GmANKTM21 Positively Regulates Drought Tolerance and Enhanced Stomatal Response through the MAPK Signaling Pathway in Soybean
Abstract
:1. Introduction
2. Results
2.1. Subcellular Localization
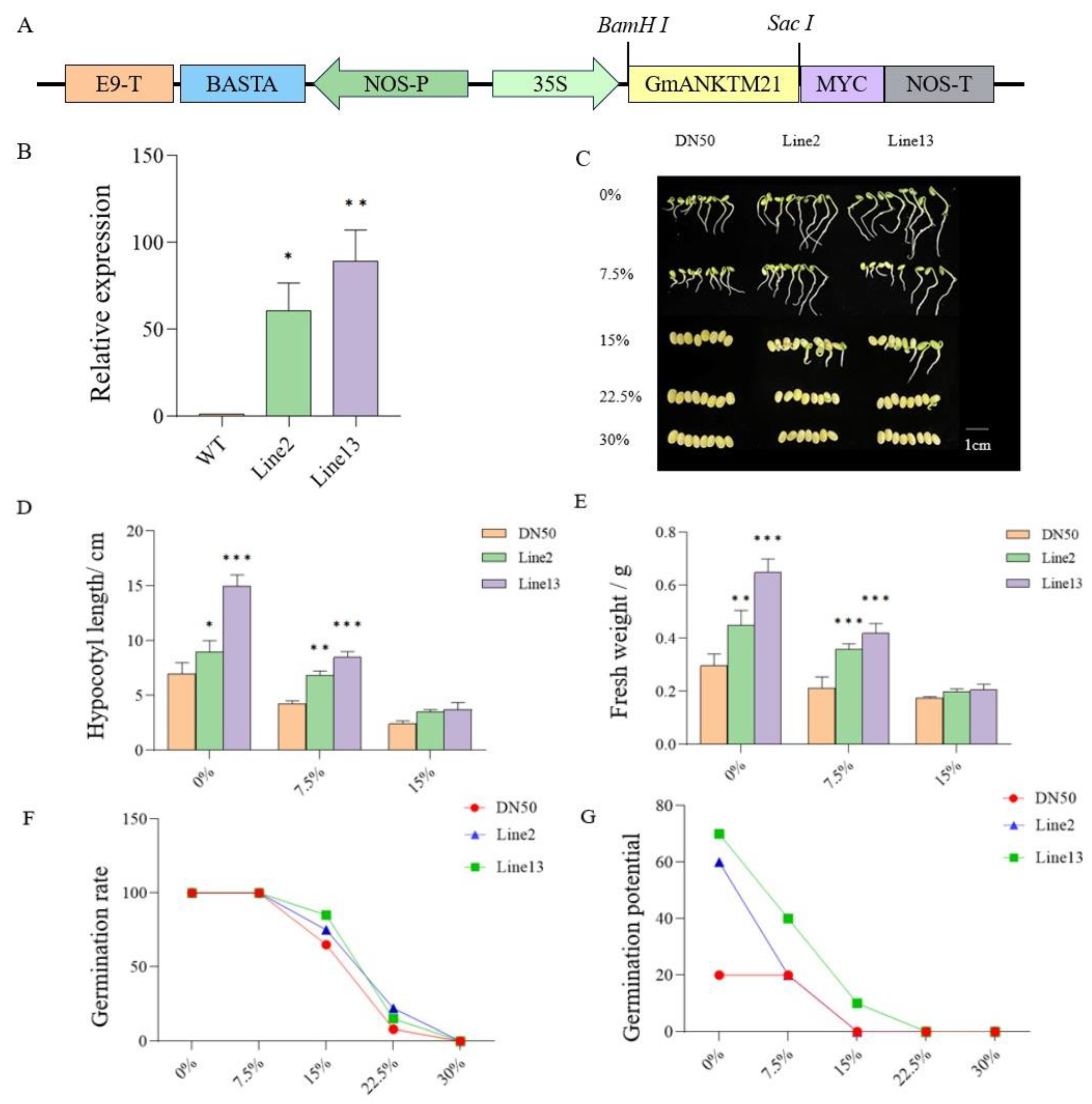
2.2. The Overexpression of GmANKTM21 Enhanced the Tolerance of Soybeans to Drought during Germination
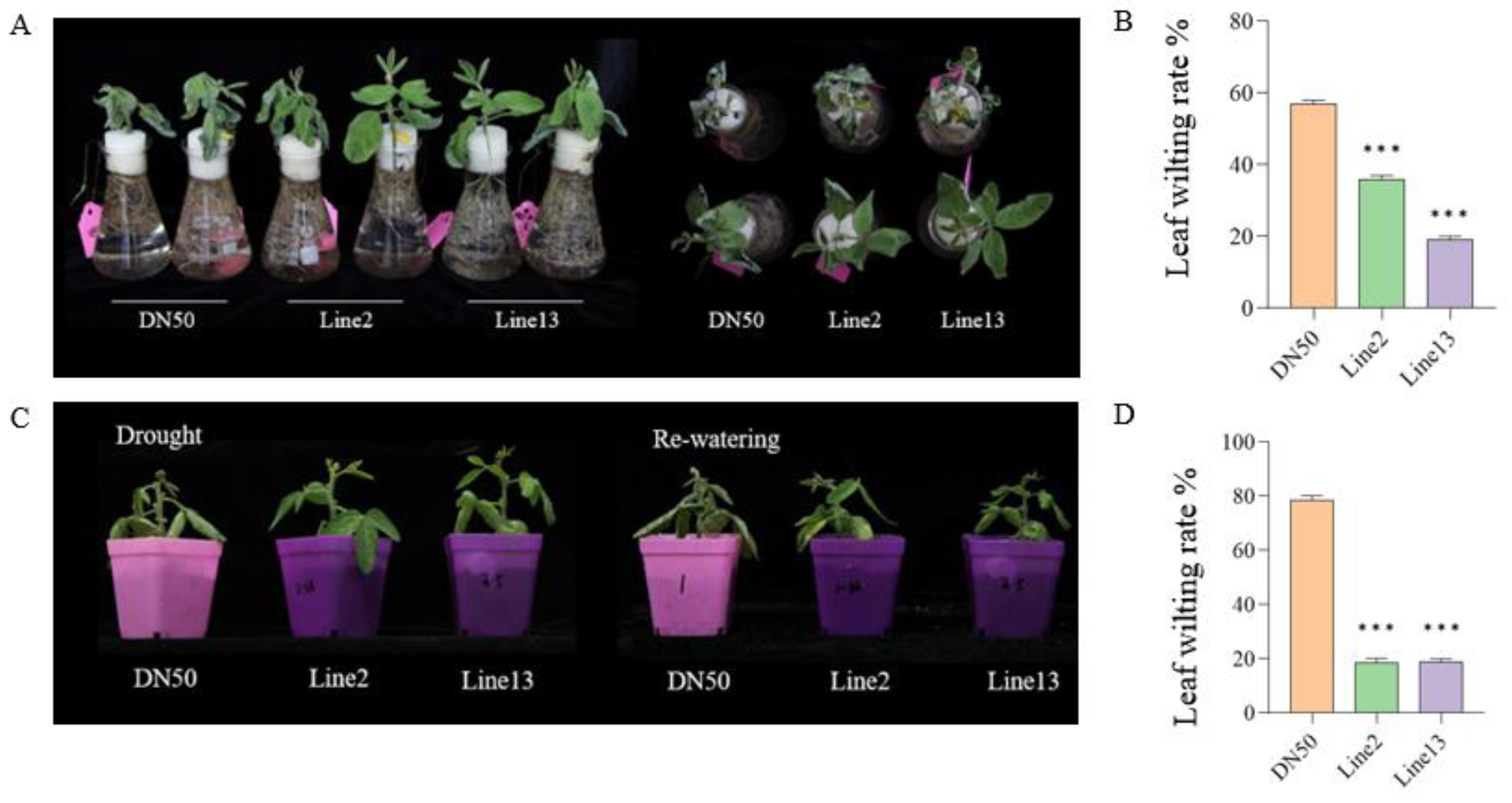
2.3. Overexpression of GmANKTM21 Enhanced Drought Tolerance in Soybean Seedlings
2.4. Overexpression of the GmANKTM21 Gene Enhanced the Water Retention Ability of Soybean
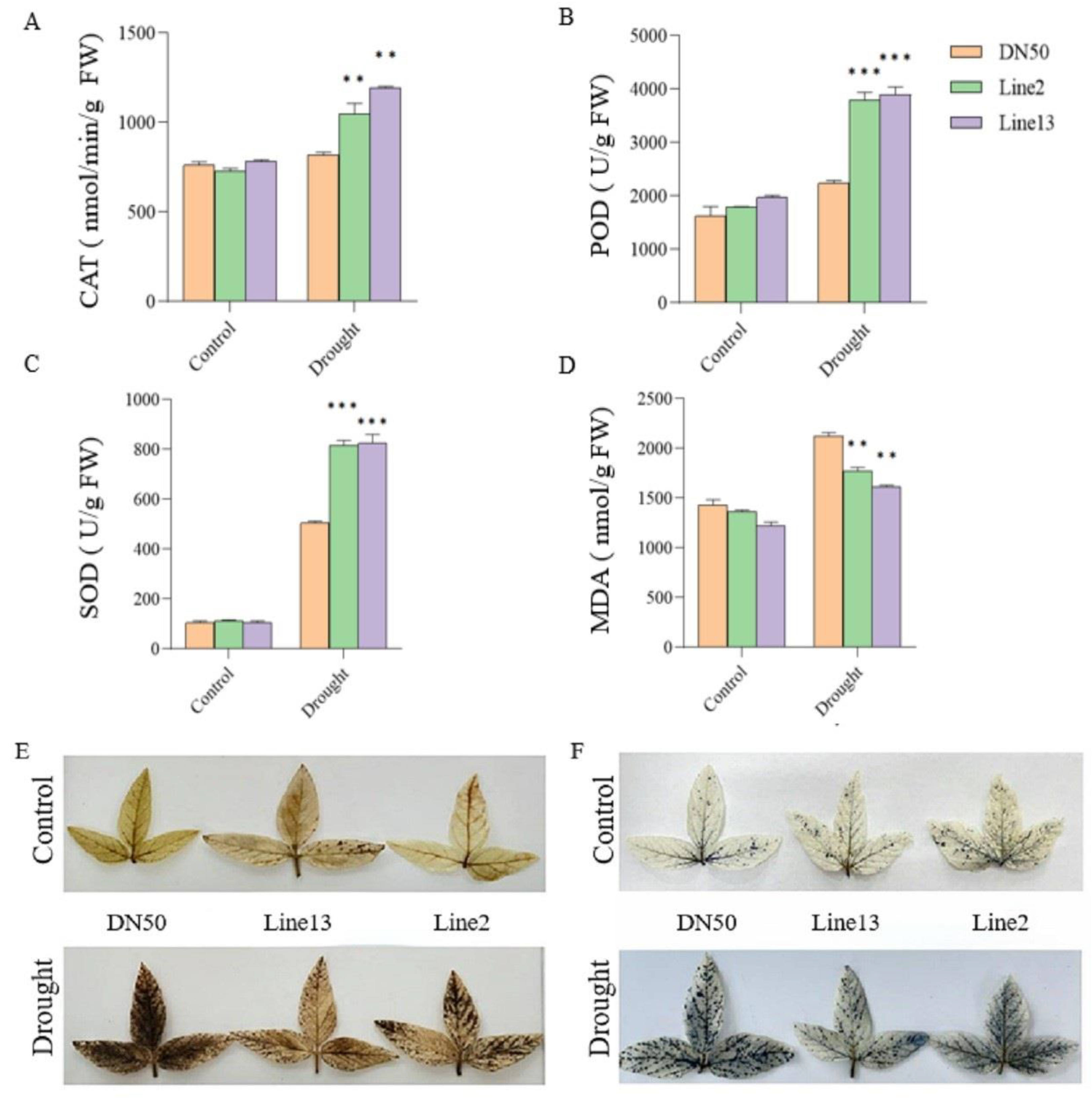
2.5. Overexpression of the GmANKTM21 Gene Enhanced the Antioxidant Capacity of Soybean
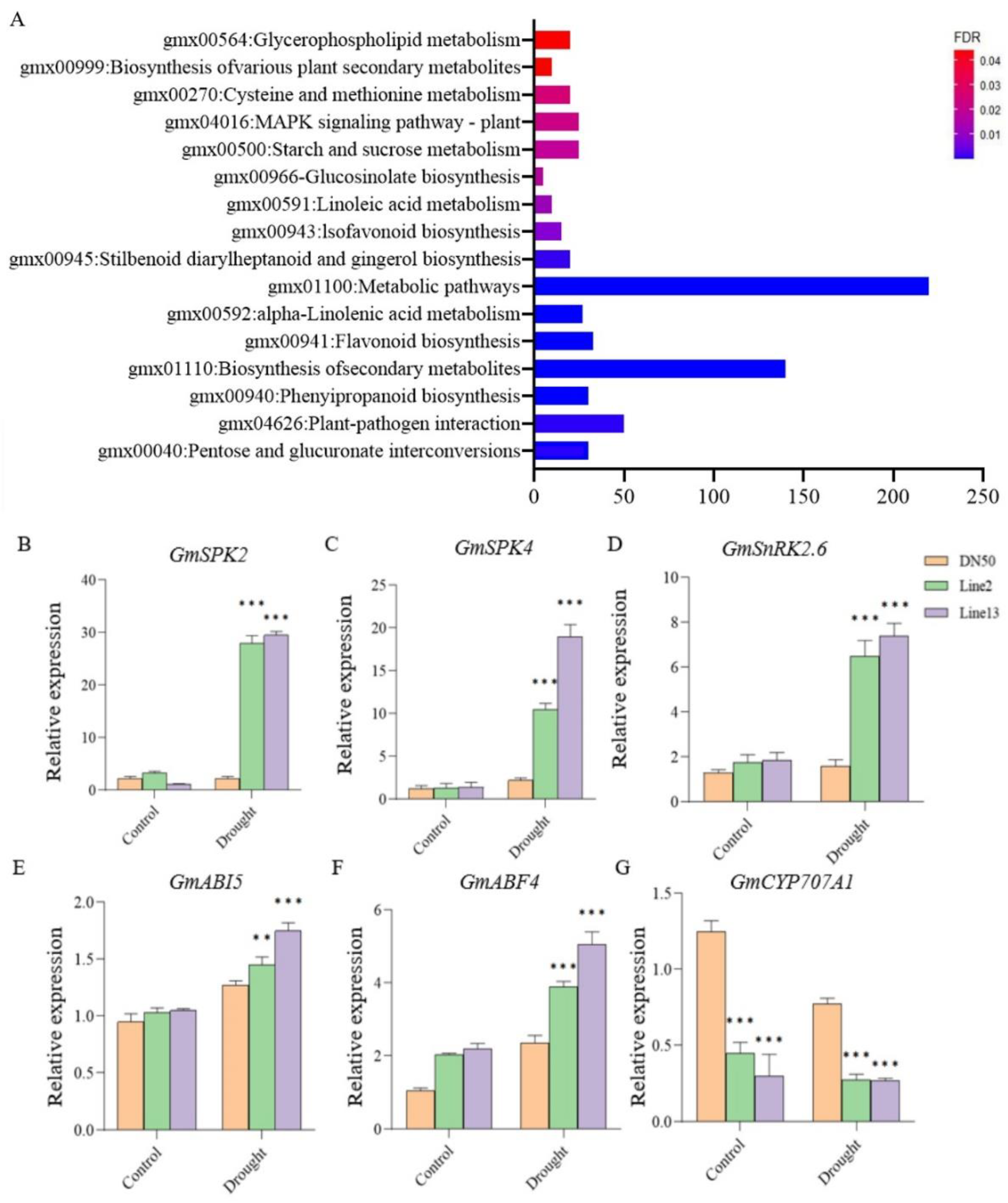
2.6. Studies on Downstream Genes of GmANKTM21 Gene Regulation
3. Discussion
4. Materials and Methods
4.1. Subcellular Localization
4.2. Plant Materials and Growth Conditions
4.3. Vector Construction and Transformation
4.4. RNA Extraction and RT-qPCR Analysis
4.5. Determination of Seed Germination Index
4.6. Leaf Water Loss Rate
4.7. Stomatal Opening and Density
4.8. Stratum Corneum Staining
4.9. Wax Observation
4.10. Determination of Antioxidant Enzyme Activity
4.11. DAB and NBT Staining
4.12. Expression Analysis of Downstream Genes by the GmANKTM21 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Merrium, S.; Ali, Z.; Tahir, M.H.N.; Habib-Ur-Rahman, M.; Hakeem, S. Leaf rolling dynamics for atmospheric moisture harvesting in wheat plant as an adaptation to arid environments. Environ. Sci. Pollut. Res. 2022, 29, 48995–49006. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bu, T.; Cheng, Q.; Dong, L.; Su, T.; Chen, Z.; Kong, F.; Gong, Z.; Liu, B.; Li, M. Two homologous LHY pairs negatively control soybean drought tolerance by repressing the abscisic acid responses. New Phytol. 2021, 229, 2660–2675. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2020, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Y.; Lu, Z.-W.; Sun, Y.; Fang, Z.-W.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S.; Min, D.-H. The Ankyrin-Repeat Gene GmANK114 Confers Drought and Salt Tolerance in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 584167. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ren, Y.; Xu, J.; Wei, Q.; Song, P.; Guan, Y.; Gao, H.; Zhang, Y.; Hu, H.; Li, C. Identification of ankyrin-transmembrane-type subfamily genes in Triticeae species reveals TaANKTM2A-5 regulates powdery mildew resistance in wheat. Front. Plant Sci. 2022, 13, 943217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wan, Q.; He, X.; Ning, L.; Huang, Y.; Xu, Z.; Liu, J.; Shao, H. Genome-wide characterization of the ankyrin repeats gene family under salt stress in soybean. Sci. Total Environ. 2016, 568, 899–909. [Google Scholar] [CrossRef]
- Li, J.; Mahajan, A.; Tsai, M.-D. Ankyrin repeat: A unique motif mediating protein−protein interactions. Biochemistry 2006, 45, 15168–15178. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L.; Qiu, X.; Wei, J.; Lu, H.; Sun, G.; Ma, X.; Yang, Z.; Zhu, C.; Hou, Y.; et al. AKR2A participates in the regulation of cotton fibre development by modulating biosynthesis of very-long-chain fatty acids. Plant Biotechnol. J. 2020, 18, 526–539. [Google Scholar] [CrossRef]
