Cell Therapies for Acute Radiation Syndrome
Abstract
:1. Introduction
1.1. Background
1.2. Preclinical Models
1.3. Combined Injury Animal Models
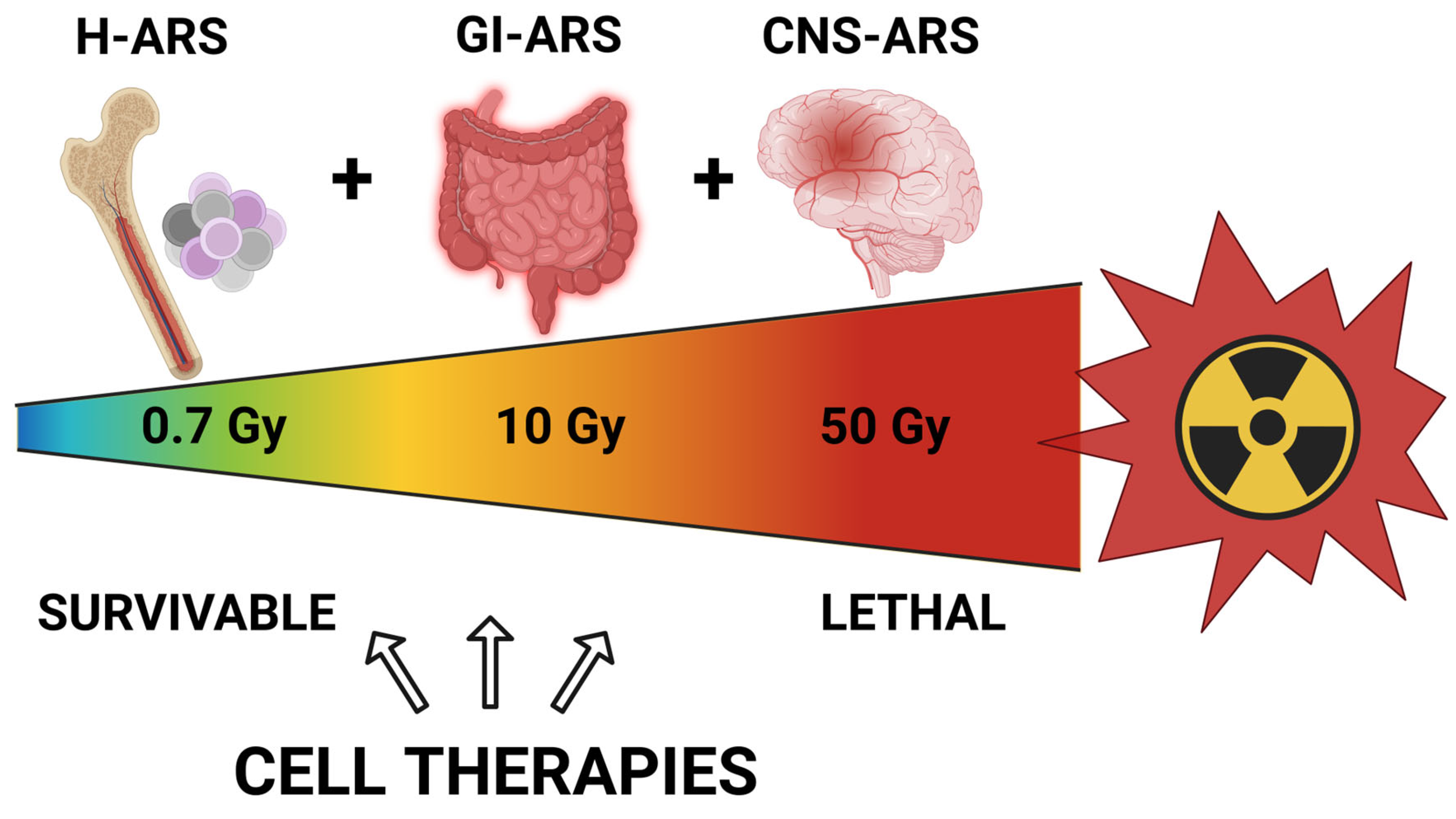
2. Acute Radiation Syndrome
3. Management of Acute Radiation Syndrome
3.1. Background
3.2. Approved Therapies
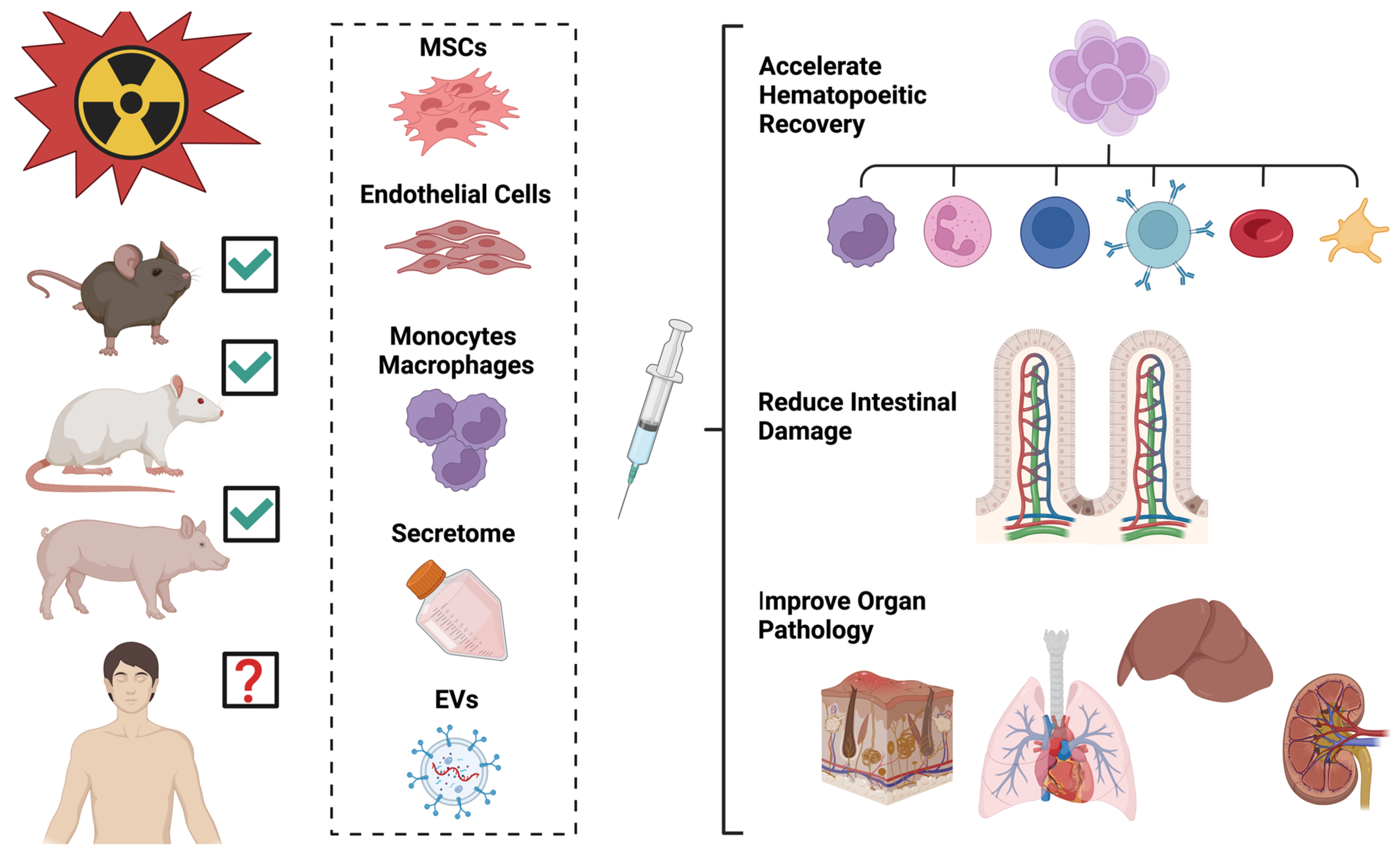
4. Cellular Therapeutics
4.1. Background
Mesenchymal Stromal Cells—Background
4.2. Cellular Therapies for Radiation in Animal Models
4.2.1. Cells
4.2.2. Cell Products
4.2.3. Countermeasure Testing in Combined Injury Models
4.2.4. Delivery of Cellular Therapeutics
5. Future Directions and Challenges
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- CBRN EU. CBRN Risk Mitigation. 2024. Available online: https://cbrn-risk-mitigation.network.europa.eu/index_en (accessed on 5 May 2024).
- CBRNE Central. Asia-Pacific. Articles on Chemical, Biological, Radiological and Nuclear (CBRN) and Explosives Threats, Preparedness and Response in the Asia-Pacific Region. 2024. Available online: https://cbrnecentral.com/tag/asia-pacific (accessed on 1 January 2024).
- Homeland Security. National Strategy for Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Standards. U.S. Department of Homeland Security: Washington, DC, USA, 2022. Available online: https://www.dhs.gov/national-strategy-chemical-biological-radiological-nuclear-and-explosives-cbrne-standards (accessed on 20 June 2024).
- CBRNEC. Chemical, Biological, Radiological, Nuclear and Explosives Resilience Strategy for Canada. Public Safety Canada: Ottawa, ON, Canada, 2018. Available online: https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/rslnc-strtg/index-en.aspx (accessed on 1 May 2024).
- Coleman, C.N.; Bader, J.L.; Koerner, J.F.; Hrdina, C.; Cliffer, K.D.; Hick, J.L.; James, J.J.; Mansoura, M.K.; Livinski, A.A.; Nystrom, S.V.; et al. Chemical, Biological, Radiological, Nuclear, and Explosive (CBRNE) Science and the CBRNE Science Medical Operations Science Support Expert (CMOSSE). Disaster Med. Public Health Prep. 2019, 13, 995–1010. [Google Scholar] [CrossRef]
- Ende, N.; Azzam, E.I. Consideration for the treatment of mass casualties based on pathology of the fatalities of HiroCDCa and Nagasaki. Int. J. Radiat. Biol. 2011, 87, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, S.; Nelson, M.A.; Brown, M.J. Dirty bomb source term characterization and downwind dispersion: Review of experimental evidence. J. Environ. Radioact. 2023, 263, 107166. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, S.; Nelson, M.A.; Brown, M.J. Review of particle deposition to and removal from clothing, skin, and hair after a radioactive airborne dispersal event. J. Environ. Radioact. 2023, 270, 107296. [Google Scholar] [CrossRef]
- Kugathasan, T.; Mothersill, C. Radiobiological and social considerations following a radiological terrorist attack; mechanisms, detection and mitigation: Review of new research developments. Int. J. Radiat. Biol. 2022, 98, 855–864. [Google Scholar] [CrossRef]
- Williams, G.; O’Malley, M. Surgical considerations in the management of combined radiation blast injury casualties caused by a radiological dirty bomb. Injury 2010, 41, 943–947. [Google Scholar] [CrossRef]
- Wolbarst, A.B.; Wiley, A.L.; Nemhauser, J.B., Jr.; Christensen, D.M.; Hendee, W.R. Medical response to a major radiologic emergency: A primer for medical and public health practitioners. Radiology 2010, 254, 660–677. [Google Scholar] [CrossRef]
- Eaton, E.B.; Varney, T.R., Jr. Mesenchymal stem cell therapy for acute radiation syndrome: Innovative medical approaches in military medicine. Mil. Med. Res. 2015, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, R. Mesenchymal stem cell therapy for acute radiation syndrome. Mil. Med. Res. 2016, 3, 17. [Google Scholar] [CrossRef]
- Ledney, G.D.; Stewart, D.A.; Exum, E.D.; Sheehy, P.A. Skin wound-enhanced survival and myelocytopoiesis in mice after whole-body irradiation. Acta Radiol. Oncol. 1981, 20, 29–38. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Mitchel, J.T. Working with the U.S. Food and Drug Administration to obtain approval of products under the Animal Rule. Ann. N. Y. Acad. Sci. 2016, 1374, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Newman, V.L.; Berg, A.N.; MacVittie, T.J. Animal models for acute radiation syndrome drug discovery. Expert Opin. Drug Discov. 2015, 10, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.S.; Carnell, L.S.; DiCarlo, A.L.; Hoffman, C.M.; Loelius, S.G.; Homer, M. Interagency approaches to animal models for acute radiation exposure. Int. J. Radiat. Biol. 2021, 97 (Suppl. S1), S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Brown, S.L.; Georges, G.E.; Hauer-Jensen, M.; Hill, R.P.; Huser, A.K.; Kirsch, D.G.; MacVittie, T.J.; Mason, K.A.; Medhora, M.M.; et al. Animal models for medical countermeasures to radiation exposure. Radiat. Res. 2010, 173, 557–578. