The White Clover TrMYB33-TrSAMS1 Module Contributes to Drought Tolerance by Modulation of Spermidine Biosynthesis via an ABA-Dependent Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Analysis of TrSAMS1 and Three Genes Involved in Spd Synthesis
2.2. TrSAMS1 Promotes Spd Synthesis and Positively Contributes to Drought Tolerance in Arabidopsis and White Clover
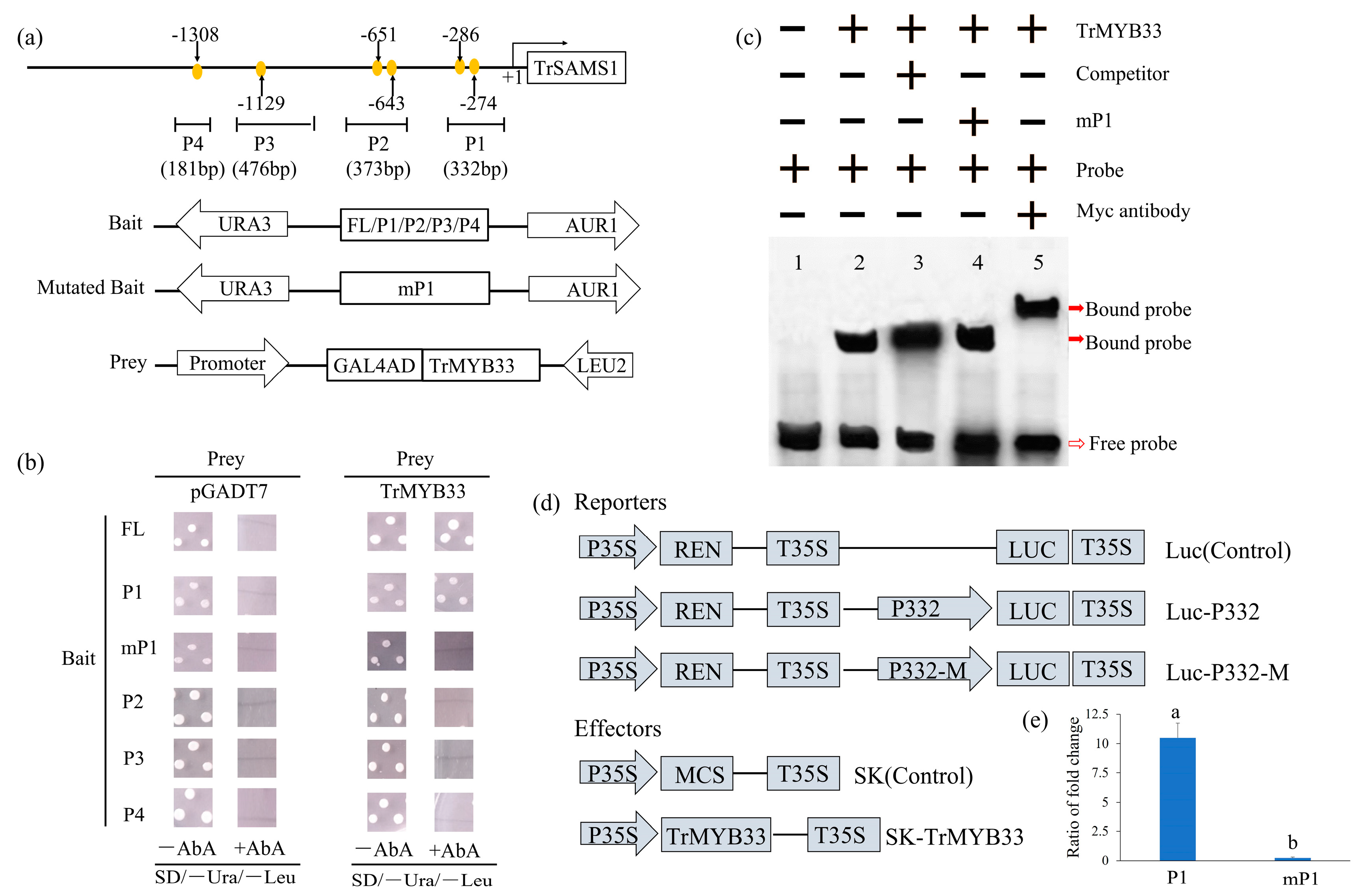
2.3. The TrMYB33 Transcription Factor Regulates TrSAMS1 Transcription
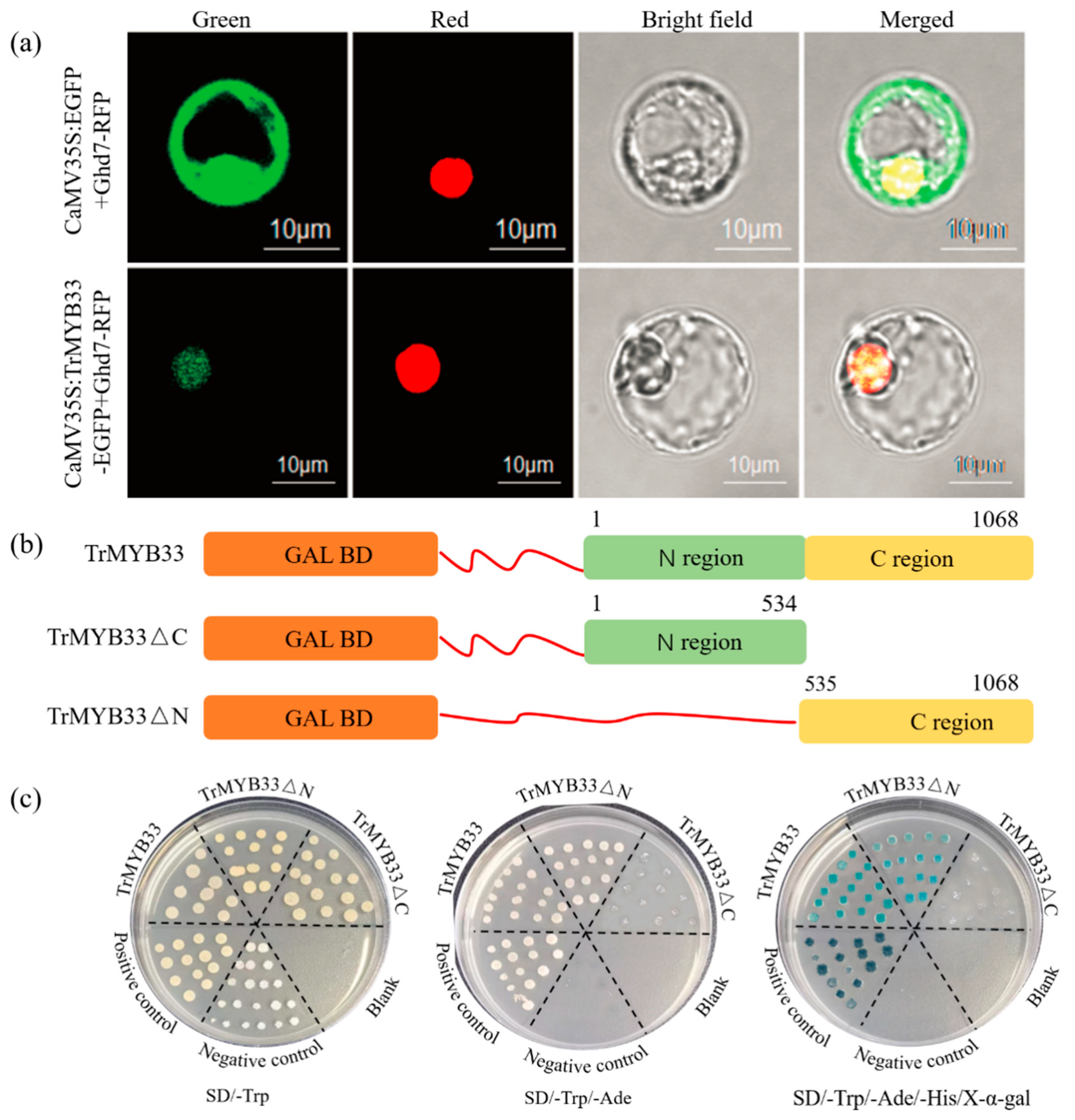
2.4. TrMYB33 Is Localized in the Nucleus and Has the Ability to Activate Transcription
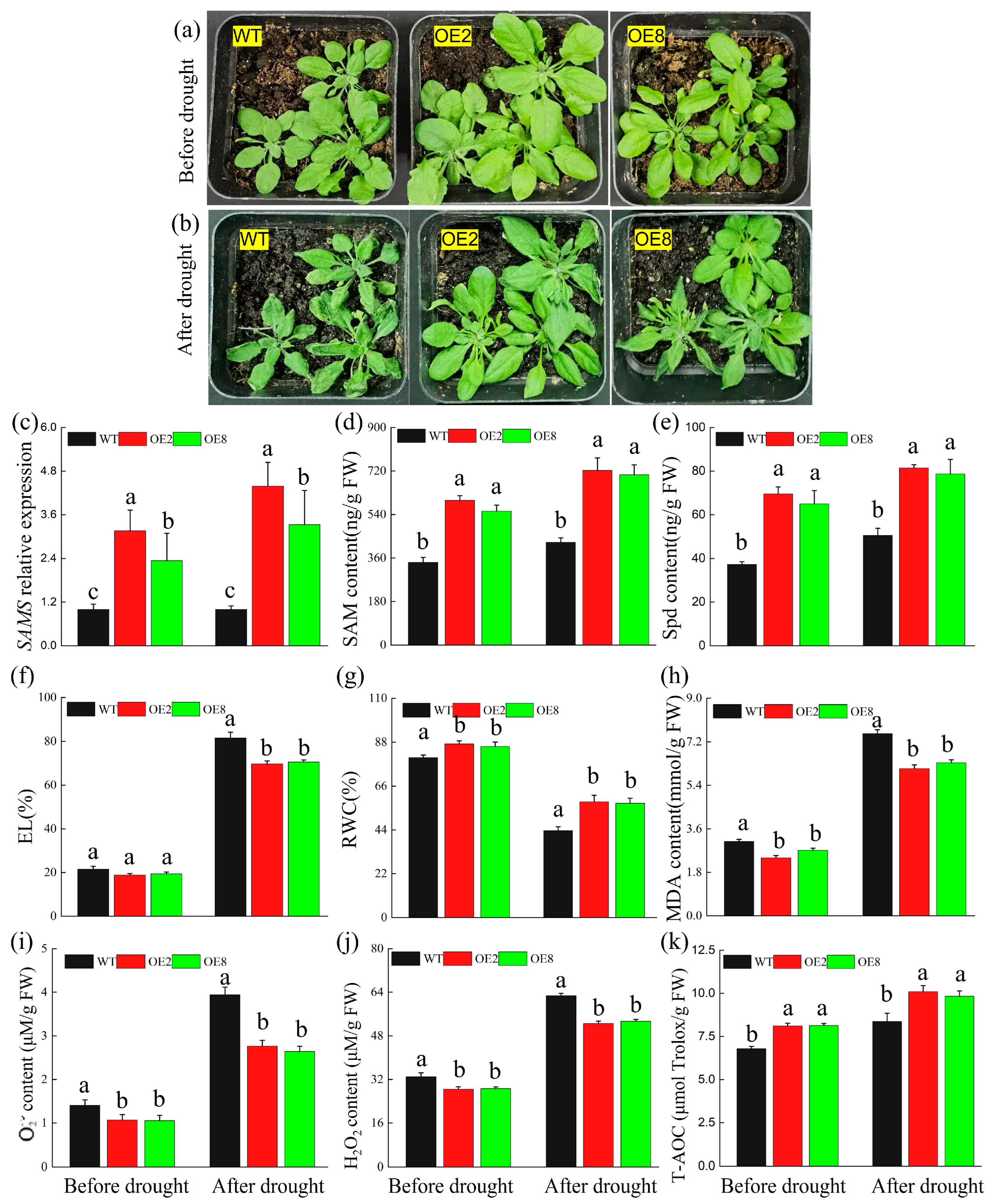
2.5. TrMYB33 Regulates Spd Accumulation and Contributes to Drought Tolerance in Arabidopsis
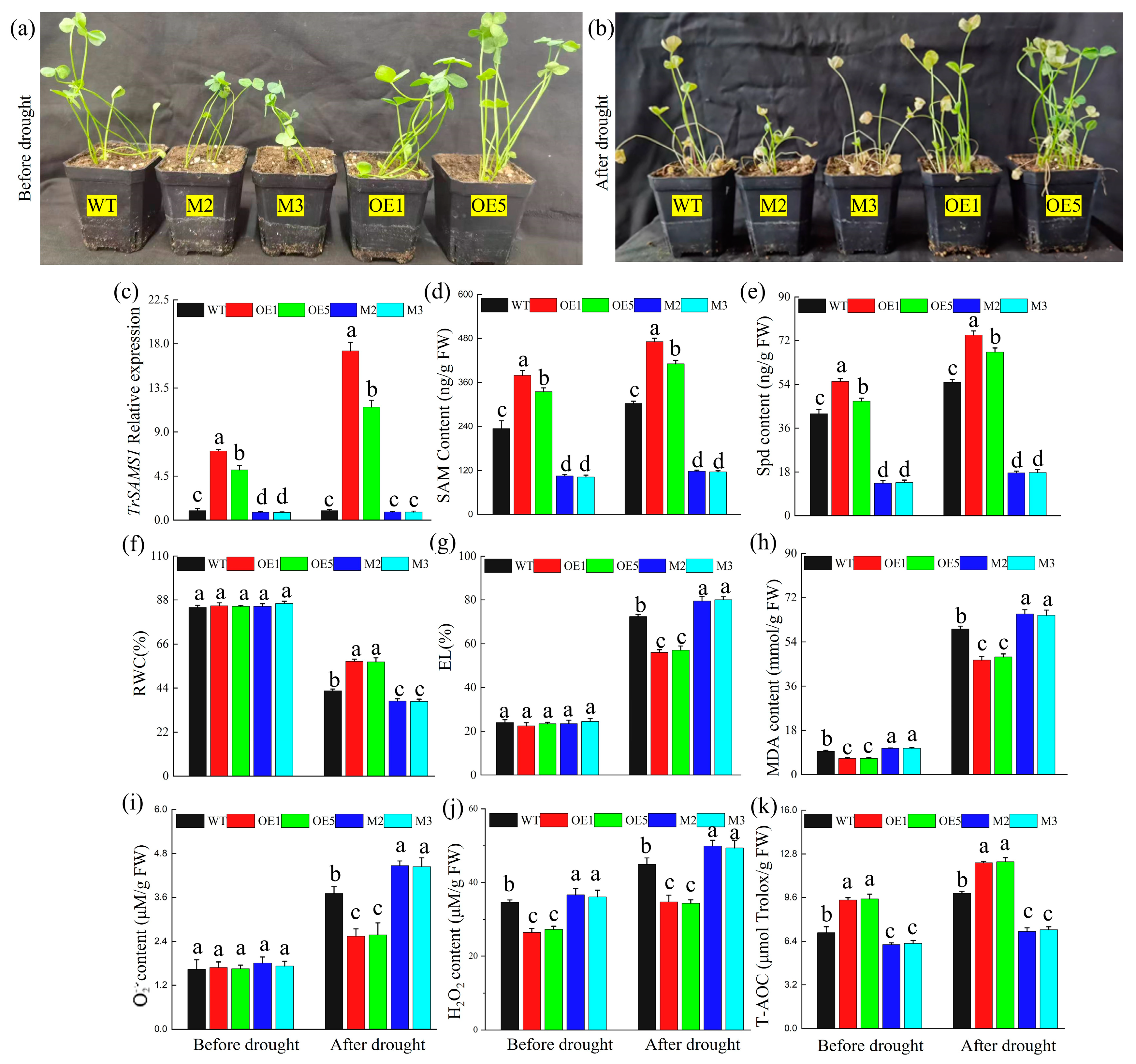
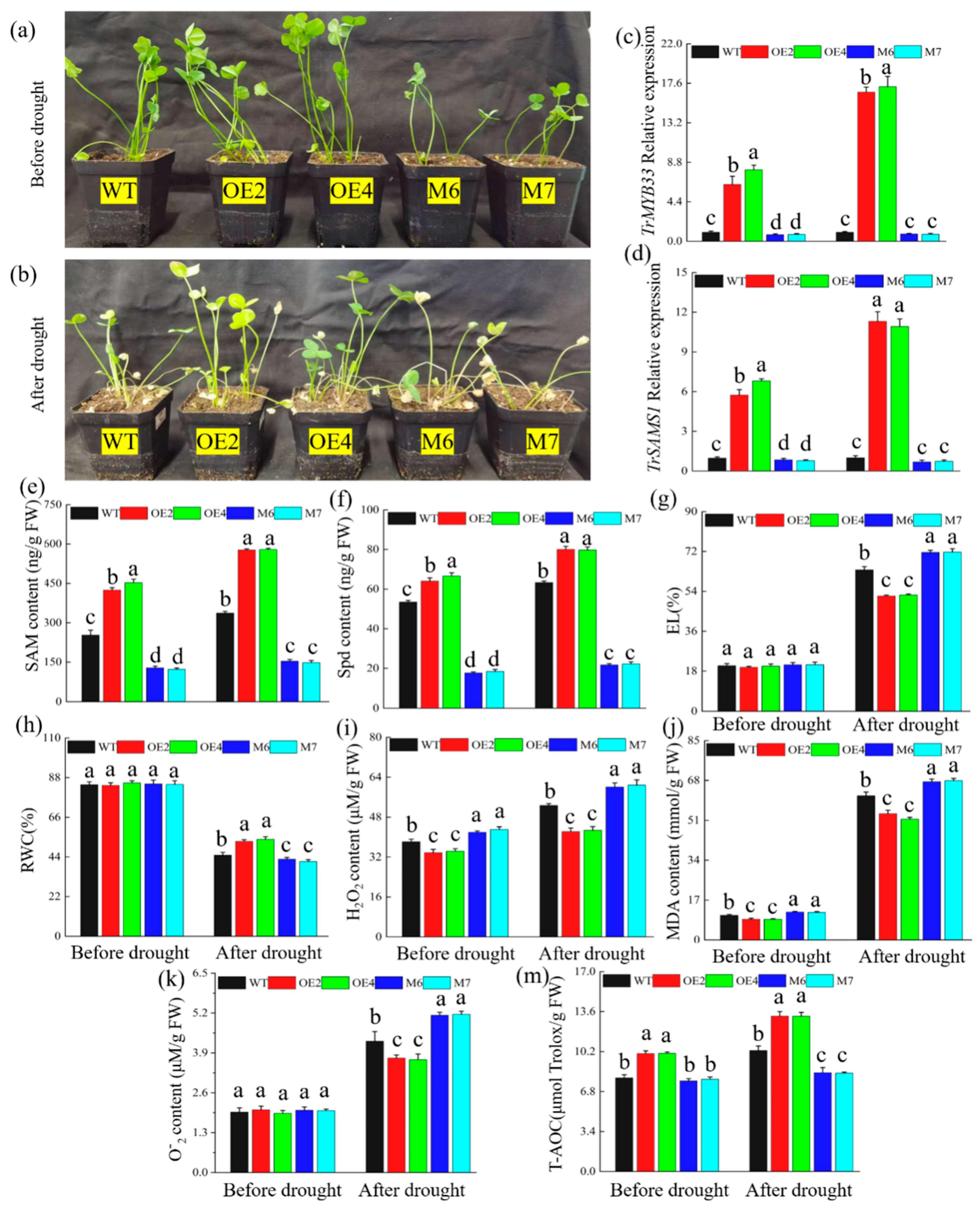
2.6. TrMYB33 Promotes SAMS Expression and Spd Synthesis in Trifolium Repens and Improves Its Drought Tolerance
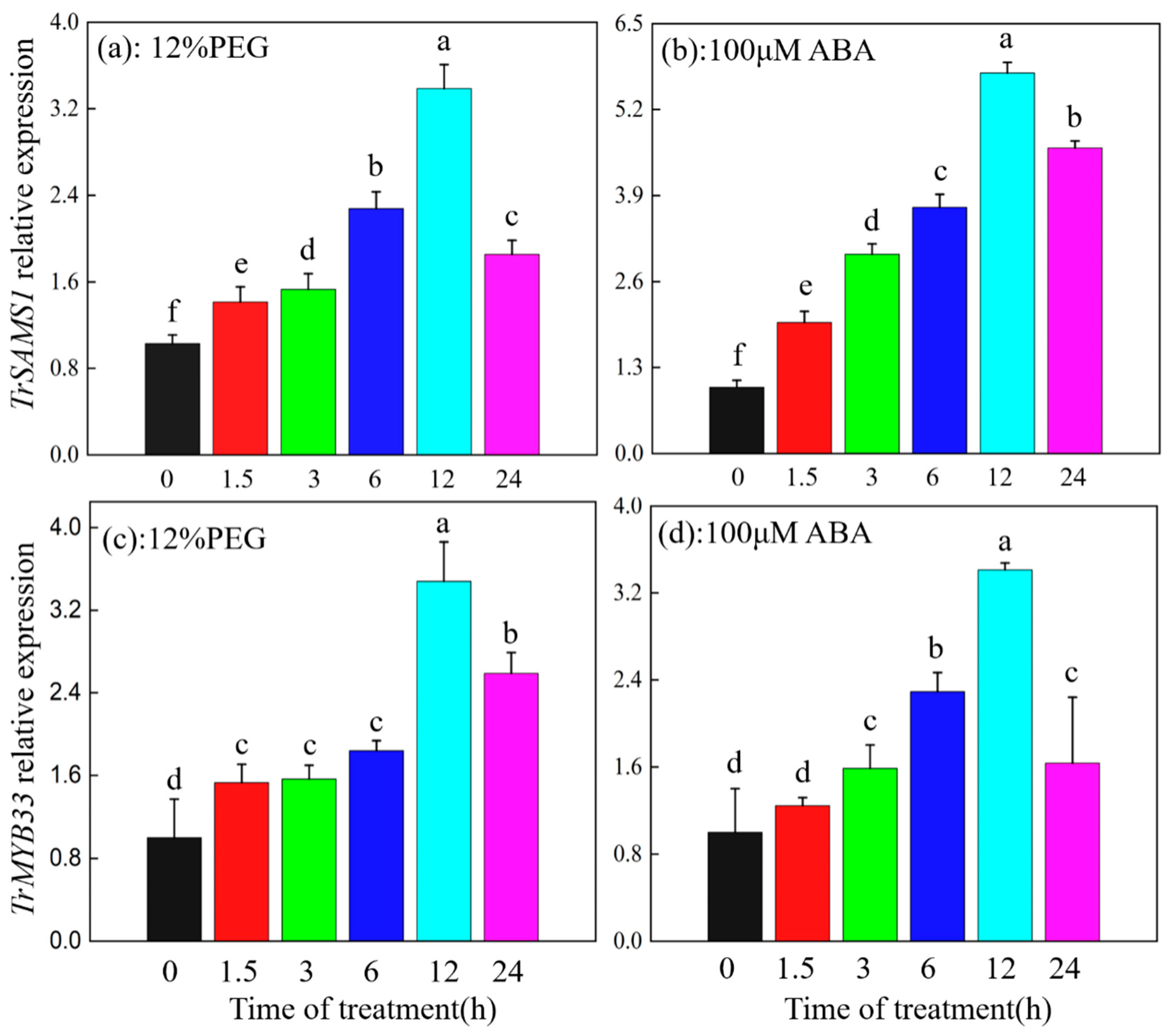
2.7. TrSAMS1 and TrMYB33 Are Induced by Drought and ABA in White Clover
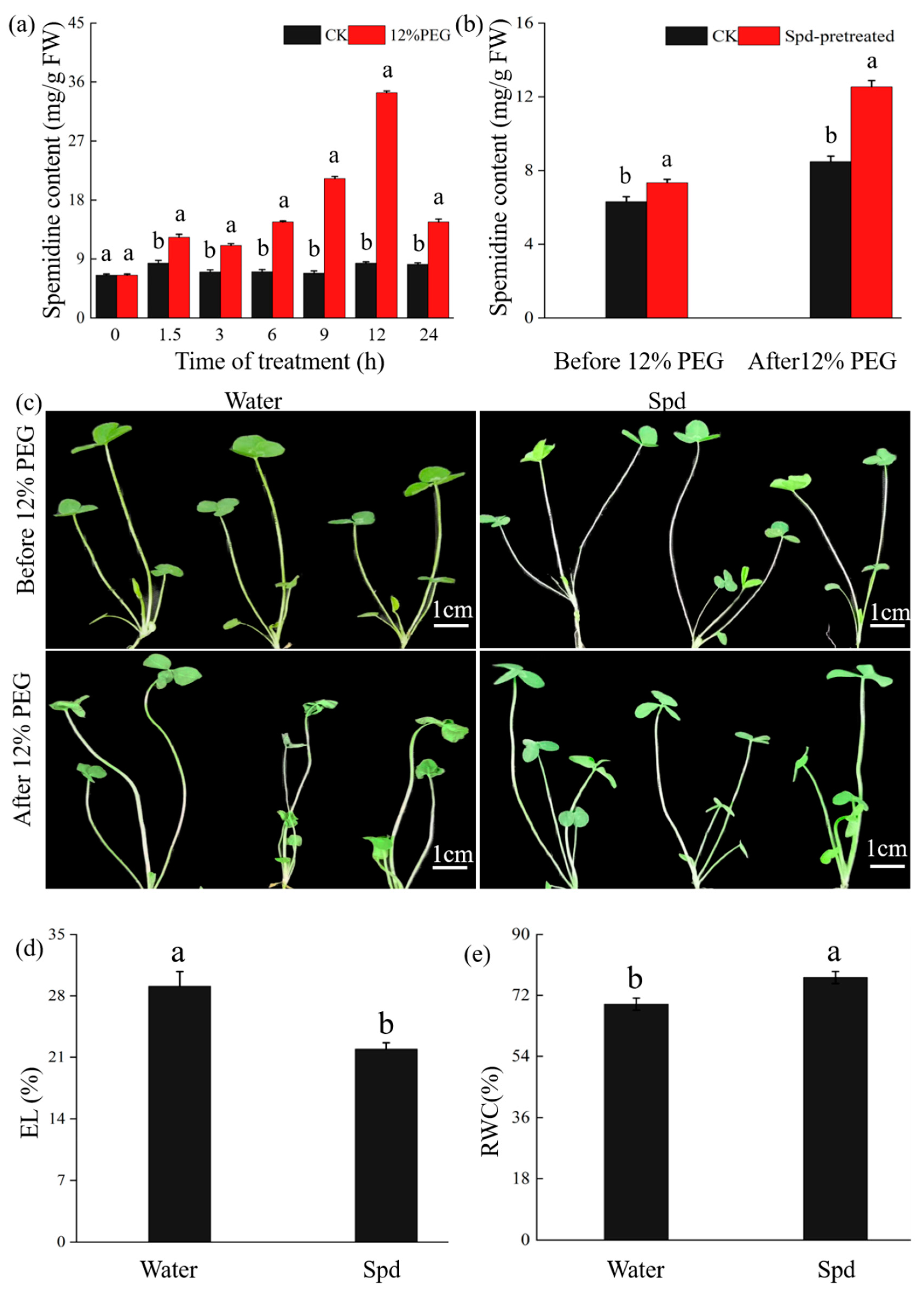
2.8. Drought Induces Spd Accumulation and Exogenous Spd Enhances Drought Tolerance of White Clover
2.9. ABA Signaling Is Involved in Spd Accumulation in White Clover under Drought Conditions
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. RNA Extraction and Quantitative Real-Time RT-PCR Analysis
3.3. Isolation and Analysis of TrSAMS1
3.4. Construction and Screening of a cDNA Library
3.5. Subcellular Localization of the TrMYB33 Protein
3.6. Plasmid Construction and Plant Transformation
3.7. Virus-Induced Gene Silencing (VIGS) of TrMYB33 or TrSAMS1 in White Clover
3.8. Yeast One-Hybrid (Y1H) Assay
3.9. Dual Luciferase Assay
