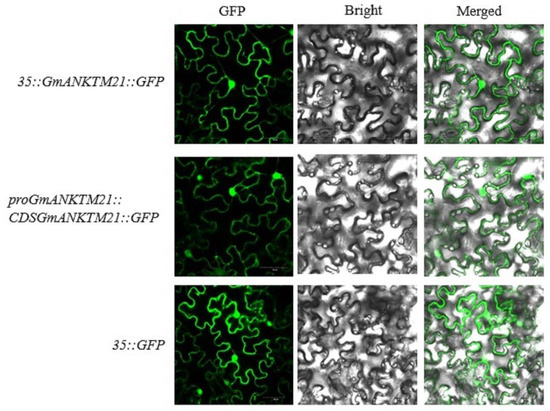
Figure 1.
Subcellular localization of GmANKTM21 in tobacco leaf lower epidermal cells. Green fluorescent protein (GFP) fluorescence, fluorescence, bright field images, and merged images are displayed from left to right. Fluorescence was observed by confocal microscopy. Scale bar is 50 μm.
2.2. The Overexpression of GmANKTM21 Enhanced the Tolerance of Soybeans to Drought during Germination
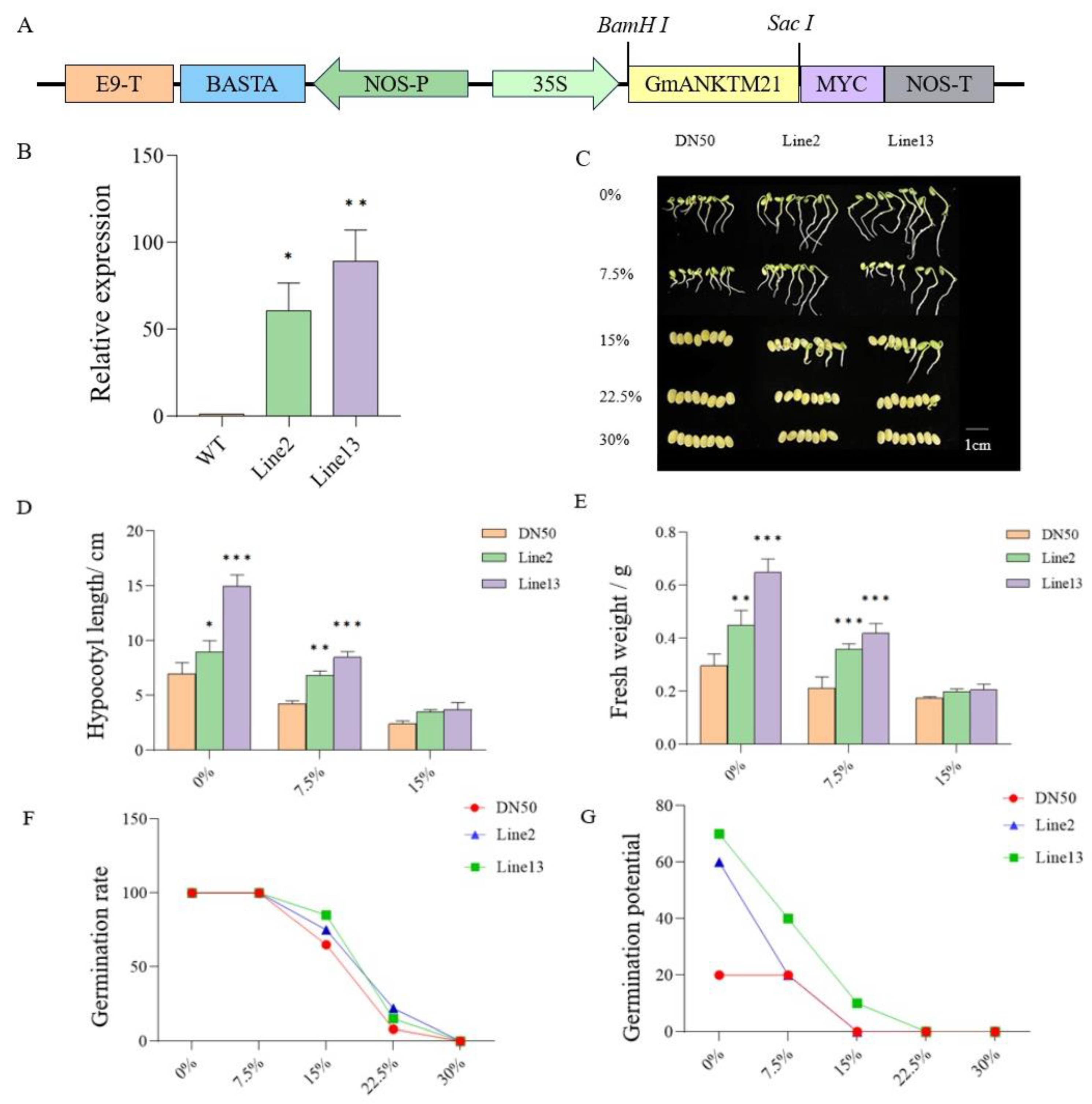
To confirm the function of GmANKTM21 in soybean drought tolerance, we generated two T3-generation GmANKTM21-OE (overexpression) transgenic lines under the control of the CaMV35S promoter (Figure 2A). In comparison with DN50 (WT) plants, expression levels of GmANKTM21 in Line2 and Line13 were significantly higher, by 53-fold in Line2 and 80-fold in Line13 (Figure 2B). The importance of GmANKTM21 in plant resistance to drought was first evaluated using a germination assay.
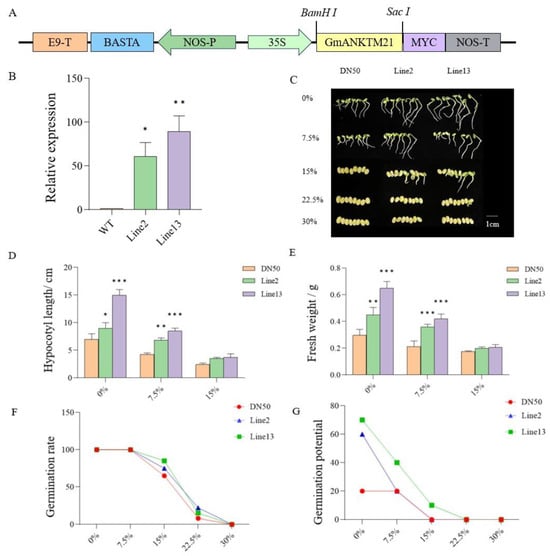
Figure 2.