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Olabisi, A.O. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opin. Drug Discov. 2017, 12, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.L.; Deburghgraeve, C.R.; Bird, M.D.; Hauer-Jensen, M.; Kovacs, E.J. Development of a combined radiation and burn injury model. J. Burn Care Res. 2011, 32, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Medhora, M.; Gasperetti, T.; Schamerhorn, A.; Gao, F.; Narayanan, J.; Lazarova, Z.; Jacobs, E.R.; Tarima, S.; Fish, B.L. Wound Trauma Exacerbates Acute, but not Delayed, Effects of Radiation in Rats: Mitigation by Lisinopril. Int. J. Mol. Sci. 2020, 21, 3908. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Fish, B.L.; Szabo, A.; Schock, A.; Narayanan, J.; Jacobs, E.R.; Moulder, J.E.; Lazarova, Z.; Medhora, M. Enhanced survival from radiation pneumonitis by combined irradiation to the skin. Int. J. Radiat. Biol. 2014, 90, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Swift, J.M.; Christensen, C.L.; Gupta, P.; Balakathiresan, N.; Maheshwari, R.K. Hemorrhage Exacerbates Radiation Effects on Survival, Leukocytopenia, Thrombopenia, Erythropenia, Bone Marrow Cell Depletion and Hematopoiesis, and Inflammation-Associated microRNAs Expression in Kidney. PLoS ONE 2015, 10, e0139271. [Google Scholar] [CrossRef]
- Kiang, J.G.; Garrison, B.R.; Burns, T.M.; Zhai, M.; Dews, I.C.; Ney, P.H.; Cary, L.H.; Fukumoto, R.; Elliott, T.B.; Ledney, G.D. Wound trauma alters ionizing radiation dose assessment. Cell Biosci. 2012, 2, 20. [Google Scholar] [CrossRef]
- Kiang, J.G.; Gorbunov, N.V. Bone Marrow Mesenchymal Stem Cells Increase Survival after Ionizing Irradiation Combined with Wound Trauma: Characterization and Therapy. J. Cell Sci. Ther. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Fukumoto, R. Ciprofloxacin increases survival after ionizing irradiation combined injury: Gamma-H2AX formation, cytokine/chemokine, and red blood cells. Health Phys. 2014, 106, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Ledney, G.D.; Exum, E.D.; Jackson, W.E., 3rd. Wound-induced alterations in survival of 60Co irradiated mice: Importance of wound timing. Experientia 1985, 41, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Ledney, G.D.; Exum, E.D.; Sheehy, P.A. Survival enhanced by skin-wound trauma in mice exposed to 60Co radiation. Experientia 1981, 37, 193–194. [Google Scholar] [CrossRef]
- Garrett, J.; Orschell, C.M.; Mendonca, M.S.; Bigsby, R.M.; Dynlacht, J.R. Subcutaneous wounding postirradiation reduces radiation lethality in mice. Radiat. Res. 2014, 181, 578–583. [Google Scholar] [CrossRef]
- Dynlacht, J.R.; Garrett, J.; Joel, R.; Lane, K.; Mendonca, M.S.; Orschell, C.M. Further Characterization of the Mitigation of Radiation Lethality by Protective Wounding. Radiat. Res. 2017, 187, 732–742. [Google Scholar] [CrossRef] [PubMed]
- A Brochure for Physicians Acute Radiation Syndrome. Available online: https://www.Cdc.gov/radiation-emergencies/media/pdfs/ARS.pdf (accessed on 23 November 2023).
- DiCarlo, A.L.; Maher, C.; Hick, J.L.; Hanfling, D.; Dainiak, N.; Chao, N.; Bader, J.L.; Coleman, C.N.; Weinstock, D.M. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med. Public Health Prep. 2011, 5 (Suppl. S1), S32–S44. [Google Scholar] [CrossRef]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Chua, H.L.; Plett, P.A.; Fisher, A.; Sampson, C.H.; Vemula, S.; Feng, H.; Sellamuthu, R.; Wu, T.; Orschell, C.M. Lifelong Residual bone Marrow Damage in Murine Survivors of the Hematopoietic Acute Radiation Syndrome (H-ARS): A Compilation of Studies Comprising the Indiana University Experience. Health Phys. 2019, 116, 546–557. [Google Scholar] [CrossRef]
- Chua, H.L.; Plett, P.A.; Sampson, C.H.; Joshi, M.; Tabbey, R.; Katz, B.P.; MacVittie, T.J.; Orschell, C.M. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012, 103, 356–366. [Google Scholar] [CrossRef]
- MacVittie, T.J.; Farese, A.M.; Jackson, W.E., 3rd. A Systematic Review of the Hematopoietic Acute Radiation Syndrome (H-ARS) in Canines and Non-human Primates: Acute Mixed Neutron/Gamma vs. Reference Quality Radiations. Health Phys. 2020, 119, 527–558. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Orschell, C.M. The delayed effects of acute radiation exposure (DEARE): Characteristics, mechanisms, animal models, and promising medical countermeasures. Int. J. Radiat. Biol. 2023, 99, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Boerma, M.; Fu, Q.; Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 2007, 13, 3047–3055. [Google Scholar] [CrossRef]
- Iddins, C.J.; DiCarlo, A.L.; Ervin, M.D.; Herrera-Reyes, E.; Goans, R.E. Cutaneous and local radiation injuries. J. Radiol. Prot. 2022, 42, 011001. [Google Scholar] [CrossRef]