3.10. Super-Shift Electrophoretic Mobility Shift Assay (EMSA)
3.11. Transcriptional Activation Assay for TrMYB33
3.12. Drought Tolerance Assays
3.13. Physiological Measurements
3.14. Quantification of SAM, Spd and ABA Content
3.15. Exogenous ABA and Spermidine Backfill Experiment
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Bashir, K.; Matsui, A.; Tanaka, M.; Seki, M. Transcriptomic Analysis of Soil-Grown Arabidopsis thaliana Roots and Shoots in Response to a Drought Stress. Front. Plant Sci. 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Rasheed, S.; Matsui, A.; Iida, K.; Tanaka, M.; Seki, M. Monitoring Transcriptomic Changes in Soil-Grown Roots and Shoots of Arabidopsis thaliana Subjected to a Progressive Drought Stress. Methods Mol. Biol. 2018, 1761, 223–230. [Google Scholar] [PubMed]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Mueller-Roeber, B.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xia, Z.; Cai, Z.; Li, L.; Cheng, Y.; Liu, J.; Nian, H. GmWRKY16 Enhances Drought and Salt Tolerance Through an ABA-Mediated Pathway in Arabidopsis thaliana. Front. Plant Sci. 2019, 9, 1979. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, B.; Wu, Q.; Min, Q.; Zeng, R.; Xie, Z.; Huang, J. OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet. 2021, 17, e1009699. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.H.; Fang, Y.L.; Zhang, J.X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xu, Y.; Ma, Q.; Xu, W.; Wang, T.; Xue, Y.; Chong, K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Tian, S.; Dong, H.; Guo, C. Pleiotropic effects of TaMYB3R1 on plant development and response to osmotic stress in transgenic Arabidopsis. Gene 2015, 558, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Graceson, A.; Hare, M.; Hall, N.; Monaghan, J. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Kwon, Y.; Kim, J.H.; Noh, H.; Hong, S.W.; Lee, H. A dual role for MYB60 in stomatal regulation and root growth of Arabidopsis thaliana under drought stress. Plant Mol. Biol. 2011, 77, 91–103. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, M.; Matus, J.T.; Francia, P.; Rusconi, F.; Cañón, P.; Medina, C.; Conti, L.; Cominelli, E.; Tonelli, C.; Arce-Johnson, P. The grapevine guard cell-related VvMYB60 transcription factor is involved in the regulation of stomatal activity and is differentially expressed in response to ABA and osmotic stress. BMC Plant Biol. 2011, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, Y.; Yang, L.; Song, J.; Yang, Z. AtMYB20 is negatively involved in plant adaptive response to drought stress. Plant Soil 2014, 376, 433–443. [Google Scholar] [CrossRef]
- Liang, Y.K.; Dubos, C.; Dodd, I.C.; Holroyd, G.H.; Hetherington, A.M.; Campbell, M.M. AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr. Biol. 2005, 15, 1201–1206. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Kim, Y.J.; Park, K.Y. Increasing polyamine contents enhanced the stress tolerance via reinforcement of antioxidative properties. Front. Plant Sci. 2019, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Campos, J.L.; Figueras Besford, R.T. Recent advances in the understanding of polyamine functions during plant development. Plant Growth Regul. 1993, 12, 331–340. [Google Scholar] [CrossRef]
- Lutts, S.; Hausman, J.F.; Quinet, M.; Lefèvre, I. Polyamines and their roles in the alleviation of ion toxicities in plants. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 315–353. [Google Scholar]
