Multi-Omics Approaches in Oil Palm Research: A Comprehensive Review of Metabolomics, Proteomics, and Transcriptomics Based on Low-Temperature Stress
Abstract
1. Introduction
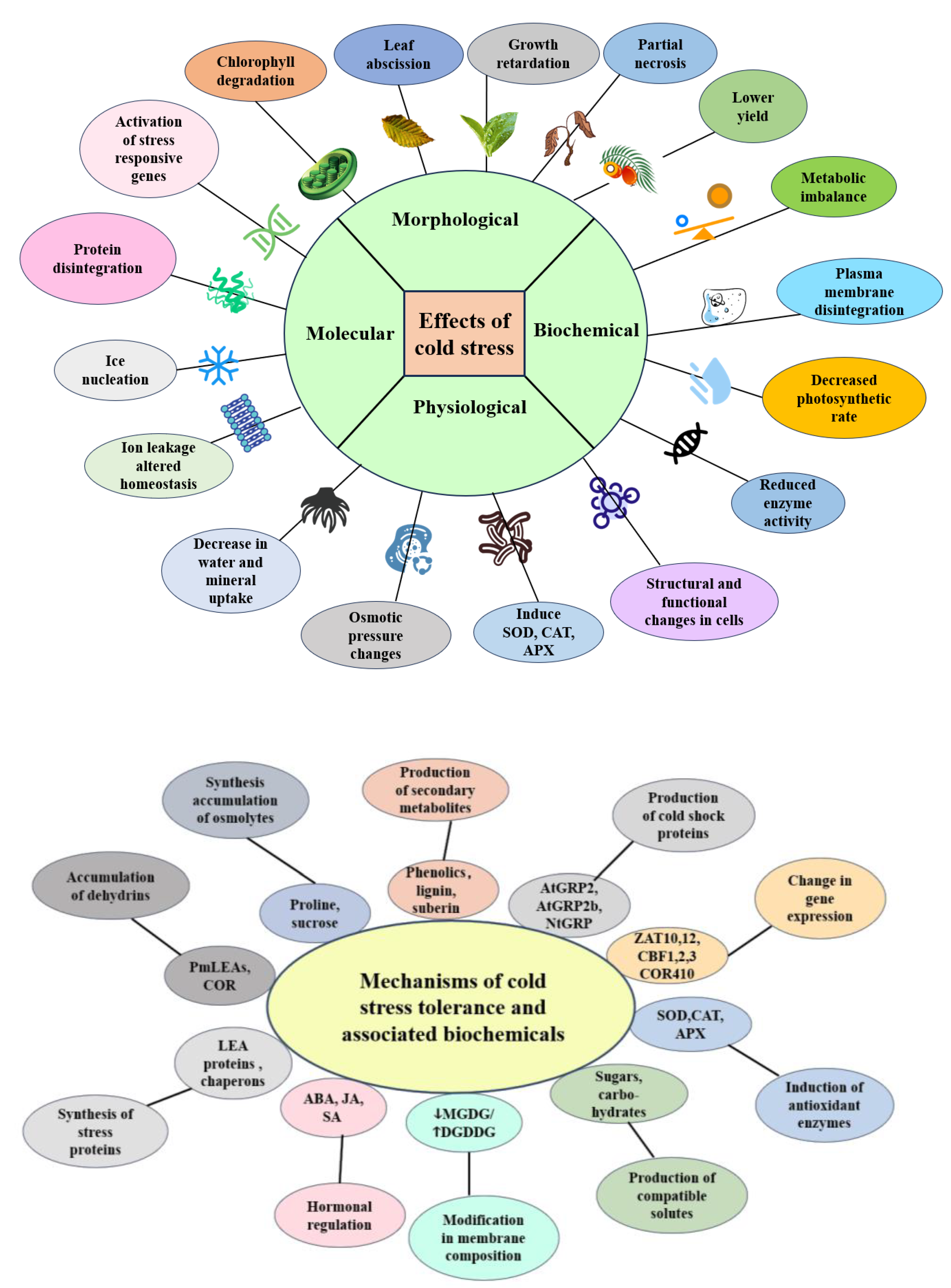
2. Cold Stress Tolerance in Oil Palm: From Physiological Responses to Molecular Mechanisms
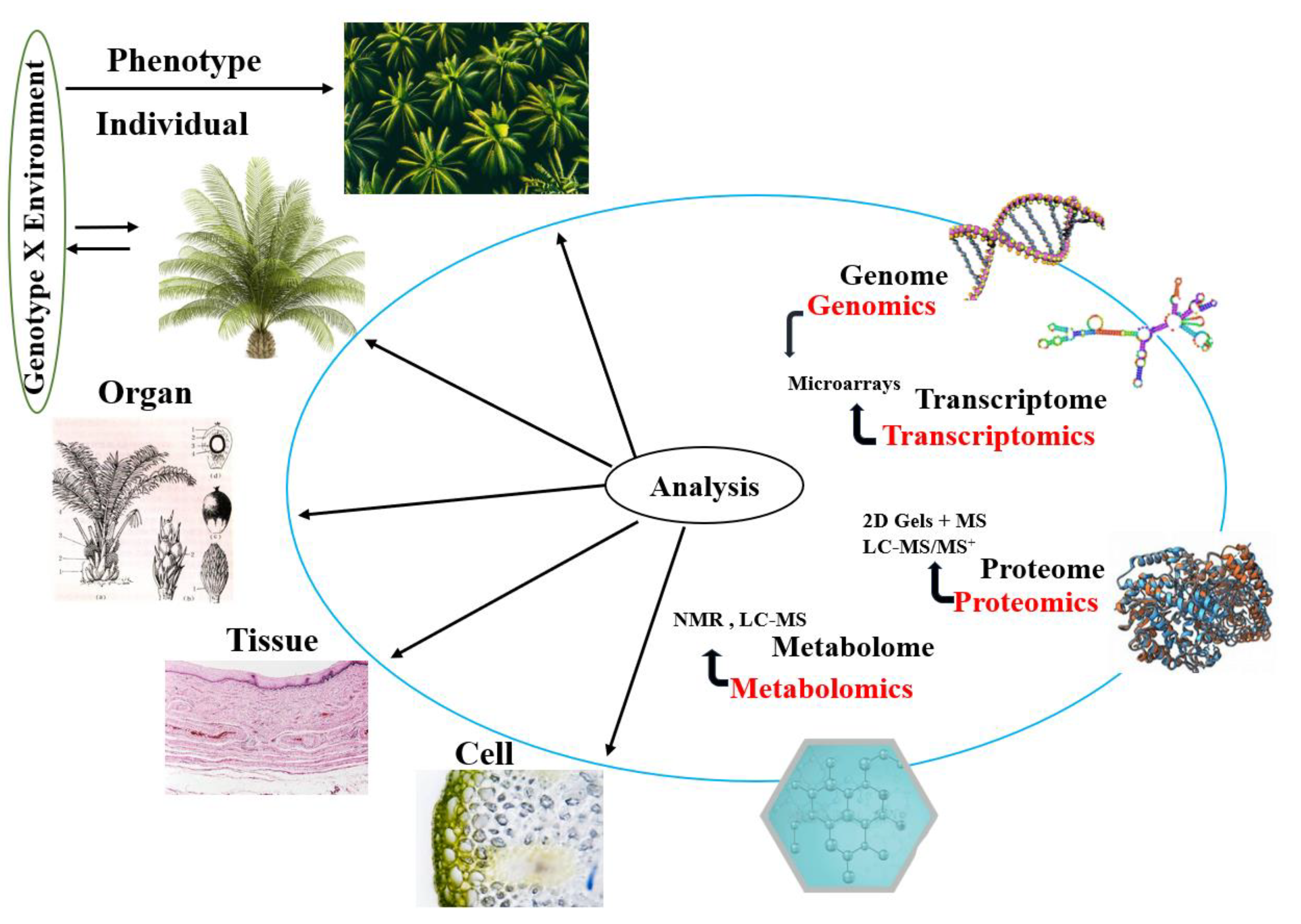
3. Multiomic and Genome Sequencing Approaches in Oil Palm
3.1. Genomics
3.2. Oil Palm Genome Sequencing
3.3. Importance Development of Oil Palm Genome Sequencing
3.4. Challenges in Oil Palm Genome Sequencing
3.5. Progress in Oil Palm Genome Sequencing
3.6. Applications of Oil Palm Genome Sequencing
- (a)
- Identify genes that are intricately linked to critical agronomic traits, including yield, disease resistance, and stress tolerance.