- Kenchegowda, D.; Bolduc, D.L.; Kurada, L.; Blakely, W.F. Severity scoring systems for radiation-induced GI injury—Prioritization for use of GI-ARS medical countermeasures. Int. J. Radiat. Biol. 2023, 99, 1037–1045. [Google Scholar] [CrossRef]
- Dainiak, N.; Albanese, J. Medical management of acute radiation syndrome. J. Radiol. Prot. 2022, 42, 031002. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Chawla, R.; Marwah, R.; Kumar, V.; Goel, R.; Arora, P.; Jaiswal, S.; Sharma, R.K. Medical radiation countermeasures for nuclear and radiological emergencies: Current status and future perspectives. J. Pharm. Bioallied Sci. 2010, 2, 202–212. [Google Scholar] [CrossRef]
- Leiterer, A.; Bardot, I.; Ménétrier, F.; Bardot, S.; Grémy, O.; Bérard, P.; Pech, A.; Favaro, P. Medical countermeasures after a radiological event: An update from the CATO project. Int. J. Radiat. Biol. 2014, 90, 1043–1047. [Google Scholar] [CrossRef]
- Nicogossian, A.E.; Rummel, J.D.; Leveton, L.; Teeter, R. Development of countermeasures for medical problems encountered in space flight. Adv. Space Res. 1992, 12, 329–337. [Google Scholar] [CrossRef]
- Singh, V.K.; Hanlon, B.K.; Santiago, P.T.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int. J. Radiat. Biol. 2017, 93, 885–906. [Google Scholar] [PubMed]
- Singh, V.K.; Garcia, M.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int. J. Radiat. Biol. 2017, 93, 870–884. [Google Scholar] [PubMed]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation countermeasure agents: An update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Romaine, P.L.; Newman, V.L.; Seed, T.M. Medical countermeasures for unwanted CBRN exposures: Part II radiological and nuclear threats with review of recent countermeasure patents. Expert Opin. Ther. Pat. 2016, 26, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Romaine, P.L.; Seed, T.M. Medical Countermeasures for Radiation Exposure and Related Injuries: Characterization of Medicines, FDA-Approval Status and Inclusion into the Strategic National Stockpile. Health Phys. 2015, 108, 607–630. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today 2022, 58, 133–145. [Google Scholar] [CrossRef]
- Micewicz, E.D.; Damoiseaux, R.D.; Deng, G.; Gomez, A.; Iwamoto, K.S.; Jung, M.E.; Nguyen, C.; Norris, A.J.; Ratikan, J.A.; Ruchala, P.; et al. Classes of Drugs that Mitigate Radiation Syndromes. Front. Pharmacol. 2021, 12, 666776. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. Entolimod as a radiation countermeasure for acute radiation syndrome. Drug Discov. Today 2021, 26, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Gleiberman, A.S.; Toshkov, I.; Aygun-Sunar, S.; Bapardekar, M.; Manderscheid-Kern, P.; Bellnier, D.; Krivokrysenko, V.I.; Feinstein, E.; Gudkov, A.V. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: Implications for head-and-neck cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 228–234. [Google Scholar] [CrossRef]
- Krivokrysenko, V.I.; Toshkov, I.A.; Gleiberman, A.S.; Krasnov, P.; Shyshynova, I.; Bespalov, I.; Maitra, R.K.; Narizhneva, N.V.; Singh, V.K.; Whitnall, M.H.; et al. The Toll-Like Receptor 5 Agonist Entolimod Mitigates Lethal Acute Radiation Syndrome in Non-Human Primates. PLoS ONE 2015, 10, e0135388. [Google Scholar] [CrossRef]
- Krivokrysenko, V.I.; Shakhov, A.N.; Singh, V.K.; Bone, F.; Kononov, Y.; Shyshynova, I.; Cheney, A.; Maitra, R.K.; Purmal, A.; Whitnall, M.H.; et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J. Pharmacol. Exp. Ther. 2012, 343, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Benderitter, M.; Caviggioli, F.; Chapel, A.; Coppes, R.P.; Guha, C.; Klinger, M.; Malard, O.; Stewart, F.; Tamarat, R.; Van Luijk, P.; et al. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid. Redox Signal. 2014, 21, 338–355. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, A.L.; Tamarat, R.; Rios, C.I.; Benderitter, M.; Czarniecki, C.W.; Allio, T.C.; Macchiarini, F.; Maidment, B.W.; Jourdain, J.-R. Cellular Therapies for Treatment of Radiation Injury: Report from a NIH/NIAID and IRSN Workshop. Radiat. Res. 2017, 188, e54–e75. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; Jourdain, J.R.; DiCarlo, A.L. Cellular Therapies for Treatment of Radiation Injury after a Mass Casualty Incident. Radiat. Res. 2017, 188, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.L.; Nemhauser, J.; Chang, F.; Mashayekhi, B.; Sczcur, M.; Knebel, A.; Hrdina, C.; Coleman, N. Radiation event medical management (REMM): Website guidance for health care providers. Prehospital Emerg. Care 2008, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Martin, M. Medical management of the acute radiation syndrome. Rep. Pract. Oncol. Radiother. 2011, 16, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, N. Medical management of acute radiation syndrome and associated infections in a high-casualty incident. J. Radiat. Res. 2018, 59 (Suppl S2), ii54–ii64. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.S. Blood platelet change in acute radiation sickness. Med. Radiol. 1956, 1, 52–56. [Google Scholar]