- Oh, M.W.; Komatsu, S. Characterization of proteins in soybean roots under flooding and drought stresses. J. Proteomics 2015, 114, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Oh, M.W.; Komatsu, S. Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses. Biol. Plant. 2016, 60, 269–278. [Google Scholar] [CrossRef]
- Sun, F.; Ma, J.; Wang, P.; Yang, Y. Genome-Wide Identification of the SAMS Gene Family in Upland Cotton (Gossypium hirsutum L.) and Expression Analysis in Drought Stress Treatments. Genes 2022, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; He, M.; Jahan, M.S.; Wu, J.; Gu, Q.; Shu, S.; Sun, J.; Guo, S. CsCDPK6, a CsSAMS1-Interacting Protein, Affects Polyamine/Ethylene Biosynthesis in Cucumber and Enhances Salt Tolerance by Overexpression in Tobacco. Int. J. Mol. Sci. 2021, 22, 11133. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Liang, L.; Zhao, Y.; Guo, Z.; Lu, S.A. Cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2018, 41, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Piano, E. Indirect selection for root development of white clover and implications for drought tolerance. J. Agron. Crop Sci. 2004, 190, 28–34. [Google Scholar] [CrossRef]
- Mercer, C.F.; Watson, R.N. Effects of nematicides and plant resistance on white clover performance and seasonal populations of nematodes parasitizing white clover in grazed pasture. J. Nematol. 2007, 39, 298–304. [Google Scholar] [PubMed]