Germination of GmANKTM21-OE (Line2 and Line13) and wild-type (DN50) seeds under different concentrations of PEG6000. (A) T-DNA region map of recombinant vector; (B) RT-qPCR detection of GmANKTM21 gene expression level in soybean overexpression plants. Values indicate the means ± SD (n = 3); (C) Germination phenotype of soybean seeds under drought; (D) Hypocotyl length; (E) Plant fresh weight, values indicate the means ± SD (n = 6); (F) Germination rate; (G) Germination potential. Values indicate the means ± SD (n = 6); the differences were statistically assessed using the Student’s t test; asterisks indicate significant differences (* p < 0.05, ** p < 0.01, *** p < 0.001) compared to those of the control samples (DN50).
Under normal conditions, Line2 and Line13 had longer hypocotyls and higher fresh weights compared to DN50. When exposed to 7.5% PEG6000, the growth of all soybean plants was found to be significantly inhibited, while Line13 grew relatively well. There were no significant differences in growth phenotypes at 15% PEG6000 (Figure 2C–E). Based on the data acquired, it became apparent that under a lower concentration of PEG6000 (7.5%), the germination rates of all three genotypes closely resembled those of their untreated counterparts (0%). However, discernible inhibitory effects of PEG6000 on soybean seed germination were evident at concentrations of 15% and 22.5%. Under these stress-inducing conditions, Line2 and Line13 seeds exhibited significantly heightened germination rates compared to DN50. Line2 and Line13 showed higher germination potential compared to DN50 under normal conditions, and Line13 showed higher germination potential under 7.5% and 15% PEG6000 conditions (Figure 2F,G).
2.3. Overexpression of GmANKTM21 Enhanced Drought Tolerance in Soybean Seedlings
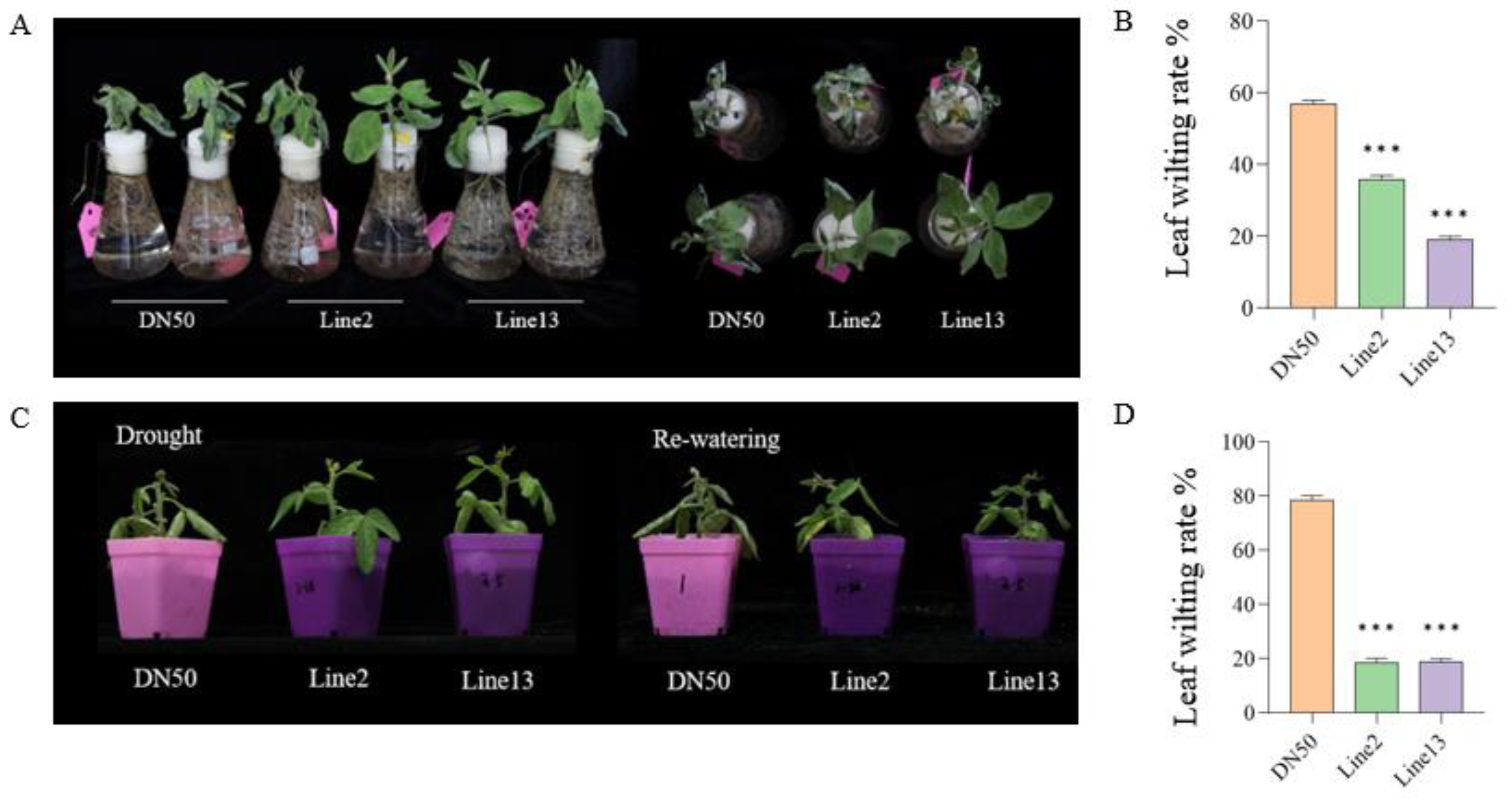
In order to further evaluate the drought tolerance of GmANKTM21-OE in soybeans, two sets of drought treatments were conducted on Lines2 and Line13. These treatments consisted of exposing the plants to 15% PEG6000 for 24 h, water deficit conditions for three days, and rehydration. Significant phenotypic differences were observed under the drought treatments (Figure 3A,C), with Line2 and Line13 plants showing lower leaf wilting compared to DN50 (Figure 3B,D), suggesting that they are more resistant to drought stress.

Figure 3.
Seedling phenotype of soybeans with overexpression of the GmANKTM21 gene under different drought simulation conditions. (A) PEG600 treatment; (C) Water cut-off treatment and restoration; (B,D) Leaf wilting rate. Values indicate the means ± SD (n = 6); asterisks indicate significant differences (*** p < 0.001) compared to that of the control samples (DN50).