- Diaz, M.F.; Horton, P.L.D.; Dumbali, S.P.; Kumar, A.; Livingston, M.; Skibber, M.A.; Mohammadalipour, A.; Gill, B.S.; Zhang, S.; Cox, C.S.; et al. Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis. Sci. Rep. 2020, 10, 22211. [Google Scholar] [CrossRef]
- Singh, V.K.; Brown, D.S.; Kao, T.C.; Seed, T.M. Preclinical development of a bridging therapy for radiation casualties. Exp. Hematol. 2010, 38, 61–70. [Google Scholar] [CrossRef]
- Weisdorf, D.; Chao, N.; Waselenko, J.K.; Dainiak, N.; Armitage, J.O.; McNiece, I.; Confer, D. Acute radiation injury: Contingency planning for triage, supportive care, and transplantation. Biol. Blood Marrow Transplant. 2006, 12, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, S.; Niehues, T.; Meisel, R.; Bernbeck, B.; Laws, H.J.; Kögler, G.; Enzmann, J.; Wernet, P.; Göbel, U.; Dilloo, D. Transplantation of haematopoietic stem cells derived from cord blood, bone marrow or peripheral blood: A single centre matched-pair analysis in a heterogeneous risk population. Klin. Padiatr. 2004, 216, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent. Years? Stem Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Danev, N.; Li, G.; Duan, J.E.; Van de Walle, G.R. Comparative transcriptomic analysis of bovine mesenchymal stromal cells reveals tissue-source and species-specific differences. iScience 2024, 27, 108886. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, J.; Han, L.; Jiang, X.; Yan, L.; Hao, J.; Wang, H. Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Res. Ther. 2019, 10, 19. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Jansen, B.J.H.; Gilissen, C.; Roelofs, H.; Schaap-Oziemlak, A.; Veltman, J.A.; Raymakers, R.A.P.; Jansen, J.H.; Kögler, G.; Figdor, C.G.; Torensma, R.; et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulos, V.; Tiffany, A.; Polanek, M.; Harley, B.A.C. Donor Variability in Human Mesenchymal Stem Cell Osteogenic Response as a Function of Passage Conditions and Donor Sex. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.A.; Herzig, M.C.; Delavan, C.; Cantu, C.; Salgado, C.; Bynum, J.A.; Cap, A.P. Human primary fibroblasts perform similarly to MSCs in assays used to evaluate MSC safety and potency. Transfusion 2019, 59 (Suppl. S2), 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Bates, P.D.; Kunugi, K.A.; Nickel, K.P.; DeWerd, L.A.; Capitini, C.M.; Galipeau, J.; Kimple, R.J. Dichotomic Potency of IFNgamma Licensed Allogeneic Mesenchymal Stromal Cells in Animal Models of Acute Radiation Syndrome and Graft Versus Host Disease. Front. Immunol. 2021, 12, 708950. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.A.; Herzig, M.C.; Delavan, C.P.; Abaasah, I.; Cantu, C.; Salgado, C.; Lovelace, S.; Garcia, L.; Jensen, K.; Montgomery, R.; et al. Use of multiple potency assays to evaluate human mesenchymal stromal cells. J. Trauma Acute Care Surg. 2020, 89 (Suppl. S2), S109–S117. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.C.; Christy, B.A.; Montgomery, R.K.; Delavan, C.P.; Jensen, K.J.; Lovelace, S.E.; Cantu, C.; Salgado, C.L.; Cap, A.P.; Bynum, J.A. Interactions of human mesenchymal stromal cells with peripheral blood mononuclear cells in a Mitogenic proliferation assay. J. Immunol. Methods 2021, 492, 113000. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.C.; Christy, B.A.; Montgomery, R.K.; Cantu-Garza, C.; Barrera, G.D.; Lee, J.H.; Mucha, N.; Talackine, J.R.; Abaasah, I.A.; Bynum, J.A.; et al. Short-term assays for mesenchymal stromal cell immunosuppression of T-lymphocytes. Front. Immunol. 2023, 14, 1225047. [Google Scholar] [CrossRef]
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2019, 21, 289–306. [Google Scholar] [CrossRef]
- Cuende, N.; Rasko, J.E.J.; Koh, M.B.C.; Dominici, M.; Ikonomou, L. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 2018, 20, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, M.R.; Ali, K.; Kamel, I.; Esteghamati, S.; Saleh, A.; Ghanim, M. The Safety and Efficacy of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Heart Failure and Myocardial Infarction: A Meta-Analysis of Clinical Trials. Cureus 2023, 15, e49645. [Google Scholar] [CrossRef] [PubMed]