- (b)
- Develop molecular markers, which serve as powerful tools for marker-assisted selection in breeding programs, enhancing the efficiency and precision of cultivar development.
- (c)
- Explore the genetic diversity and population structure of oil palm, providing insights into the species’ natural variability and breeding potential.
- (d)
- Investigate the evolutionary history and domestication process of oil palm, furthering our understanding of its adaptation and improvement over time.
- (e)
- Develop genome-editing tools that enable targeted improvements in oil palm cultivars, potentially enhancing their productivity, resilience, and adaptability.
4. QTLs Contributing to Cold Stress Tolerance
5. Marker-Trait Association (MTA) for Cold Tolerance
6. Genomics in Oil Palm Response to Abiotic Stress
7. Applications of Transcriptomics for Plant Sciences
8. Application of Proteomics for Plant Sciences
9. Application of Metabolomics for Plant Science
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The Chemistry of Stress: Understanding the ‘Cry for Help’ of Plant Roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Audil, G.; Ajaz Ahmad, L.; Noor Ul Islam, W. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; de Oliveira, A.B., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. Ch. 1. [Google Scholar] [CrossRef]
- Méndez Espinoza, C.; Vallejo Reyna, M.Á. Mecanismos de respuesta al estrés abiótico: Hacia una perspectiva de las especies forestales. Rev. Mex. Cienc. For. 2019, 10, 33–64. [Google Scholar] [CrossRef]
- Murphy, D.J. Future prospects for oil palm in the 21st century: Biological and related challenges. Eur. J. Lipid Sci. Technol. 2007, 109, 296–306. [Google Scholar] [CrossRef]
- Watson-Hernández, F.; Serrano-Núñez, V.; Gómez-Calderón, N.; Pereira da Silva, R. Quantification and Evaluation of Water Requirements of Oil Palm Cultivation for Different Climate Change Scenarios in the Central Pacific of Costa Rica Using APSIM. Agronomy 2023, 13, 19. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H.M. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Lei, X.; Xiao, Y.; Xia, W.; Mason, A.S.; Yang, Y.; Ma, Z.; Peng, M. RNA-Seq Analysis of Oil Palm under Cold Stress Reveals a Different C-Repeat Binding Factor (CBF) Mediated Gene Expression Pattern in Elaeis guineensis Compared to Other Species. PLoS ONE 2014, 9, e114482. [Google Scholar] [CrossRef] [PubMed]
- Mirza, B.; Wang, W.; Wang, J.; Choi, H.; Chung, N.C.; Ping, P. Machine Learning and Integrative Analysis of Biomedical Big Data. Genes 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Tafti, A.; Dahdivan, N.; Ardakani, S.A. Physicochemical properties and applications of date seed and its oil. Int. Food Res. J. 2017, 24, 1399–1406. [Google Scholar]
- Wei, Q.; Shi, P.; Khan, F.S.; Htwe, Y.M.; Zhang, D.; Li, Z.; Wei, X.; Yu, Q.; Zhou, K.; Wang, Y. Cryopreservation and Cryotolerance Mechanism in Zygotic Embryo and Embryogenic Callus of Oil Palm. Forests 2023, 14, 966. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Iqbal, A.; Qadri, R.; Shi, P.; Wang, Y.; Wu, Y.; Fan, H.; Wu, G. Correlation analysis of cold-related gene expression with physiological and biochemical indicators under cold stress in oil palm. PLoS ONE 2019, 14, e0225768. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, N.; Jadhav, H.; Ahlawat, A.; Kalaivendan, R.G.T.; Annapure, U. Effect of cold plasma processing on physicochemical characteristics and thermal properties of palm oil. Future Foods 2023, 7, 100231. [Google Scholar] [CrossRef]