- Li, Z.; Zhang, Y.; Xu, Y.; Zhang, X.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Physiological and iTRAQ-Based Proteomic Analyses Reveal the Function of Spermidine on Improving Drought Tolerance in White Clover. J. Proteome Res. 2016, 15, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Li, Z.; Liu, L.; Zhou, J.; Feng, G.; et al. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, J.; Zhang, Y.; Zeng, W.; Cheng, B.; Hassan, M.J.; Zhang, Y.; Pu, Q.; Peng, Y. Spermine Regulates Water Balance Associated with Ca2+-Dependent Aquaporin (TrTIP2-1, TrTIP2-2 and TrPIP2-7) Expression in Plants under Water Stress. Plant Cell Physiol. 2020, 61, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.M.; Watson, R.J. Nucleotide preferences in sequence-specific recognition of DNA by c-myb protein. Nucleic Acids Res. 1991, 19, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Weston, K. Extension of the DNA binding consensus of the chicken c-Myb and v-Myb proteins. Nucleic Acids Res. 1992, 20, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Conservation and diversification of three–repeat Myb transcription factors in plants. J. Plant Res. 2005, 118, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E.; Drummond, B.J.; Bowen, B. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 1994, 76, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Gubler, F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 2005, 17, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liu, Z.; Dai, X.; Xiang, F. Primary root growth in Arabidopsis thaliana is inhibited by the miR159 mediated repression of MYB33, MYB65 and MYB101. Plant Sci. 2017, 262, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Lee, D.J. Exogenously Applied Polyamines Increase Drought Tolerance of Rice by Improving Leaf Water Status, Photosynthesis and Membrane Properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Ghassemi, S.; Farhangi-Abriz, S.; Faegi-Analou, R.; Ghorbanpour, M.; Lajayer, B.A. Monitoring Cell Energy, Physiological Functions and Grain Yield in Field-Grown Mung Bean Exposed to Exogenously Applied Polyamines under Drought Stress. Soil Sci. Plant Nutr. 2018, 18, 1108–1125. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Zheng, B. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished Story of Polyamines: Role of Conjugation, Transport and Light-Related Regulation in The Polyamine Metabolism in Plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H. Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, A.; Aziz; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Li, Z.Y.; Chen, S.Y. Differential accumulation of the S-adenosylmethionine decarboxylase transcript in rice seedlings in response to salt and drought stress. Theor. Appl. Genet. 2000, 100, 782–788. [Google Scholar] [CrossRef]
- Shen, W.; Nada, K.; Tachibana, S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000, 124, 431–439. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Nada, K.; Kasukabe, Y.; Tachibana, S. Enhanced susceptibility of photosynthesis to low-temperature photoinhibition due to interruption of chill-induced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.). Plant Cell Physiol. 2002, 43, 196–206. [Google Scholar] [CrossRef]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Ihara, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Audran, C.; Liotenberg, S.; Gonneau, M.; North, H.; Marion-Poll, A. Localization and expression of zeaxanthin epoxidase mRNA in Arabidopsis in response to drought stress and during seed development. Funct. Plant Biol. 2001, 28, 1161–1173. [Google Scholar] [CrossRef]
- Koiwai, H.; Nakaminami, K.; Seo, M.; Mitsuhashi, W.; Toyomasu, T.; Koshiba, T. Tissue-Specific Localization of an Abscisic Acid Biosynthetic Enzyme, AAO3, in Arabidopsis. Plant Physiol. 2004, 134, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. Cell Mol. Biol. 2010, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Klueva, N.Y.; Zheng, H.G.; Nieto-Sotelo, J.; Nguyen, H.T. Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochim. Biophys. Acta Gene Struct. Expr. 2001, 1517, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y. Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. Cell Mol. Biol. 2010, 7, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Ishida, J.; Narusaka, M.; Fujita, M.; Nanjo, T.; Umezawa, T.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct. Integr. Genom. 2002, 2, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Djilianov, D.; Onckelen, H.V.; Atanassov, A. Abscisic acid changes in osmotic stressed leaves of alfalfa genotypes varying in drought tolerance. J. Plant Physiol. 1997, 150, 224–227. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiol. Plant. 2010, 126, 458–468. [Google Scholar] [CrossRef]
- Avice, J.C.; Dily, F.; Goulas, E. Vegetative storage proteins in overwintering storage organs of forage legumes: Roles and regulation. Can. J. Bot. 2003, 81, 1198–1212. [Google Scholar] [CrossRef]
- Pu, Q.; Li, Z.; Nie, G.; Zhou, J.; Liu, L.; Peng, Y. Selection and Validation of Reference Genes for Quantitative Real-Time PCR in White Clover (Trifolium repens L.) Involved in Five Abiotic Stresses. Plants 2020, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Huang, K.; Zhang, X.; Yu, X.; Huang, P.; An, C. The LSD1-Type Zinc Finger Motifs of Pisum sativa LSD1 Are a Novel Nuclear Localization Signal and Interact with Importin Alpha. PLoS ONE 2011, 6, e22131. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.P.; Seth, S.; Erskine, W.; Kaur, P. An Improved Protocol for Agrobacterium-Mediated Transformation in Subterranean Clover (Trifolium subterraneum L.). Int. J. Mol. Sci. 2021, 22, 4181. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Dahro, B.; Wang, F.; Peng, T.; Liu, J.H. PtrA/NINV, an alkaline/neutral invertase gene of Poncirus trifoliata, confers enhanced tolerance to multiple abiotic stresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 2016, 29, 76. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxyl ammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qin, X.; He, Z.; Zhang, Y.; Li, Z.; Nie, G.; Zhao, J.; Feng, G.; Peng, Y. The White Clover TrMYB33-TrSAMS1 Module Contributes to Drought Tolerance by Modulation of Spermidine Biosynthesis via an ABA-Dependent Pathway. Int. J. Mol. Sci. 2024, 25, 6974. https://doi.org/10.3390/ijms25136974
Zhang Y, Qin X, He Z, Zhang Y, Li Z, Nie G, Zhao J, Feng G, Peng Y. The White Clover TrMYB33-TrSAMS1 Module Contributes to Drought Tolerance by Modulation of Spermidine Biosynthesis via an ABA-Dependent Pathway. International Journal of Molecular Sciences. 2024; 25(13):6974. https://doi.org/10.3390/ijms25136974
Chicago/Turabian StyleZhang, Youzhi, Xiaofang Qin, Zhirui He, Yan Zhang, Zhou Li, Gang Nie, Junming Zhao, Guangyan Feng, and Yan Peng. 2024. "The White Clover TrMYB33-TrSAMS1 Module Contributes to Drought Tolerance by Modulation of Spermidine Biosynthesis via an ABA-Dependent Pathway" International Journal of Molecular Sciences 25, no. 13: 6974. https://doi.org/10.3390/ijms25136974