- Hum, C.; Tahir, U.; Mei, S.H.; Champagne, J.; Fergusson, D.A.; Lalu, M.; Stewart, D.J.; Walley, K.; Marshall, J.; Dos Santos, C.C.; et al. Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cell Therapy in Preclinical Models of Sepsis: A Systematic Review and Meta-analysis. Stem Cells Transl. Med. 2024, 13, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, F.; Zhong, Y.; Wang, G.; Hu, L.; Zhang, Y.; Xie, J. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells for COVID-19 pneumonia: A meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2023, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhang, M.; Zhang, T.; Zeng, L.; Yang, K.; Yang, T.; Yu, G.; Li, J.; Wu, Y.; Chen, H. The Effectiveness and Safety of Mesenchymal Stem Cells in the Treatment of Osteoarthritis: A Systematic Review and Meta-analysis of 28 Randomized Controlled Trials. Stem Cells Int. 2022, 2022, 6151866. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Wang, B.; Li, J.; Peng, Z. The safety and efficacy of mesenchymal stromal cells in ARDS: A meta-analysis of randomized controlled trials. Crit. Care 2023, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Chance, T.C.; Rathbone, C.R.; Kamucheka, R.M.; Peltier, G.C.; Cap, A.P.; Bynum, J.A. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 87 (Suppl. S1), S74–S82. [Google Scholar] [CrossRef]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83 (Suppl. S1), S164–S169. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Prabhakara, K.; Toledano-Furman, N.E.; Wang, Y.-W.; Gill, B.S.; Wade, C.E.; Olson, S.D.; Cox, C.S. Clinical Cellular Therapeutics Accelerate Clot Formation. Stem Cells Transl. Med. 2018, 7, 731–739. [Google Scholar] [CrossRef]
- Gleeson, B.M.; Martin, K.; Ali, M.T.; Kumar, A.H.; Pillai, M.G.K.; Kumar, S.P.; O’Sullivan, J.F.; Whelan, D.; Stocca, A.; Khider, W.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells 2015, 33, 2726–2737. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Schriner, J.B.; Triolo, F.; Gill, B.S.; Cardenas, J.C.; Olson, S.D.; Cox, C.S., Jr. Low molecular weight heparin decreases pro-coagulant activity in clinical MSC products. Cytotherapy 2024, 26, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Snyder, O.L.; He, H.; Christenson, L.K.; Fleming, S.; Weiss, M.L. Procoagulant Activity of Umbilical Cord-Derived Mesenchymal Stromal Cells’ Extracellular Vesicles (MSC-EVs). Int. J. Mol. Sci. 2023, 24, 9216. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.R.; Bhonde, R.; Pal, R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.R.; Rosu-Myles, M. Uncovering the secretes of mesenchymal stem cells. Biochimie 2013, 95, 2212–2221. [Google Scholar] [CrossRef]
- Lyamina, S.; Baranovskii, D.; Kozhevnikova, E.; Ivanova, T.; Kalish, S.; Sadekov, T.; Klabukov, I.; Maev, I.; Govorun, V. Mesenchymal Stromal Cells as a Driver of Inflammaging. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Stoilova, I.; Gazeau, F.; Herbeuval, J.P.; Fourniols, T. Mesenchymal stromal cell derived extracellular vesicles as a therapeutic tool: Immune regulation, MSC priming, and applications to SLE. Front. Immunol. 2024, 15, 1355845. [Google Scholar]
- Quaedackers, M.E.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur. J. Immunol. 2009, 39, 3436–3446. [Google Scholar] [CrossRef]
- Lohrmann, H.P.; Graw, C.M.; Graw, R.G., Jr. Stimulated lymphocyte cultures: Responder recruitment and cell cycle kinetics. J. Exp. Med. 1974, 139, 1037–1048. [Google Scholar] [CrossRef]
- Andreeva, E.; Bobyleva, P.; Gornostaeva, A.; Buravkova, L. Interaction of multipotent mesenchymal stromal and immune cells: Bidirectional effects. Cytotherapy 2017, 19, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipoor, A.; Lee, R.H.; Prockop, D.J.; Bartosh, T.J. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl. Res. 2016, 177, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Costa, L.A.; Eiro, N. New era of mesenchymal stem cell-based medicine: Basis, challenges and prospects. Rev. Clin. Esp. 2023, 223, 619–628. [Google Scholar] [CrossRef]
- Oliva, J.; Pacini, S.; Canals, J.M.; Lim, M. Editorial: Mesenchymal Stromal Cells: Preclinical and Clinical Challenges. Front. Cell Dev. Biol. 2022, 10, 969178. [Google Scholar] [CrossRef]
- Najar, M.; Melki, R.; Khalife, F.; Lagneaux, L.; Bouhtit, F.; Agha, D.M.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Merimi, M. Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization. Front. Cell Dev. Biol. 2021, 9, 716853. [Google Scholar] [CrossRef] [PubMed]
- Sipos, F.; Muzes, G. Disagreements in the therapeutic use of mesenchymal stem cell-derived secretome. World J. Stem Cells 2022, 14, 365–371. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Hershfield, M.R.; Linsenbardt, H.R.; Smith, J.; Mack, J.; Natesan, S.; Averitt, D.L.; Stark, T.R.; Sosanya, N.M. Biological function of Extracellular Vesicles (EVs): A review of the field. Mol. Biol. Rep. 2023, 50, 8639–8651. [Google Scholar] [CrossRef]
- Goo, J.; Lee, Y.; Lee, J.; Kim, I.S.; Jeong, C. Extracellular Vesicles in Therapeutics: A Comprehensive Review on Applications, Challenges, and Clinical Progress. Pharmaceutics 2024, 16, 311. [Google Scholar] [CrossRef]