- Goswami, A.K.; Maurya, N.K.; Goswami, S.; Bardhan, K.; Singh, S.K.; Prakash, J.; Pradhan, S.; Kumar, A.; Chinnusamy, V.; Kumar, P.; et al. Physio-biochemical and molecular stress regulators and their crosstalk for low-temperature stress responses in fruit crops: A review. Front. Plant Sci. 2022, 13, 1022167. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Meng, X.; Song, X.; Zhang, D.; Kou, L.; Zhang, J.; Jing, Y.; Liu, G.; Liu, H.; Huang, X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov. 2022, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X.; Li, H.; Dong, J.; Li, J.; Gong, Z.; et al. Cold-Induced CBF-PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the phyB Thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Batley, J.; Edwards, D. Use of multiple ‘omics techniques to accelerate the breeding of abiotic stress tolerant crops. Curr. Plant Biol. 2022, 32, 100262. [Google Scholar] [CrossRef]
- Jeon, D.; Kang, Y.; Lee, S.; Choi, S.; Sung, Y.; Lee, T.-H.; Kim, C. Digitalizing breeding in plants: A new trend of next-generation breeding based on genomic prediction. Front. Plant Sci. 2023, 14, 1092584. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Minamikawa, M.F.; Kajiya-Kanegae, H.; Ishimori, M.; Hayashi, T. Genomics-assisted breeding in fruit trees. Breed. Sci. 2016, 66, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Joiret, M.; Mahachie John, J.M.; Gusareva, E.S.; Van Steen, K. Confounding of linkage disequilibrium patterns in large scale DNA based gene-gene interaction studies. BioData Min. 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Alamin, M.; Sultana, M.H.; Lou, X.; Jin, W.; Xu, H. Dissecting Complex Traits Using Omics Data: A Review on the Linear Mixed Models and Their Application in GWAS. Plants 2022, 11, 3277. [Google Scholar] [CrossRef]
- Lovina, I.U.; Willie Peggy, O.; Chiebuka, U. Single Nucleotide Polymorphisms: A Modern Tool to Screen Plants for Desirable Traits. In Plant Breeding; Ibrokhim, Y.A., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 12. [Google Scholar] [CrossRef]
- He, J.; Zhao, X.; Laroche, A.; Lu, Z.X.; Liu, H.; Li, Z. Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front. Plant Sci. 2014, 5, 484. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Wang, L.; Zhang, Y.J.; Lee, M.; Rahmadsyah, R.; Alfiko, Y.; Ye, B.Q.; Purwantomo, S.; Suwanto, A.; Chua, N.H.; et al. Developing genome-wide SNPs and constructing an ultrahigh-density linkage map in oil palm. Sci. Rep. 2018, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Hayashi, T.; Terakami, S.; Takada, N.; Sawamura, Y.; Yamamoto, T. Potential assessment of genome-wide association study and genomic selection in Japanese pear Pyrus pyrifolia. Breed. Sci. 2013, 63, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Wang, L.; Zhu, G.; Fang, W.; Chen, C.; Luo, J. Genetic diversity, linkage disequilibrium, and association mapping analyses of peach (Prunus persica) landraces in China. Tree Genet. Genomes 2012, 8, 975–990. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Guo-Liang, J. Molecular Markers and Marker-Assisted Breeding in Plants. In Plant Breeding from Laboratories to Fields; Sven Bode, A., Ed.; IntechOpen: Rijeka, Croatia, 2013; p. Ch. 3. [Google Scholar] [CrossRef]
- Singh, R.; Ong-Abdullah, M.; Low, E.T.; Manaf, M.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.; Ooi, S.E.; Chan, K.L.; Halim, M.A.; et al. Oil palm genome sequence reveals divergence of interfertile species in Old and New worlds. Nature 2013, 500, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lee, M.; Bai, B.; Sun, Y.; Qu, J.; Rahmadsyah; Alfiko, Y.; Lim, C.H.; Suwanto, A.; Sugiharti, M.; et al. Draft genome sequence of an elite Dura palm and whole-genome patterns of DNA variation in oil palm. DNA Res. 2016, 23, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Low, E.T.; Ooi, L.C.; Ong-Abdullah, M.; Ting, N.C.; Nagappan, J.; Nookiah, R.; Amiruddin, M.D.; Rosli, R.; Manaf, M.A.; et al. The oil palm SHELL gene controls oil yield and encodes a homologue of SEEDSTICK. Nature 2013, 500, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Low, E.T.; Ooi, L.C.; Ong-Abdullah, M.; Nookiah, R.; Ting, N.C.; Marjuni, M.; Chan, P.L.; Ithnin, M.; Manaf, M.A.; et al. The oil palm VIRESCENS gene controls fruit colour and encodes a R2R3-MYB. Nat. Commun. 2014, 5, 4106. [Google Scholar] [CrossRef] [PubMed]