2.4. Overexpression of the GmANKTM21 Gene Enhanced the Water Retention Ability of Soybean
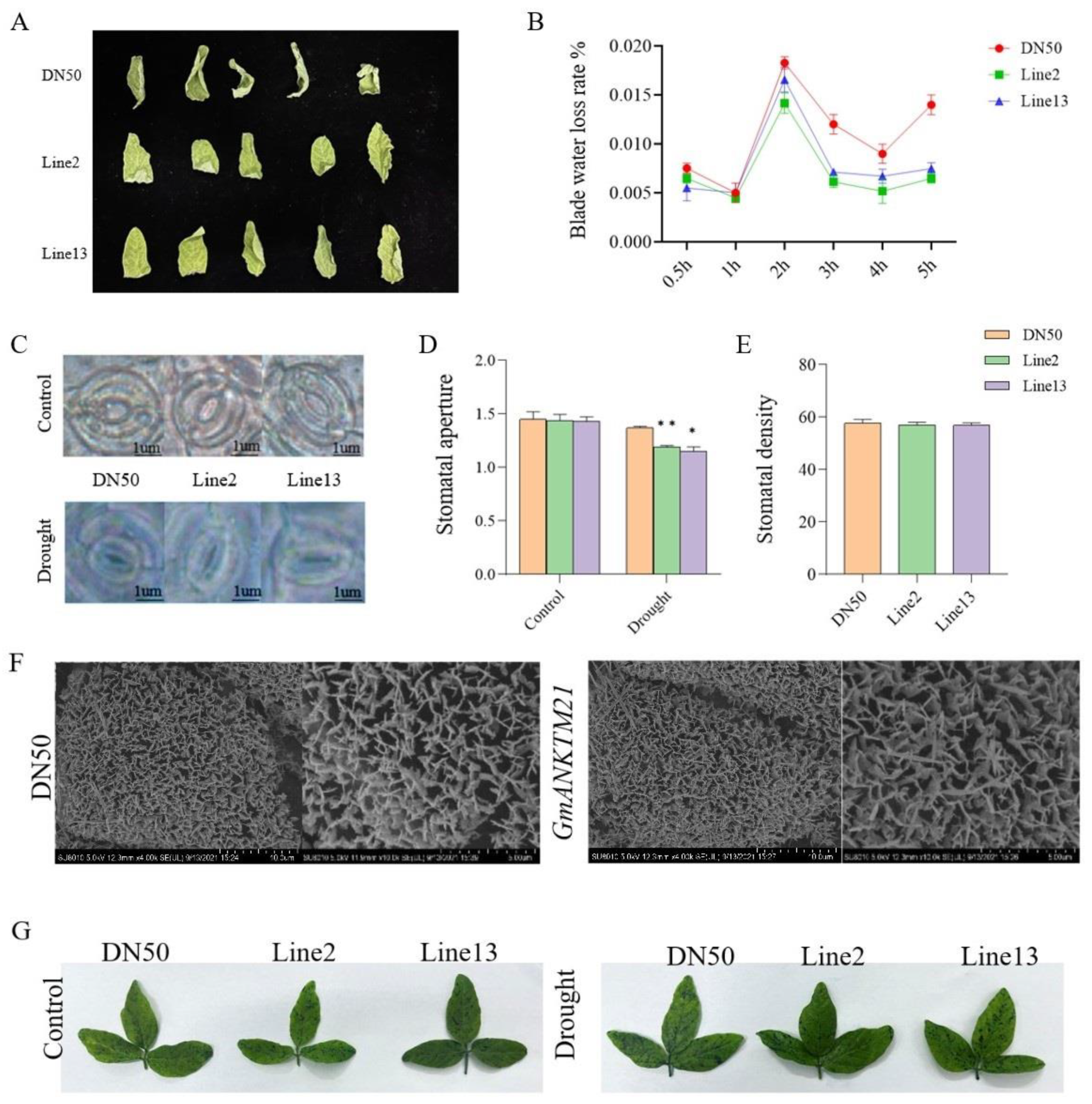
Since plant water retention capacity contributes to drought resistance [16], we measured the fresh weights of the detached second trifoliate leaves of DN50 and GmANKTM21-OE plants over 5 h following their detachment from the plants. Compared to that in DN50, water loss was slower in the leaves from GmANKTM21-OE plants (Figure 4A,B). We also observed the stomata on the second trifoliolate leaves of DN50 and GmANKTM21-OE plants during drought treatment. Before drought treatment, the stomatal aperture of GmANKTM21-OE plants was not significantly different from that of DN50 plants. After drought treatment, the stomatal aperture of GmANKTM21-OE plants was significantly smaller than that of DN50, but the stomatal density was not significantly different (Figure 4C–E).
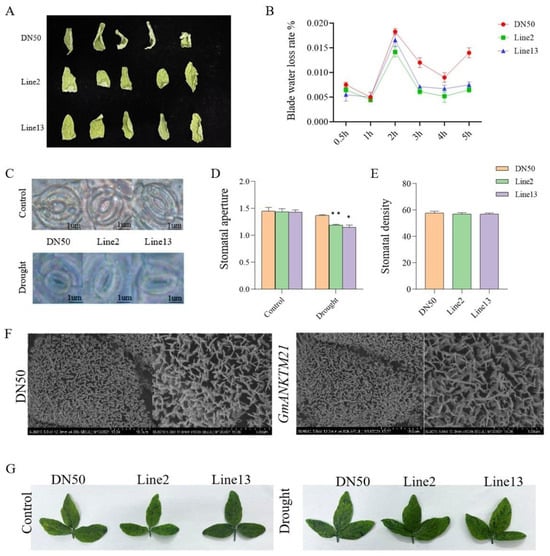
Figure 4.
Water retention ability of plants overexpressing the GmANKTM21 gene. (A) Leaf wilting phenotype; (B) Water loss rate; values indicate the means ± SD (n = 6); (C) Stomatal phenotype before and after drought treatment; (D) Stomatal opening. In total, 100 stomata were evaluated in each replicate; (E) Stomatal density; (F) Wax observation; (G) Toluidine blue staining. Values indicate the means ± SD (n = 6). The differences were statistically assessed using the Student’s t test; asterisks indicate significant differences (* p < 0.05, ** p < 0.01) compared to those of the control samples (DN50).
The accumulation of cuticular wax can enhance the water-retaining capacity of plants [17]. We examined the deposition of epidermal wax crystals on the leaf surfaces of DN50 and GmANKTM21-OE plants. No significant change in the number of Line2 and Line13 epidermal wax crystals was observed by scanning electron microscopy (SEM) compared to DN50 (Figure 4F). The wax layer directly determines cuticle permeability [18], so the cuticle permeability of GmANKTM21-OE and DN50 was examined. The results showed that there was no significant difference in the degree of staining by toluidine blue (TB) in the leaves of GmANKTM21-OE compared to DN50 (Figure 4G).