- Gemayel, J.; Chaker, D.; El Hachem, G.; Mhanna, M.; Salemeh, R.; Hanna, C.; Harb, F.; Ibrahim, A.; Chebly, A.; Khalil, C. Mesenchymal stem cells-derived secretome and extracellular vesicles: Perspective and challenges in cancer therapy and clinical applications. Clin. Transl. Oncol. 2023, 25, 2056–2068. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front. Cell Dev. Biol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Bruno, S.; Deregibus, M.C.; Camussi, G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol. Lett. 2015, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Thebaud, B. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Neonatal Lung Disease: Tiny Particles, Major Promise, Rigorous Requirements for Clinical Translation. Cells 2022, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Pincela Lins, P.M.; Pirlet, E.; Szymonik, M.; Bronckaers, A.; Nelissen, I. Manufacture of extracellular vesicles derived from mesenchymal stromal cells. Trends Biotechnol. 2023, 41, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Shaz, B.H.; Schäfer, R.; Fontaine, M.J.; Norris, P.J.; McKenna, D.H.; Jin, P.; Reems, J.-A.; Stroncek, D.; Tanashi, M.; Marks, D.; et al. Local manufacturing processes contribute to variability in human mesenchymal stromal cell expansion while growth media supplements contribute to variability in gene expression and cell function: A Biomedical Excellence for Safer Transfusion (BEST) collaborative study. Cytotherapy 2023, 26, 531–539. [Google Scholar] [PubMed]
- Stroncek, D.; Dinh, A.; Rai, H.; Zhang, N.; Somerville, R.; Panch, S. The need for uniform and coordinated practices involving centrally manufactured cell therapies. J. Transl. Med. 2022, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Brunswig-Spickenheier, B.; Cappallo-Obermann, H.; Eggert, K.; Gehling, U.M.; Rudolph, C.; Schlegelberger, B.; Cornils, K.; Zustin, J.; Spiess, A.-N.; et al. Radiation rescue: Mesenchymal stromal cells protect from lethal irradiation. PLoS ONE 2011, 6, e14486. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Balakrishnan, I.; Torok-Storb, B.; Pillai, M.M. Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion. Adv. Hematol. 2012, 2012, 142530. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Birman, E.; Forner, K.A.; Gaboury, L.; Galipeau, J. Adoptive transfer of mesenchymal stromal cells accelerates intestinal epithelium recovery of irradiated mice in an interleukin-6-dependent manner. Cytotherapy 2012, 14, 1164–1170. [Google Scholar] [CrossRef]
- Chinnapaka, S.; Yang, K.S.; Samadi, Y.; Epperly, M.W.; Hou, W.; Greenberger, J.S.; Ejaz, A.; Rubin, J.P. Allogeneic adipose-derived stem cells mitigate acute radiation syndrome by the rescue of damaged bone marrow cells from apoptosis. Stem Cells Transl. Med. 2021, 10, 1095–1114. [Google Scholar] [CrossRef]
- Piryani, S.O.; Jiao, Y.; Kam, A.Y.; Liu, Y.; Vo-Dinh, T.; Chen, B.J.; Chao, N.J.; Doan, P.L. Endothelial Cell-Derived Extracellular Vesicles Mitigate Radiation-Induced Hematopoietic Injury. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 291–301. [Google Scholar] [CrossRef]
- Schoefinius, J.S.; Brunswig-Spickenheier, B.; Speiseder, T.; Krebs, S.; Just, U.; Lange, C. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Provide Long-Term Survival After Total Body Irradiation Without Additional Hematopoietic Stem Cell Support. Stem Cells 2017, 35, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, O.A.; Azzam, E.I.; Ende, N. Human umbilical-cord-blood mononucleated cells enhance the survival of lethally irradiated mice: Dosage and the window of time. J. Radiat. Res. 2013, 54, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Lee, S.B.; Lee, J.G.; Jang, W.S.; Lee, S.J.; Park, S.; Lee, S.S. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp. Hematol. 2013, 41, 346–353.e2. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Bouchlaka, M.N.; Moffitt, A.B.; Kim, J.; Kink, J.A.; Bloom, D.D.; Love, C.; Dave, S.; Hematti, P.; Capitini, C.M. Human Mesenchymal Stem Cell-Educated Macrophages Are a Distinct High IL-6-Producing Subset that Confer Protection in Graft-versus-Host-Disease and Radiation Injury Models. Biol. Blood Marrow Transplant. 2017, 23, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shim, S.; Kim, M.J.; Myung, J.K.; Park, S. Mesenchymal stem cell-mediated Notch2 activation overcomes radiation-induced injury of the hematopoietic system. Sci. Rep. 2018, 8, 9277. [Google Scholar] [CrossRef] [PubMed]
- Kink, J.A.; Forsberg, M.H.; Reshetylo, S.; Besharat, S.; Childs, C.J.; Pederson, J.D.; Gendron-Fitzpatrick, A.; Graham, M.; Bates, P.D.; Schmuck, E.G.; et al. Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol. Blood Marrow Transplant. 2019, 25, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.H.; Kink, J.A.; Thickens, A.S.; Lewis, B.M.; Childs, C.J.; Hematti, P.; Capitini, C.M. Exosomes from primed MSCs can educate monocytes as a cellular therapy for hematopoietic acute radiation syndrome. Stem Cell Res. Ther. 2021, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wu, C.T.; Geng, P.; Liu, C.; Xiao, F.; Wang, L.S.; Wang, H. Dental Pulp Stem Cell-Derived Extracellular Vesicles Mitigate Haematopoietic Damage after Radiation. Stem Cell Rev. Rep. 2021, 17, 318–331. [Google Scholar] [CrossRef]