- Yu, F.; Shi, J.; Zhou, J.; Gu, J.; Chen, Q.; Li, J.; Cheng, W.; Mao, D.; Tian, L.; Buchanan, B.B.; et al. ANK6, a mitochondrial ankyrin repeat protein, is required for male-female gamete recognition in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 22332–22337. [Google Scholar] [CrossRef]
- Wang, H.; Zou, S.; Li, Y.; Lin, F.; Tang, D. An ankyrin-repeat and WRKY-domain-containing immune receptor confers stripe rust resistance in wheat. Nat. Commun. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, S.; Liu, S.; Yu, M.; Su, H.; Shu, H.; Li, X. Global analysis of ankyrin repeat domain C3HC4-Type RING finger gene family in plants. PLoS ONE 2013, 8, e58003. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.S.; Cho, H.S.; Choi, D.; Joung, Y.H.; Lim, C.K.; Hur, J.H.; Wang, M.-H. Tomato plants overexpressing CaKR1 enhanced tolerance to salt and oxidative stress. Biochem. Biophys. Res. Commun. 2007, 363, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Garcion, C.; Guilleminot, J.; Kroj, T.; Parcy, F.; Giraudat, J.; Devic, M. AKRP and EMB506 are two ankyrin repeat proteins essential for plastid differentiation and plant development in Arabidopsis. Plant J. 2006, 48, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, M.C.; Singla, J.; Sánchez-Martín, J.; Zbinden, H.; Šimková, H.; Karafiátová, M.; Doležel, J.; Gronnier, J.; Poretti, M.; Glauser, G.; et al. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Sintaha, M.; Man, C.-K.; Yung, W.-S.; Duan, S.; Li, M.-W.; Lam, H.-M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, X.; An, J.; Liu, X.; Ren, H. CsCER6 and CsCER7 Influence Fruit Glossiness by Regulating Fruit Cuticular Wax Accumulation in Cucumber. Int. J. Mol. Sci. 2023, 24, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Francocci, F.; Gully, K.; Martens, H.J.; De Lorenzo, G.; Nawrath, C.; Ferrari, S. Impaired Cuticle Functionality and Robust Resistance to Botrytis cinerea in Arabidopsis thaliana Plants With Altered Homogalacturonan Integrity Are Dependent on the Class III Peroxidase AtPRX71. Front. Plant Sci. 2021, 12, 696955. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Chen, M.; Ni, L.; Chen, J.; Sun, M.; Qin, C.; Zhang, G.; Zhang, A.; Jiang, M. Rice calcium/calmodulin-dependent protein kinase directly phosphorylates a mitogen-activated protein kinase kinase to regulate abscisic acid responses. Plant Cell 2021, 33, 1790–1812. [Google Scholar] [CrossRef]
- Cai, X.; Jia, B.; Sun, M.; Sun, X. Insights into the regulation of wild soybean tolerance to salt-alkaline stress. Front. Plant Sci. 2022, 13, 1002302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Y.; Xian, W.; Wang, J.; Liao, H. Identification and expression analysis of the Glycine max CYP707A gene family in response to drought and salt stresses. Ann. Bot. 2012, 110, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Hellal, F.; El-Shabrawi, H.; El-Hady, M.A.; Khatab, I.; El-Sayed, S.