These results suggest that overexpression of GmANKTM21 enhances drought tolerance by adjusting stomata to enhance water retention in plants, rather than altering leaf cuticle and wax properties.
2.5. Overexpression of the GmANKTM21 Gene Enhanced the Antioxidant Capacity of Soybean
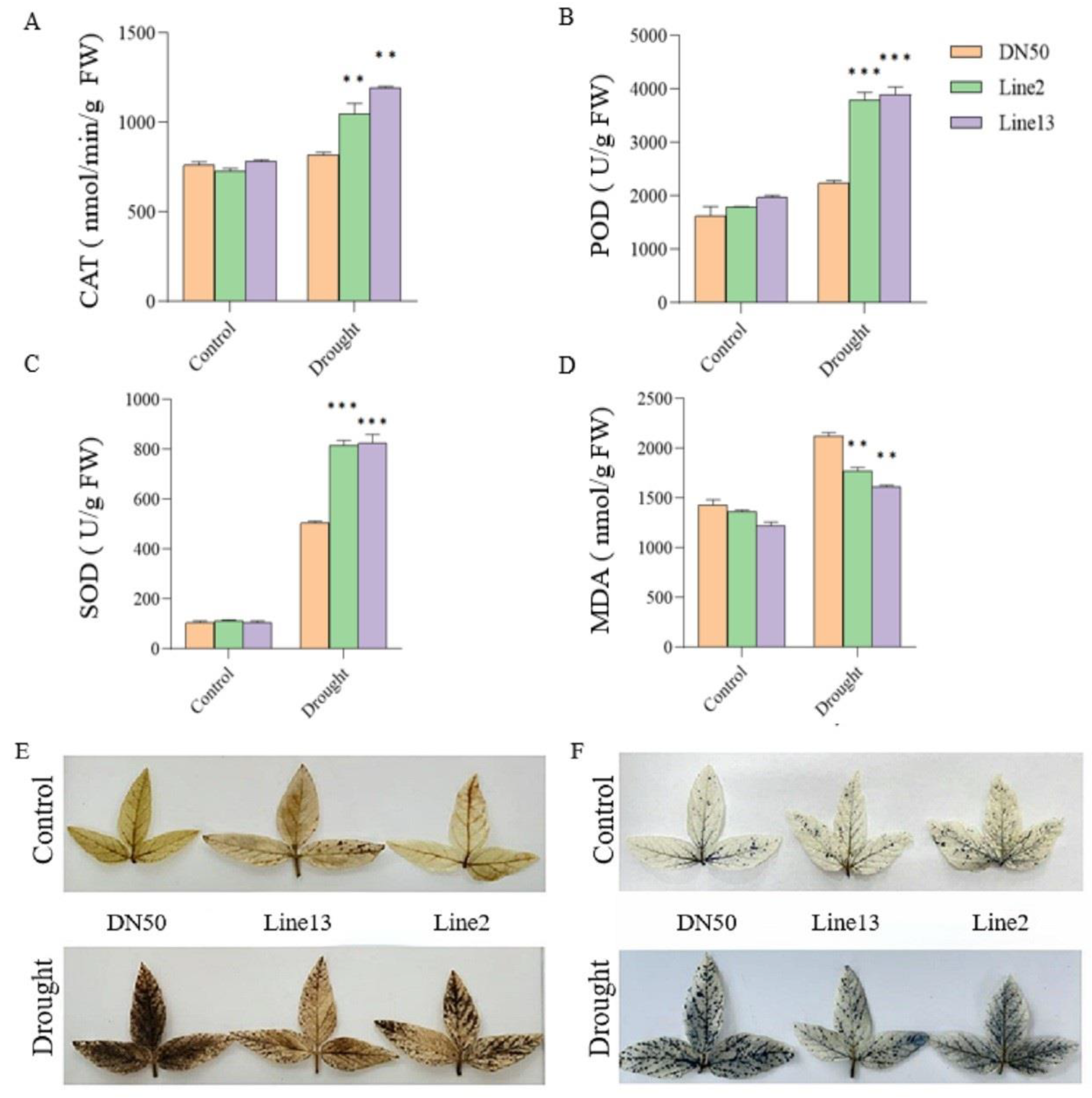
To further investigate the role of GmANKTM21 in regulating drought tolerance, we measured superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities as well as the content of MDA, to reflect the response ability of plants for resilience. The enzymatic activities of SOD, POD, and CAT scavenge intracellular ROS and reduce H2O2 production, thereby enhancing drought tolerance. The SOD, POD, and CAT activities of DN50 plants were significantly lower than those of the GmANKTM21-OE plants (Figure 5A–C). The MDA content is used to indicate lipid peroxidation products and to reflect the extent of plant damage caused by adversity. Compared with the WT plants, the MDA content in GmANKTM21-OE plants significantly decreased after drought stress (Figure 5D). Finally, we stained soybean leaves with nitro blue tetrazolium (NBT) and 3,3-diaminobenzidine (DAB) to detect H2O2 content under normal or drought treatment conditions in GmANKTM21-OE lines and DN50 plants. No substantial difference was observed under normal conditions with NBT and DAB staining; however, the color depth of DN50 plants was substantially higher than that of the GmANKTM21-OE lines under drought treatment. In contrast, the leaf color depth of the GmANKTM21-OE lines was substantially lower than that of DN50 plants (Figure 5E,F). These results suggest that GmANKTM21 overexpression in soybeans enhanced drought tolerance by reducing the MDA content and increasing the enzyme activities of SOD, POD, and CAT to reduce ROS production.
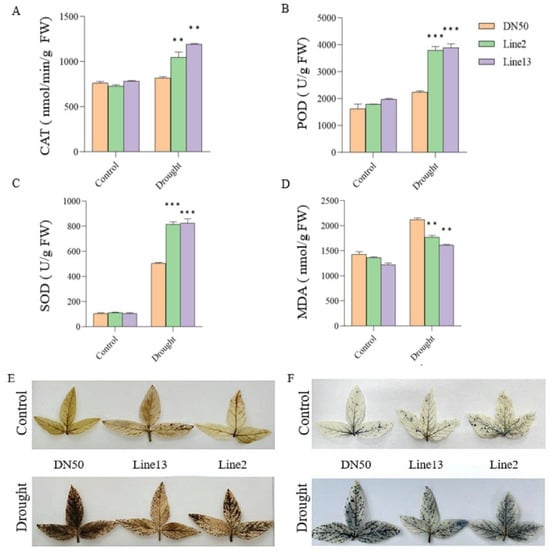
Figure 5.