- Chen, H.; Min, X.-H.; Wang, Q.-Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.-M.; An, G.; Sha, W.-H.; et al. Pre-activation of mesenchymal stem cells with TNF-alpha, IL-1beta and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zeng, Z.C.; Sun, J.; Zeng, H.Y.; Huang, Y.; Zhang, Z.Y. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015, 56, 700–708. [Google Scholar] [CrossRef]
- Plett, P.A.; Sampson, C.H.; Chua, H.L.; Joshi, M.; Booth, C.; Gough, A.; Johnson, C.S.; Katz, B.P.; Farese, A.M.; Parker, J.; et al. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012, 103, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Plett, P.A.; Sampson, C.H.; Chua, H.L.; Jackson, W.; Vemula, S.; Sellamuthu, R.; Fisher, A.; Feng, H.; Wu, T.; MacVittie, T.J.; et al. The H-ARS Dose Response Relationship (DRR): Validation and Variables. Health Phys. 2015, 109, 391–398. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Ball, L.M.; Cometa, A.M.; Roelofs, H.; Zecca, M.; Avanzini, M.A.; Bertaina, A.; Vinti, L.; Lankester, A.; Maccario, R.; et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011, 46, 200–207. [Google Scholar] [CrossRef]
- Le Blanc, K.; Samuelsson, H.; Gustafsson, B.; Remberger, M.; Sundberg, B.; Arvidson, J.; Ljungman, P.; Lönnies, H.; Nava, S.; Ringdén, O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007, 21, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.X.; Sun, Q.Y.; Guo, M.; Ai, H.S. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br. J. Radiol. 2010, 83, 52–58. [Google Scholar] [CrossRef]
- Fichtel, P.; von Bonin, M.; Kuhnert, R.; Mobus, K.; Bornhauser, M.; Wobus, M. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Modulate Hematopoietic Stem and Progenitor Cell Viability and the Expression of Cell Cycle Regulators in an Age-dependent Manner. Front. Bioeng. Biotechnol. 2022, 10, 892661. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Shekari, F.; Nazari, A.; Assar Kashani, S.; Hajizadeh-Saffar, E.; Lim, R.; Baharvand, H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: A systematic review. Cytotherapy 2021, 23, 277–284. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/beta-catenin signaling. Stem Cell Res. Ther. 2019, 10, 30. [Google Scholar] [CrossRef]
- Kink, J.A.; Bellio, M.A.; Forsberg, M.H.; Lobo, A.; Thickens, A.S.; Lewis, B.M.; Ong, I.M.; Khan, A.; Capitini, C.M.; Hematti, P. Large-scale bioreactor production of extracellular vesicles from mesenchymal stromal cells for treatment of acute radiation syndrome. Stem Cell Res. Ther. 2024, 15, 72. [Google Scholar] [CrossRef]
- Foubert, P.; Doyle-Eisele, M.; Gonzalez, A.; Berard, F.; Weber, W.; Zafra, D.; Alfonso, Z.; Zhao, S.; Tenenhaus, M.; Fraser, J.K. Development of a combined radiation and full thickness burn injury minipig model to study the effects of uncultured adipose-derived regenerative cell therapy in wound healing. Int. J. Radiat. Biol. 2017, 93, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Bolduc, D.L.; Smith, J.T.; Anderson, M.N.; Ho, C.; Lin, B.; Jiang, S. Combined Therapy of Pegylated G-CSF and Alxn4100TPO Improves Survival and Mitigates Acute Radiation Syndrome after Whole-Body Ionizing Irradiation Alone and Followed by Wound Trauma. Radiat. Res. 2017, 188, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Ho, C.; Gorbunov, N.V.; Elliott, T.B. Thrombopoietin Receptor Agonist Mitigates Hematopoietic Radiation Syndrome and Improves Survival after Whole-Body Ionizing Irradiation Followed by Wound Trauma. Mediators Inflamm. 2017, 2017, 7582079. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Elliott, T.B.; Gorbunov, N.V. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: Amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxid. Med. Cell Longev. 2014, 2014, 215858. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Bolduc, D.L.; Elliott, T.B.; Gorbunov, N.V. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxid. Med. Cell. Longev. 2014, 2014, 481392. [Google Scholar] [CrossRef]
- Giri, J.; Galipeau, J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020, 4, 1987–1997. [Google Scholar] [CrossRef]
| Species | Advantages | Disadvantages |
|---|---|---|
| Mouse | Well-characterized | Physiology distinct from human |
| Multiple inbred strains | No prodromal phase | |
| Genetic modification feasible | Skin wound healing different from human | |
| Many reagents available | ||
| Short generation time | ||
| Lower costs | ||
| Rat | Low costs similar to mouse | Fewer reagents available than for mouse |
| Larger size better for surgical studies | More difficult to genetically modify | |
| More blood and tissue available | Less genetically homogenous than mouse | |