- Ong-Abdullah, M.; Ordway, J.M.; Jiang, N.; Ooi, S.E.; Kok, S.Y.; Sarpan, N.; Azimi, N.; Hashim, A.T.; Ishak, Z.; Rosli, S.K.; et al. Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 2015, 525, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, M.; Yi Wan, Z.; Bai, B.; Ye, B.; Alfiko, Y.; Rahmadsyah, R.; Purwantomo, S.; Song, Z.; Suwanto, A.; et al. A Chromosome-level Reference Genome of African Oil Palm Provides Insights into Its Divergence and Stress Adaptation. Genom. Proteom. Bioinform. 2023, 21, 440–454. [Google Scholar] [CrossRef]
- Suraninpong, P.; Nuanlaong, S. Comparative transcriptome profiling and molecular marker development for oil palm fruit color. Sci. Rep. 2022, 12, 15507. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.K.; Mathur, R.K.; Anitha, P.; Ravichandran, G.; Bhagya, H.P. Phenomics, genomics of oil palm (Elaeis guineensis Jacq.): Way forward for making sustainable and high yielding quality oil palm. Physiol. Mol. Biol. Plants 2021, 27, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Ramli, U.; Othman, A.; Tahir, N.; Lau, B.; Shahwan, S.; Hassan, H.; Zain, N.; Dzulkafli, S.; Rozali, N.; Ishak, N.; et al. Omics—A Potential Tool for Oil Palm Improvement and Productivity. In The Oil Palm Genome; Springer: Cham, Switzerland, 2020; pp. 141–157. [Google Scholar] [CrossRef]
- Kumar, P.N.; Babu, B.K.; Mathur, R.K.; Ramajayam, D. Chapter 9—Genetic Engineering of Oil Palm. In Genetic Engineering of Horticultural Crops; Rout, G.R., Peter, K.V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 169–191. [Google Scholar] [CrossRef]
- Florence, J.; Cros, D.; Cochard, B. Agrigenomics in the breeder’s toolbox: Latest advances towards an optimal implementation of genomic selection in oil palm. In Proceedings of the International Seminar on 100 Years of Technological Advancement in Oil Palm Breeding and Seed Production (ISOPB 2017), Kuala Lumpur, Malaysia, 13 November 2017; Malaysian palm oil board-International Society for Oil Palm Breeders: Kuala Lumpur, Malaysia, 2017. 21p. [Google Scholar]
- Rajesh, Y.; Cao, H.; Longfei, J.; Mengdi, Y.; Zhou, L. CRISPR/Cas Mediated Base Editing: A Practical Approach for Genome Editing in Oil Palm. 3Biotech 2020, 7, 306. [Google Scholar] [CrossRef]
- Ithnin, M.; Kushairi, A. The Oil Palm Genome; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Nugroho, Y.A.; Tanjung, Z.A.; Yono, D.; Mulyana, A.S.; Simbolon, H.M.; Ardi, A.S.; Yong, Y.Y.; Utomo, C.; Liwang, T. Genome-wide SNP-discovery and analysis of genetic diversity in oil palm using double digest restriction site associated DNA sequencing. IOP Conf. Ser. Earth Environ. Sci. 2019, 293, 012041. [Google Scholar] [CrossRef]
- Huang, X.; Hilscher, J.; Stoger, E.; Christou, P.; Zhu, C. Modification of cereal plant architecture by genome editing to improve yields. Plant Cell Rep. 2021, 40, 953–978. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.; Goher, F.; Zhang, D.; Shi, P.; Li, Z.; Htwe, Y.M.; Wang, Y. Is CRISPR/Cas9 a way forward to fast-track genetic improvement in commercial palms? Prospects and limits. Front. Plant Sci. 2022, 13, 1042828. [Google Scholar] [CrossRef]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef] [PubMed]