Analysis of physiological parameters of different soybean plants under drought treatment. (A) CAT activity; (B) POD activity; (C) SOD activity; (D) MDA content; (E) NBT staining; (F) DAB dyeing. Values indicate the means ± SD (n = 6). The differences were statistically assessed using the Student’s t test; asterisks indicate significant differences (** p < 0.01, *** p < 0.001) compared to those of the control samples (DN50).
2.6. Studies on Downstream Genes of GmANKTM21 Gene Regulation
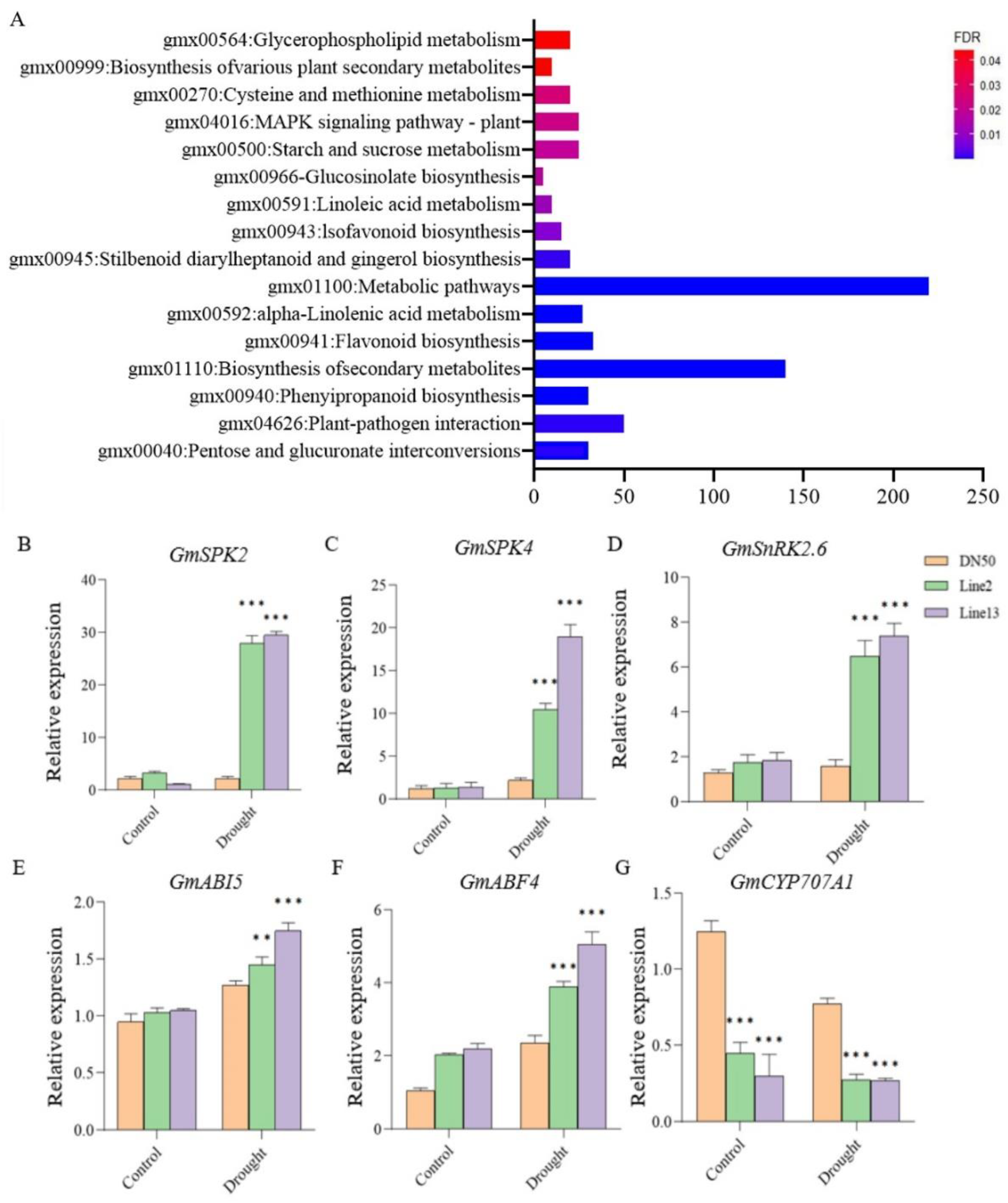
To further investigate the drought resistance mechanism of GmANKTM21, we performed a comparative transcriptome analysis using leaves from plants of GmANKTM21-OE lines and DN50 plants. Based on the RNA-seq data, we identified differential expression genes (DEGs) in the GmANKTM21-OE plants compared to DN50. To examine the functions of these DEGs, KEGG pathway analysis was performed. KEGG enrichment analysis showed that the differential genes were mainly enriched in the plant MAPK signaling pathway, starch and sucrose metabolism, and biosynthesis of secondary metabolites (Figure 6A).
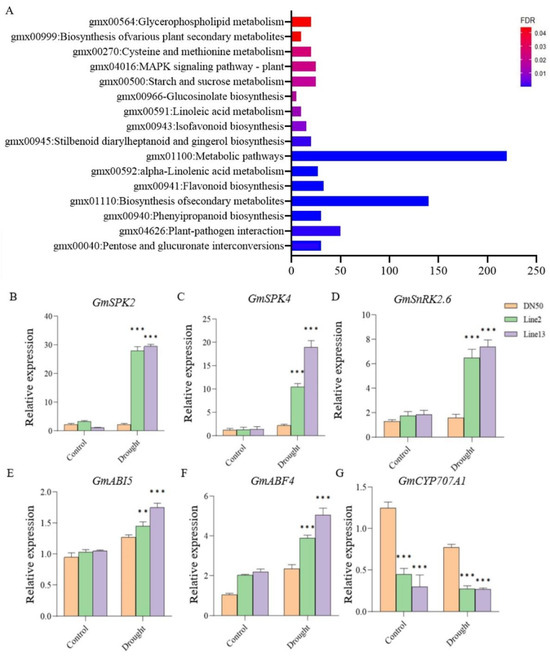
Figure 6.
Analysis of the KEGG pathway and verification of drought tolerance genes. (A) KEGG pathway analysis of DEGs of GmANKTM21-OE and DN50 under the drought treatment; (B–G) Analysis of expression patterns of selected genes under the drought treatment by RT-qPCR. Values indicate the means ± SD (n = 3). The differences were statistically assessed using the Student’s t test; asterisks indicate significant differences (** p < 0.01, *** p < 0.001) compared to those of the control samples (DN50).