| Guinea Pig | Rapid response to radiation | Less characterized |
| Fewer reagents available than for mouse or rat | ||
| Ferret | Good model for prodromal phase | Less characterized |
| Fewer reagents available than for mouse or rat | ||
| Rabbit | Easy to handle | Less characterized |
| Fewer reagents available than for mouse or rat | ||
| Swine | Good model for skin wounds, burns | More expensive to purchase and maintain |
| Good model for H-ARS | More difficult to use large number of animals | |
| Fewer reagents available than for mouse or rat | ||
| Canine | Medium size | Pulmonary system different from human |
| Long lifespan | Companion animal status | |
| Good model for GI-ARS | ||
| Immune system similarities to human | ||
| NHP | Physiology most comparable to human | More sentient species |
| Well-characterized | More expensive to purchase and maintain | |
| Radiation response similar to human | More difficult to use large number of animals | |
| Gold standard model for FDA |
| Reference | Animal Model | Cellular Therapeutic | Admin. Method | Timing | Results/Outcomes |
|---|---|---|---|---|---|
| Lange, 2011 [121] | mouse | mouse BM-MSCs | IV | within 8 h | Increased survival, accelerated hematopoeitic recovery |
| whole body rad | of irrad | ||||
| Yang, 2012 [122] | mouse | mouse MSCs | IV | 16–24 h | Increased survival |
| whole body rad | post-irrad | ||||
| Francois, 2012 [123] | mouse | mouse MSCs | IP | 1 day | Increased survival, intestinal epith damage reduced; |
| whole body rad | (non-MHC matched) | post-irrad | antiobiotics improved survival | ||
| Schoefinius, 2017 [126] | mouse | mouse BM-MSCs/MSC-EVs | IV | shortly after | MSCs increased short & long-term survival; |
| whole body rad | post-irrad | EVs only increased long-term survival | |||
| Piryani, 2019 [125] | mouse | mouse ECs/EC-EVs | IV | 4 daily doses | Increased survival, increased BM cellularity |
| whole body rad | post-irrad | ||||
| Chinnapaka, 2021 [124] | mouse | mouse ASCs | IP | 24 h | Increased survival, accelerated hematopoeitic recovery; |
| whole body rad | post-irrad | cells migrated to BM | |||
| Chinnadurai, 2021 [81] | mouse | mouse MSCs (activated) | IP | 1 day & 8 days | Increased survival |
| whole body rad | post-irrad | ||||
| Kovelenko, 2013 [127] | mouse | human UCB-MNCs | RO | 4 doses @24–52 h | Increased survival |
| whole body rad | post-irrad | ||||
| Shim, 2013 [128] | mouse | human UCB-MSCs | IV | 4 h | Accelerated hematopoietic & BM recovery |
| whole body rad | post-irrad | ||||
| Wen, 2016 [129] | mouse | human MSC-EVs | IV | 6–72 h | Increased WBC & granulocyte numbers at 3 weeks |
| whole body rad | post-irrad | ||||
| Bouchlaka, 2017 [130] | mouse | human macrophages | IV | 3 h | Increased/prolonged survival |
| whole body rad | (after MSC co-culture) | post-irrad | |||
| Kim, 2018 [131] | mouse | human UCB-MSCs | IV | 3 h or 30 h | Increased survival, accelerated hematopoeitic recovery; |
| whole body rad | post-irrad | Increased prolif in BM | |||
| Kink, 2019 [132] | mouse | human macrophages | IV | 4 h | Increased survival, hematopoeitic recovery if macrophages |
| whole body rad | (MSC-EV-educated) | post-irrad | pretreated with EVs from LPS-primed human BM-MSCs | ||
| Diaz, 2020 [64] | mouse | human BM-MSCs | RO | 3 h or 30 h | Increased survival, improved gut recovery, |
| whole body rad | post-irrad | no effect on pancytopenia | |||
| Forsberg, 2021 [133] | mouse | human monocytes | IV | 4 h, 24 h, 48 h | Increased survival, hematopoetic recovery if injected at 4–24 h |
| whole body rad | (MSC-EV-educated) | post-irrad | IL-6 required for female mice but not male mice | ||
| Kong, 2021 [134] | mouse | human DPSC-EVs | IV | 7 daily doses | Inhibited decrease in WBCs, better recovery at 19–25 days |
| whole body rad | post-irrad | ||||
| Chen H, 2015 [135] | rat | rat MSC-CM ± activation | IP/IV | IP Continuous | Improved intestinal damage & survival; |
| partial rad (abd) | & IV for 3 days | activated MSC-CM more potent | |||
| Chen Y-X, 2015 [136] | rat | rat MSC-CM | IV | Pre-irradiation | Improved liver pathology |
| partial rad (liver) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christy, B.A.; Herzig, M.C.; Wu, X.; Mohammadipoor, A.; McDaniel, J.S.; Bynum, J.A. Cell Therapies for Acute Radiation Syndrome. Int. J. Mol. Sci. 2024, 25, 6973. https://doi.org/10.3390/ijms25136973
Christy BA, Herzig MC, Wu X, Mohammadipoor A, McDaniel JS, Bynum JA. Cell Therapies for Acute Radiation Syndrome. International Journal of Molecular Sciences. 2024; 25(13):6973. https://doi.org/10.3390/ijms25136973
Chicago/Turabian StyleChristy, Barbara A., Maryanne C. Herzig, Xiaowu Wu, Arezoo Mohammadipoor, Jennifer S. McDaniel, and James A. Bynum. 2024. "Cell Therapies for Acute Radiation Syndrome" International Journal of Molecular Sciences 25, no. 13: 6973. https://doi.org/10.3390/ijms25136973