- Korf, I. Gene finding in novel genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-L.; Rosli, R.; Tatarinova, T.V.; Hogan, M.; Firdaus-Raih, M.; Low, E.-T.L. Seqping: Gene prediction pipeline for plant genomes using self-training gene models and transcriptomic data. BMC Bioinform. 2017, 18, 1426. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-L.; Tatarinova, T.V.; Rosli, R.; Amiruddin, N.; Azizi, N.; Halim, M.A.A.; Sanusi, N.S.N.M.; Jayanthi, N.; Ponomarenko, P.; Triska, M.; et al. Evidence-based gene models for structural and functional annotations of the oil palm genome. Biol. Direct 2017, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Schöffmann, O.; Morgenstern, B.; Waack, S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinform. 2006, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Yandell, M. MAKER2: An annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 2011, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, M.; Wan, Z.; Bai, B.; Ye, B.; Alfiko, Y.; Ramadsyah, R.; Purwantomo, S.; Song, Z.; Suwanto, A.; et al. Chromosome-level Reference Genome Provides Insights into Divergence and Stress Adaptation of the African Oil Palm. bioRxiv 2022. [CrossRef] [PubMed]
- Jamaludin, N.; Mat Yunus, A.M.; Fizree, P.; Bahariah, B.; Shaharuddin, N.; Ho, C.-L.; Rasid, O.; Ghulam Kadir, A.P. DNA-free CRISPR/Cas9 genome editing system for oil palm protoplasts using multiple ribonucleoproteins (RNPs) complexes. Ind. Crops Prod. 2024, 208, 117795. [Google Scholar] [CrossRef]
- Lineesha, K.; Antony, G. Genome Editing: Prospects and Challenges. In The Coconut Genome; Springer: Cham, Switzerland, 2021; pp. 191–203. [Google Scholar] [CrossRef]
- Mahmoud, A. Genome Sequence of Oil Palm, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2021; p. XXIV, 439. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Xia, W.; Mason, A.S.; Yang, Y.; Ma, Z.; Peng, M. Exploiting transcriptome data for the development and characterization of gene-based SSR markers related to cold tolerance in oil palm (Elaeis guineensis). BMC Plant Biol. 2014, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- John Martin, J.J.; Rajesh, Y. Oil Palm Breeding in the Modern Era: Challenges and Opportunities. Plants 2022, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-X.; Sun, C.-X.; Shao, H.; Lei, X.-T. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr. J. Biotechnol. 2011, 10, 2630–2637. [Google Scholar] [CrossRef]
- Yeap, W.C.; Ooi, T.E.; Namasivayam, P.; Kulaveerasingam, H.; Ho, C.L. EgRBP42 encoding an hnRNP-like RNA-binding protein from Elaeis guineensis Jacq. is responsive to abiotic stresses. Plant Cell Rep. 2012, 31, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Bajay, S.K.; Ferreira, R.C.U.; Pimenta, R.J.G.; Mancini, M.; Aono, A.H.; Niederauer, G.F.; Horta, M.A.C.; de Souza, A.P. Chapter 9—Transcriptomics in agricultural sciences: Capturing changes in gene regulation during abiotic or biotic stress. In Transcriptome Profiling; Ajmal Ali, M., Lee, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 257–283. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, D.; Mathur, S.; Singh, A.; Ranjan, R. Upcoming progress of transcriptomics studies on plants: An overview. Front. Plant Sci. 2022, 13, 1030890. [Google Scholar] [CrossRef] [PubMed]
- Soniya, E.V.; Srinivasan, A.; Menon, A.; Kattupalli, D. Chapter 10—Transcriptomics in response of biotic stress in plants. In Transcriptome Profiling; Ajmal Ali, M., Lee, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 285–303. [Google Scholar] [CrossRef]
- Ali, M.; Yang, T.; He, H.; Zhang, Y. Plant biotechnology research with single-cell transcriptome: Recent advancements and prospects. Plant Cell Rep. 2024, 43, 75. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Tian, X.; Xu, J. Single-Cell Transcriptome Analysis in Plants: Advances and Challenges. Mol. Plant 2021, 14, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Wang, L.; Wang, H.R. Identification and characterization of a plastidial ω-3 fatty acid desaturase EgFAD8 from oil palm (Elaeis guineensis Jacq.) and its promoter response to light and low temperature. PLoS ONE 2018, 13, e0196693. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.K.; Zhao, C. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; John Martin, J.J.; Zhang, H.; Zhang, R.; Cao, H. Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm. Plants 2021, 10, 2622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yarra, R.; Jin, L.; Cao, H. Genome-wide identification and expression analysis of MYB gene family in oil palm (Elaeis guineensis Jacq.) under abiotic stress conditions. Environ. Exp. Bot. 2020, 180, 10245. [Google Scholar] [CrossRef]