The MAPK signaling pathway is involved in plant growth and development, phytohormone signal pathways, and ABA-regulated signal pathways consisting of PYR/PYL/RCAR-PP2C-SnRK2 [19,20]. Based on these results, we suggest that GmANKTM21 plays an important role in the drought stress response by regulating various drought-related genes and may be involved in the MAPK signaling pathway. To further investigate whether the role of GmANKTM21 in drought tolerance is associated with the MAPK signaling pathway, we analyzed the expression of genes that function in drought tolerance and genes that are related to the MAPK signaling pathway in DN50 and GmANKTM21-OE plants. The results showed that GmSPK2, GmSPK4, GmSnRK2.6, GmABI5, and GmABF4 were up-regulated in Line2 and Line13 after drought treatment. However, in DN50, the expression levels of the GmSPK2, GmSPK4, and GmSnRK2.6 genes did not change significantly before and after drought treatment. In addition, GmCYP707A1 gene expression in Line2 and Line13 plants was significantly lower than that of DN50 before and after drought treatment (Figure 6B–G). In the light of previous studies, it has been observed that the GmSPK2 and GmSPK4 genes can be activated in response to hyperosmotic and drought stresses, exerting resistance through pertinent mechanisms [21]. Additionally, the GmCYP707A1 gene, a member of the CYP707A1 family, plays a role in the degradation of ABA [22]. Drawing upon the outcomes of our current experiment, we suggest that the GmANKTM21 gene governs plant drought resistance by modulating the expression of various genes within the MAPK signaling pathway, including GmSPK2, GmSPK4, and GmCYP707A1.
3. Discussion
Drought significantly impacts soybean production and quality. ANK proteins facilitate protein-protein interactions, which are crucial for defense responses and, occasionally, growth and development. Understanding ANK protein function sheds light on plant stress responses, aiding environmental adaptation research in plants.
Water is essential for seed germination; however, while other conditions may be ideal, drought stress inhibits the imbibition of seeds and, consequently, hinders germination [23]. Our results revealed improvements in seed germination percentage, root growth, and drought tolerance in the transgenic soybean with GmANKTM21 in comparison to those of WT plants under drought stress simulated by PEG6000 (Figure 2 and Figure 3), and our results showed that overexpression of GmANKTM21 in soybeans could also improve plant tolerance to drought stress.
Stomatal closure, recognized as an early response to drought stress, is commonly utilized as a crucial indicator for swiftly assessing plant water status [24,25]. Our investigation validated that the overexpression of GmANKTM21 facilitates prompt stomatal closure, thereby diminishing transpiration and mitigating leaf water loss during drought conditions (Figure 4C–E). Despite the pivotal role of cuticular waxes in providing the initial defense against environmental stresses [26,27,28], our findings indicate that the overexpression of GmANKTM21 does not significantly influence the quantity of wax or keratin (Figure 4F,G). This underscores that GmANKTM21 primarily attenuates water loss by enhancing stomatal closure, independent of cuticular wax modulation.
In biological systems, adverse environmental conditions like drought stress intensify ROS production, posing risks to plant health. Antioxidant enzymes play a crucial role in mitigating ROS levels and reducing oxidative stress [29]. In Arabidopsis plants overexpressing VvNAC17, increased activities of SOD, POD, and CAT, alongside with elevated proline content and reduced MDA levels, were observed [30]. Similarly, our study with transgenic soybeans overexpressing GmANKTM21 demonstrated enhanced drought tolerance, supported by elevated enzymatic activities of SOD, POD, and CAT, and decreased MDA levels (Figure 5).
To characterize the enriched pathways and genes affected by overexpressing GmANKTM21, we performed transcriptomic analyses in leaves and investigated significantly enriched biological processes and pathways by KEGG analysis. The results showed that differentially expressed genes were enriched into the MAPK signaling pathway (Figure 6A). The MAPK signaling cascade pathway is widely involved in multiple developmental stages of plants and has recently been shown to play a key role in abiotic stress signaling [31]. Recently, several MAPK family members have been reported to be directly or indirectly activated by the ABA pathway [32]. To validate the data, the levels of some differentially expressed genes were assessed by RT-qPCR in leaves exposed to drought stress (Figure 6B–G). The results showed that GmANKTM21 regulated the expression of GmSPK2.6, GmSPK4, and GmCYP707A1 under drought stress. Previous reports have indicated that GmSPK2 and GmSPK4 are induced under hypertonic and dehydration stress, while GmCYP707A1 responds to both salt and dehydration stresses. Consequently, the RT-qPCR results confirmed that GmANKTM21 regulates the expression levels of certain genes within the MAPK signaling pathway, thereby affecting plant drought tolerance.
4. Materials and Methods
4.1. Subcellular Localization
The full-length cDNA sequence of GmANKTM21 was ligated to the GFP gene carrying the CaMV35S promoter, and the full-length cDNA sequence of GmANKTM21 was ligated to the GFP gene carrying the GmANKTM21 promoter. The recombinant vector was transferred into the Agrobacterium tumefaciens GV3101 strain, injected into tobacco epidermal cells, placed in a dark room at 25 °C for 12 h, and then restored to light conditions for 48 h of culturing and observed using laser confocal microscopy.
4.2. Plant Materials and Growth Conditions
Soybean (Glycine max) cultivars Dongnong50 and GmANKTM21-overexpressing transgenic soybean were used to analyze. Plants were grown in an artificial climate chamber under LD conditions (16-h:8-h light/dark) at 28 °C, relative humidity 55–65%, and light intensity 1000. On the 18th day after emergence, fully expanded trifoliate leaves were sampled 2 h after dawn from three individual plants for RT-qPCR analysis.
4.3. Vector Construction and Transformation
To generate the GmANKTM21-overexpressing transgenic soybean, the GmANKTM21 coding DNA sequence (CDS) was amplified, as were the PCR fragments from Dongnong50 leaves, which contained BamH I and Sac I, respectively. The PCR product was then inserted downstream of the CaMV 35S promoter in the pBA-myc vector. The resulting construct was then introduced into Agrobacterium strain EHA105 and transformed into the Dongnong50 cultivar. The resulting construct also contained the selectable marker BAR for glufosinate resistance. T0, T1, and T2 plants were selected by smearing the expanded leaves with 160 mg·L−1 glufosinate. The glufosinate-resistant plants were used for RT-qPCR.