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2017, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, W.; Wang, G.; Hu, Y.; Zhong, X.; Tang, G. Physiological Regulation of Photosynthetic-Related Indices, Antioxidant Defense, and Proline Anabolism on Drought Tolerance of Wild Soybean (Glycine soja L.). Plants 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Luang, S.; Li, Y.; Bazanova, N.; Borisjuk, N.; Hrmova, M.; Lopato, S. Wheat drought-responsive WXPL transcription factors regulate cuticle biosynthesis genes. Plant Mol. Biol. 2017, 94, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Gai, X.; Ren, J.; Liu, X.; Cai, Y.; Wang, Q.; Ren, H. Cucumis sativus L. WAX2Plays a Pivotal Role in Wax Biosynthesis, Influencing Pollen Fertility and Plant Biotic and Abiotic Stress Responses. Plant Cell Physiol. 2015, 56, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, C.; Luo, N.; Jiang, Y.; Wang, Y.; Liu, Y.; Chen, C.; Wang, Y.; Gan, Q.; Jin, S.; et al. Brassica napus BnaC9.DEWAX1 Negatively Regulates Wax Biosynthesis via Transcriptional Suppression of BnCER1-2. Int. J. Mol. Sci. 2023, 24, 4287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, X.; Tang, M.; Wang, Y.; Zhang, Q.; Li, H.; Zhou, Y.; Sun, F.; Cui, X. Molecular Characterization and Drought Resistance of GmNAC3 Transcription Factor in Glycine max (L.) Merr. Int. J. Mol. Sci. 2022, 23, 12378. [Google Scholar] [CrossRef]