- Zhou, L.X.; Cao, H.X. Analysis of the expression characteristics of oil palm WRKY transcription factor genes under low temperature stress. South. Agric. J. 2018, 49, 1490–1497. [Google Scholar]
- Zhou, L.; Yarra, R. Genome-Wide Identification and Characterization of AP2/ERF Transcription Factor Family Genes in Oil Palm under Abiotic Stress Conditions. Int. J. Mol. Sci. 2021, 22, 2821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yarra, R. Genome-wide identification and expression analysis of bZIP transcription factors in oil palm (Elaeis guineensis Jacq.) under abiotic stress. Protoplasma 2022, 259, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yarra, R.; Zhou, L.; Cao, H. The auxin response factor (ARF) gene family in Oil palm (Elaeis guineensis Jacq.): Genome-wide identification and their expression profiling under abiotic stresses. Protoplasma 2022, 259, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, K.; Kuczyńska, A.; Krajewski, P.; Kempa, M.; Witaszak, N. Global Proteome Profiling Revealed the Adaptive Reprogramming of Barley Flag Leaf to Drought and Elevated Temperature. Cells 2023, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Kausar, R.; Wang, X.; Komatsu, S. Crop Proteomics under Abiotic Stress: From Data to Insights. Plants 2022, 11, 2877. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Gillet, L.; Rosenberger, G.; Amon, S.; Collins, B.C.; Aebersold, R. Data-independent acquisition-based SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Mustafa, G. Plant Proteomics. In Plant Omics: Advances in Big Data Biology; CABI: Wallingford, UK, 2022; pp. 30–49. [Google Scholar] [CrossRef]
- Kumar, J.; Jain, K.; Ranjan, R.; Mohanty, A.; Kumar, A.; Ranjan, T. Proteomic Approaches in Physiological Studies of Plant Abiotic Stress. In Omics Analysis of Plants under Abiotic Stress; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 19–53. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Yang, J.; Zhou, W.; Dai, S. Exploring the diversity of plant proteome. J. Integr. Plant Biol. 2021, 63, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Saand, M.A.; Li, J.; Wu, Y.; Zhou, L.; Cao, H.; Yang, Y. Integrative Omics Analysis of Three Oil Palm Varieties Reveals (Tanzania × Ekona) TE as a Cold-Resistant Variety in Response to Low-Temperature Stress. Int. J. Mol. Sci. 2022, 23, 14926. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.M.; Choudhary, S.; Ramchiary, N. Proteomic Responses to Cold Stress. In Cold Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Herath, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 111–125. [Google Scholar] [CrossRef]
- Gao, F.; Ma, P.; Wu, Y.; Zhou, Y.; Zhang, G. Quantitative Proteomic Analysis of the Response to Cold Stress in Jojoba, a Tropical Woody Crop. Int. J. Mol. Sci. 2019, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Khandwal, D.; Choudhary, B.; Ardeshana, D.; Jha, R.K.; Tanna, B.; Yadav, S.; Mishra, A.; Varshney, R.K.; Siddique, K.H.M. Differential Physio-Biochemical and Metabolic Responses of Peanut (Arachis hypogaea L.) under Multiple Abiotic Stress Conditions. Int. J. Mol. Sci. 2022, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.C.R.; Vieira, L.R.; de Aquino Ribeiro, J.A.; de Sousa, C.A.F.; Júnior, M.T.S.; Abdelnur, P.V. Metabolic effect of drought stress on the leaves of young oil palm (Elaeis guineensis) plants using UHPLC-MS and multivariate analysis. Sci. Rep. 2021, 11, 18271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Li, S.; Zhang, S.; Yang, X. Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat. Crop J. 2019, 7, 857–866. [Google Scholar] [CrossRef]