4.4. RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from mature leaves of seedlings using the Ultrapure RNA Kit (CWBIO, Shanghai, China) according to the manufacturer’s instructions, with three independent biological replicates. Total RNA was reverse transcribed into first-strand cDNA using the Reverse Transcription System and SYBR Premix Top Mix (Transgen, Beijing, China). The measured Ct values were transformed into relative copy numbers utilizing the ∆∆Ct methodology. Relative fold enrichment was computed by normalizing the quantity of the target DNA fragment against the reference gene, TUA5. Three independent biological replicates (n = 3 plants) were collected and subjected to RT-qPCR with technical triplicates. Raw data underwent standardization as previously outlined. Details regarding the primers are provided in Supplementary Table S1.
4.5. Determination of Seed Germination Index
In this experiment, the relevant indexes of seed germination of GmANKTM21-OE and Dongnong50 soybean seeds under stress treatment were analyzed, and the seeds were treated with 0%, 7.5%, 15%, 22.5%, and 30% PEG6000 solution, put into the same volume of culture box, and grown for a total of seven days. Each group of experiments was subjected to three technical replicates and three biological replicates. The germination and growth indices are measured as follows:
Germination rate = number of germinated seeds/number of tested seeds × 100%
Germination potential = number of germinated seeds within the specified date/number of seeds tested × 100%
The length of the hypocotyls of the plants was measured with a digital vernier caliper, and the electronic balance accurately weighed the fresh weight of the plant.
4.6. Leaf Water Loss Rate
GmANKTM21-OE and Dongnong50 leaves with similar shape and growth stage were selected to measure the fresh weight of the leaves at 0.5 h, 1 h, 2 h, 4 h and 5 h respectively and finally put into the oven to dry to a constant weight. Record the dry weight and calculate the leaf water loss rate. Six replications were performed for each group of experiments.
4.7. Stomatal Opening and Density
The stomatal opening and stomatal density of soybean with GmANKTM21-OE and Dongnong50, leaves with similar shape and growth stage were selected from normal irrigation and water interruption for one week, and the stomatal opening and stomatal density per unit area at different locations were measured under the microscope, and the average value was finally taken. The test was designed with three replications, and 100 stomata were examined in each replication.
4.8. Stratum Corneum Staining
GmANKTM21-OE and Dongnong50 leaves with similar shape and growth stage were selected. The materials were soaked in the same volume of 5% concentration toluidine blue solution freshly prepared, incubated at 37 °C for 2 h, and after the staining was completed, rinsed with distilled water many times and taken with a camera. Each group of experiments was subjected to three technical replicates and three biological replicates.
4.9. Wax Observation
GmANKTM21-OE and wild type (Dongnong50) leaves with similar shape and growth stage were selected and fixed in a fixative solution (75% ethanol, 5% acetic acid, 5% glycerol, 5% formaldehyde, and 10% deionized water) for at least one day, then the critical point was dried, sputtered gold-plated, and observed under SEM (JEM-6380LV, JEOL Ltd., Tokyo, Japan).
4.10. Determination of Antioxidant Enzyme Activity
The antioxidants, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), were extracted from approximately 0.1 g of the leaves of GmANKTM21-OE and Dongnong50 using a 1 mL extraction solution. The malondialdehyde (MDA) in the leaves was measured spectrophotometrically following the manufacturer’s instructions (Comin, Suzhou, China). Each recorded value represented three biological replicates and three technical replicates.
4.11. DAB and NBT Staining
The fully expanded leaves of GmANKTM21-OE and Dongnong50 after drought treatment for 6 days were immediately vacuum-infiltrated with diaminobenzidine tetrahydrochloride (DAB) solution (1 mg/mL 3,3′-diaminobenzidine-4HCl, pH 3.8) and NBT (nitroblue-tetrazole) solution (0.5 mg/mL NBT, 10 mM potassium phosphate, pH 7.8, 10 mM sodium azide), incubated in the dark for 8–10 h, and decolorized in 95% ethanol. Subsequently, the stained leaves were observed directly.
4.12. Expression Analysis of Downstream Genes by the GmANKTM21 Gene
The leaves and stem tips were taken at the R1 stage (the initial flowering stage of DN50), and biological replicates were performed three times for each sample. The samples were sequenced on the Illumina sequencing platform by Annoroad Biotechnology Co., Ltd. Differentially expressed genes were analyzed, and KEGG pathway analysis was performed. Subsequently, the differentially expressed genes were verified by RT-qPCR. All primers are listed in Supplementary Table S1.
5. Conclusions
In summary, we demonstrated the subcellular localization of GmANKTM21 in the cell membrane and nucleus. Overexpression of GmANKTM21 in soybeans resulted in drought tolerance compared with wild-type (WT) plants, which was associated with stomatal closure and increased activity of antioxidant enzymes during drought stress. Additionally, KEGG and RT-qPCR analyses revealed that GmANKTM21 positively regulates the expression of GmSPK2 and GmSPK4, while negatively regulating the expression of GmCYP707A1 in response to drought stress. These findings provide new insights into the function of GmANKTM21 and may contribute to the enhancement of plant abiotic-stress tolerance through genetic manipulation in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25136972/s1.
Author Contributions
Conceptualization, Y.Z.; methodology, S.W.; software, X.M.; validation, Y.Z., S.W. and Y.H.; formal analysis, J.Z.; investigation, J.X.; resources, X.K.; data curation, J.Z. and S.J.; writing—original draft preparation, Y.Z. and J.Z.; writing—review and editing, Y.Z. and S.J.; visualization, S.J.; supervision, J.Z.; project administration, X.B. and X.W.; funding acquisition, X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the National Natural Science Foundation of China (U23A20192).