- Cui, X.; Tang, M.; Li, L.; Chang, J.; Yang, X.; Chang, H.; Zhou, J.; Liu, M.; Wang, Y.; Zhou, Y.; et al. Expression Patterns and Molecular Mechanisms Regulating Drought Tolerance of Soybean [Glycine max (L.) Merr.] Conferred by Transcription Factor Gene GmNAC19. Int. J. Mol. Sci. 2024, 25, 2396. [Google Scholar] [CrossRef]
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and Metabolomic Analysis of Seedling-Stage Soybean Responses to PEG-Simulated Drought Stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, X.; Zhang, Y.; Zhao, S.; Liu, J.; Wu, G.; Yan, X.; Luo, J. Transcriptomic Profiling Highlights the ABA Response Role of BnSIP1-1 in Brassica napus. Int. J. Mol. Sci. 2023, 24, 10641. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, S.; Ma, X.; He, Y.; Zhou, J.; Jiao, S.; Xun, J.; Kong, X.; Wu, X.; Bai, X. GmANKTM21 Positively Regulates Drought Tolerance and Enhanced Stomatal Response through the MAPK Signaling Pathway in Soybean. Int. J. Mol. Sci. 2024, 25, 6972. https://doi.org/10.3390/ijms25136972
Zhao Y, Wang S, Ma X, He Y, Zhou J, Jiao S, Xun J, Kong X, Wu X, Bai X. GmANKTM21 Positively Regulates Drought Tolerance and Enhanced Stomatal Response through the MAPK Signaling Pathway in Soybean. International Journal of Molecular Sciences. 2024; 25(13):6972. https://doi.org/10.3390/ijms25136972
Chicago/Turabian StyleZhao, Yue, Sinan Wang, Xiaofei Ma, Yu He, Jingwen Zhou, Shuang Jiao, Jianing Xun, Xiaoyu Kong, Xiaoxia Wu, and Xi Bai. 2024. "GmANKTM21 Positively Regulates Drought Tolerance and Enhanced Stomatal Response through the MAPK Signaling Pathway in Soybean" International Journal of Molecular Sciences 25, no. 13: 6972. https://doi.org/10.3390/ijms25136972