- Li, F.; Lu, X.; Duan, P.; Liang, Y.; Cui, J. Integrating transcriptome and metabolome analyses of the response to cold stress in pumpkin (Cucurbita maxima). PLoS ONE 2021, 16, e0249108. [Google Scholar] [CrossRef] [PubMed]

| Gene Family | Gene Name | Temperature | Descriptions | Reference |
|---|---|---|---|---|
| CBF | CBF1, CBF2 | 4 °C | At 4 °C, CBF1 (R2 = 0.68), CBF2 (R2 = 0.680.91) gene expression was significantly linked to sucrose. | [11] |
| CBF1, CBF3 | 12 °C | Expression of CBFs were clear link with ICE1, SIZ1, ZAT10, COR413, and ZAT12 expression. | ||
| MYB | EgMYB111, EgMYB157 | 8 °C | Overexpression of EgMYB111 and/or EgMYB157 significantly increases abiotic tolerance in transgenic Arabidopsis plants. | [68] |
| EgMYB38, EgMYB43, EgMYB57, EgMYB76, EgMYB82, EgMYB91, EgMYB104, EgMYB106, EgMYB111, EgMYB127, EgMYB133, EgMYB146, EgMYB151, EgMYB155 | A total of 14 MYB genes were significantly up-regulated under all abiotic stress conditions (cold, salinity, and drought); EgMYB146, EgMYB151, and EgMYB155 were all significantly up-regulated | [69] | ||
| WRKY | EgWRKY03, EgWRKY06, EgWRKY07, EgWRKY11, EgWRKY16, EgWRKY25, EgWRKY26, EgWRKY28, EgWRKY29, EgWRKY35, EgWRKY52, EgWRKY59, EgWRKY61, EgWRKY72, EgWRKY76, EgWRKY80, EgWRKY88 | 8 °C | 17 EgWRKYs with greater than two-fold change in expression under cold stress | [70] |
| AP2/ERF/RAV | EgAP2.15, EgAP2.34, EgERF23, EgERF104, EgERF130 | 8 °C | Increase expression of AP2/ERF genes in response to cold exposure. | [71] |
| bZIP | EgbZIP1, EgbZIP4, EgbZIP27, EgbZIP44, EgbZIP52, EgbZIP68, EgbZIP77, EgbZIP85, EgbZIP86, EgbZIP89, EgbZIP95 | NA | The bZIP genes were up-regulated in response to cold, salt, or drought stress, suggesting that EgbZIP plays a significant role in stress response. | [72] |
| ARF | EgARF4, EgARF5, EgARF6, EgARF9, EgARF10, EgARF12, EgARF13, EgARF15, EgARF21, EgARF22 (up-regulated) | 8 °C | Different types of abiotic stresses can induce the expression of EgARFs (cold, drought, and salt). The ARF gene functional investigations in oil palm serve as a genetic resource platform for oil palm abiotic stress resistance breeding. | [73] |
| EgARF1, EgARF3, EgARF8, EgARF14, EgARF17, EgARF18, EgARF19, and EgARF20 (down-regulated) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John Martin, J.J.; Song, Y.; Hou, M.; Zhou, L.; Liu, X.; Li, X.; Fu, D.; Li, Q.; Cao, H.; Li, R. Multi-Omics Approaches in Oil Palm Research: A Comprehensive Review of Metabolomics, Proteomics, and Transcriptomics Based on Low-Temperature Stress. Int. J. Mol. Sci. 2024, 25, 7695. https://doi.org/10.3390/ijms25147695
John Martin JJ, Song Y, Hou M, Zhou L, Liu X, Li X, Fu D, Li Q, Cao H, Li R. Multi-Omics Approaches in Oil Palm Research: A Comprehensive Review of Metabolomics, Proteomics, and Transcriptomics Based on Low-Temperature Stress. International Journal of Molecular Sciences. 2024; 25(14):7695. https://doi.org/10.3390/ijms25147695
Chicago/Turabian StyleJohn Martin, Jerome Jeyakumar, Yuqiao Song, Mingming Hou, Lixia Zhou, Xiaoyu Liu, Xinyu Li, Dengqiang Fu, Qihong Li, Hongxing Cao, and Rui Li. 2024. "Multi-Omics Approaches in Oil Palm Research: A Comprehensive Review of Metabolomics, Proteomics, and Transcriptomics Based on Low-Temperature Stress" International Journal of Molecular Sciences 25, no. 14: 7695. https://doi.org/10.3390/ijms25147695
APA StyleJohn Martin, J. J., Song, Y., Hou, M., Zhou, L., Liu, X., Li, X., Fu, D., Li, Q., Cao, H., & Li, R. (2024). Multi-Omics Approaches in Oil Palm Research: A Comprehensive Review of Metabolomics, Proteomics, and Transcriptomics Based on Low-Temperature Stress. International Journal of Molecular Sciences, 25(14), 7695. https://doi.org/10.3390/ijms25147695