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merrium, S.; Ali, Z.; Tahir, M.H.N.; Habib-Ur-Rahman, M.; Hakeem, S. Leaf rolling dynamics for atmospheric moisture harvesting in wheat plant as an adaptation to arid environments. Environ. Sci. Pollut. Res. 2022, 29, 48995–49006. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bu, T.; Cheng, Q.; Dong, L.; Su, T.; Chen, Z.; Kong, F.; Gong, Z.; Liu, B.; Li, M. Two homologous LHY pairs negatively control soybean drought tolerance by repressing the abscisic acid responses. New Phytol. 2021, 229, 2660–2675. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2020, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Y.; Lu, Z.-W.; Sun, Y.; Fang, Z.-W.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S.; Min, D.-H. The Ankyrin-Repeat Gene GmANK114 Confers Drought and Salt Tolerance in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 584167. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ren, Y.; Xu, J.; Wei, Q.; Song, P.; Guan, Y.; Gao, H.; Zhang, Y.; Hu, H.; Li, C. Identification of ankyrin-transmembrane-type subfamily genes in Triticeae species reveals TaANKTM2A-5 regulates powdery mildew resistance in wheat. Front. Plant Sci. 2022, 13, 943217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wan, Q.; He, X.; Ning, L.; Huang, Y.; Xu, Z.; Liu, J.; Shao, H. Genome-wide characterization of the ankyrin repeats gene family under salt stress in soybean. Sci. Total Environ. 2016, 568, 899–909. [Google Scholar] [CrossRef]
- Li, J.; Mahajan, A.; Tsai, M.-D. Ankyrin repeat: A unique motif mediating protein−protein interactions. Biochemistry 2006, 45, 15168–15178. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L.; Qiu, X.; Wei, J.; Lu, H.; Sun, G.; Ma, X.; Yang, Z.; Zhu, C.; Hou, Y.; et al. AKR2A participates in the regulation of cotton fibre development by modulating biosynthesis of very-long-chain fatty acids. Plant Biotechnol. J. 2020, 18, 526–539. [Google Scholar] [CrossRef]
- Yu, F.; Shi, J.; Zhou, J.; Gu, J.; Chen, Q.; Li, J.; Cheng, W.; Mao, D.; Tian, L.; Buchanan, B.B.; et al. ANK6, a mitochondrial ankyrin repeat protein, is required for male-female gamete recognition in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 22332–22337. [Google Scholar] [CrossRef]
- Wang, H.; Zou, S.; Li, Y.; Lin, F.; Tang, D. An ankyrin-repeat and WRKY-domain-containing immune receptor confers stripe rust resistance in wheat. Nat. Commun. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, S.; Liu, S.; Yu, M.; Su, H.; Shu, H.; Li, X. Global analysis of ankyrin repeat domain C3HC4-Type RING finger gene family in plants. PLoS ONE 2013, 8, e58003. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.S.; Cho, H.S.; Choi, D.; Joung, Y.H.; Lim, C.K.; Hur, J.H.; Wang, M.-H. Tomato plants overexpressing CaKR1 enhanced tolerance to salt and oxidative stress. Biochem. Biophys. Res. Commun. 2007, 363, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Garcion, C.; Guilleminot, J.; Kroj, T.; Parcy, F.; Giraudat, J.; Devic, M. AKRP and EMB506 are two ankyrin repeat proteins essential for plastid differentiation and plant development in Arabidopsis. Plant J. 2006, 48, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, M.C.; Singla, J.; Sánchez-Martín, J.; Zbinden, H.; Šimková, H.; Karafiátová, M.; Doležel, J.; Gronnier, J.; Poretti, M.; Glauser, G.; et al. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Sintaha, M.; Man, C.-K.; Yung, W.-S.; Duan, S.; Li, M.-W.; Lam, H.-M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, X.; An, J.; Liu, X.; Ren, H. CsCER6 and CsCER7 Influence Fruit Glossiness by Regulating Fruit Cuticular Wax Accumulation in Cucumber. Int. J. Mol. Sci. 2023, 24, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Francocci, F.; Gully, K.; Martens, H.J.; De Lorenzo, G.; Nawrath, C.; Ferrari, S. Impaired Cuticle Functionality and Robust Resistance to Botrytis cinerea in Arabidopsis thaliana Plants With Altered Homogalacturonan Integrity Are Dependent on the Class III Peroxidase AtPRX71. Front. Plant Sci. 2021, 12, 696955. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Chen, M.; Ni, L.; Chen, J.; Sun, M.; Qin, C.; Zhang, G.; Zhang, A.; Jiang, M. Rice calcium/calmodulin-dependent protein kinase directly phosphorylates a mitogen-activated protein kinase kinase to regulate abscisic acid responses. Plant Cell 2021, 33, 1790–1812. [Google Scholar] [CrossRef]
- Cai, X.; Jia, B.; Sun, M.; Sun, X. Insights into the regulation of wild soybean tolerance to salt-alkaline stress. Front. Plant Sci. 2022, 13, 1002302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Y.; Xian, W.; Wang, J.; Liao, H. Identification and expression analysis of the Glycine max CYP707A gene family in response to drought and salt stresses. Ann. Bot. 2012, 110, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Hellal, F.; El-Shabrawi, H.; El-Hady, M.A.; Khatab, I.; El-Sayed, S.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2017, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, W.; Wang, G.; Hu, Y.; Zhong, X.; Tang, G. Physiological Regulation of Photosynthetic-Related Indices, Antioxidant Defense, and Proline Anabolism on Drought Tolerance of Wild Soybean (Glycine soja L.). Plants 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Luang, S.; Li, Y.; Bazanova, N.; Borisjuk, N.; Hrmova, M.; Lopato, S. Wheat drought-responsive WXPL transcription factors regulate cuticle biosynthesis genes. Plant Mol. Biol. 2017, 94, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Gai, X.; Ren, J.; Liu, X.; Cai, Y.; Wang, Q.; Ren, H. Cucumis sativus L. WAX2Plays a Pivotal Role in Wax Biosynthesis, Influencing Pollen Fertility and Plant Biotic and Abiotic Stress Responses. Plant Cell Physiol. 2015, 56, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, C.; Luo, N.; Jiang, Y.; Wang, Y.; Liu, Y.; Chen, C.; Wang, Y.; Gan, Q.; Jin, S.; et al. Brassica napus BnaC9.DEWAX1 Negatively Regulates Wax Biosynthesis via Transcriptional Suppression of BnCER1-2. Int. J. Mol. Sci. 2023, 24, 4287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, X.; Tang, M.; Wang, Y.; Zhang, Q.; Li, H.; Zhou, Y.; Sun, F.; Cui, X. Molecular Characterization and Drought Resistance of GmNAC3 Transcription Factor in Glycine max (L.) Merr. Int. J. Mol. Sci. 2022, 23, 12378. [Google Scholar] [CrossRef]
- Cui, X.; Tang, M.; Li, L.; Chang, J.; Yang, X.; Chang, H.; Zhou, J.; Liu, M.; Wang, Y.; Zhou, Y.; et al. Expression Patterns and Molecular Mechanisms Regulating Drought Tolerance of Soybean [Glycine max (L.) Merr.] Conferred by Transcription Factor Gene GmNAC19. Int. J. Mol. Sci. 2024, 25, 2396. [Google Scholar] [CrossRef]
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and Metabolomic Analysis of Seedling-Stage Soybean Responses to PEG-Simulated Drought Stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, X.; Zhang, Y.; Zhao, S.; Liu, J.; Wu, G.; Yan, X.; Luo, J. Transcriptomic Profiling Highlights the ABA Response Role of BnSIP1-1 in Brassica napus. Int. J. Mol. Sci. 2023, 24, 10641. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).