Growth Properties and Metabolomic Analysis Provide Insight into Drought Tolerance in Barley (Hordeum vulgare L.)
Abstract
1. Introduction
2. Results
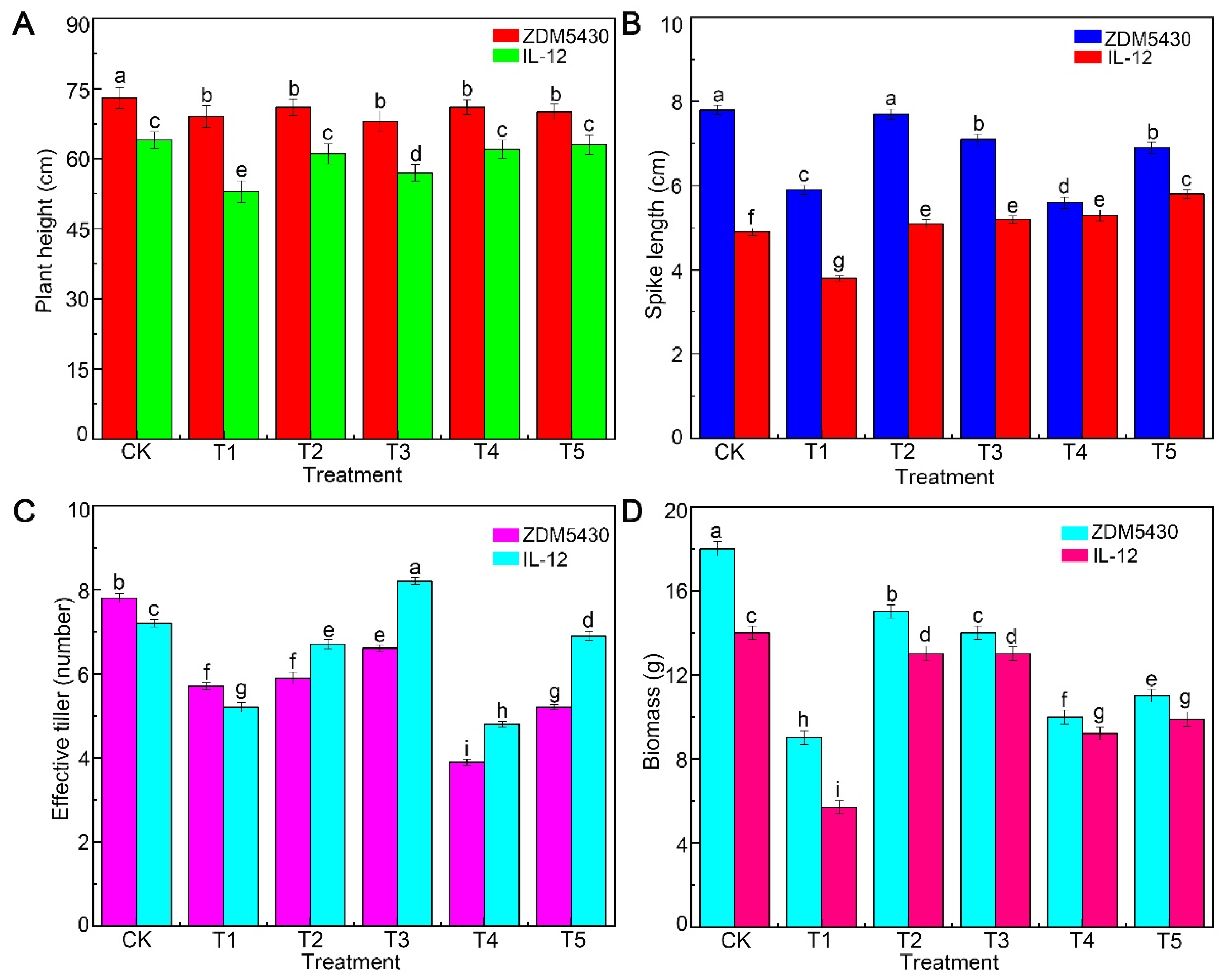
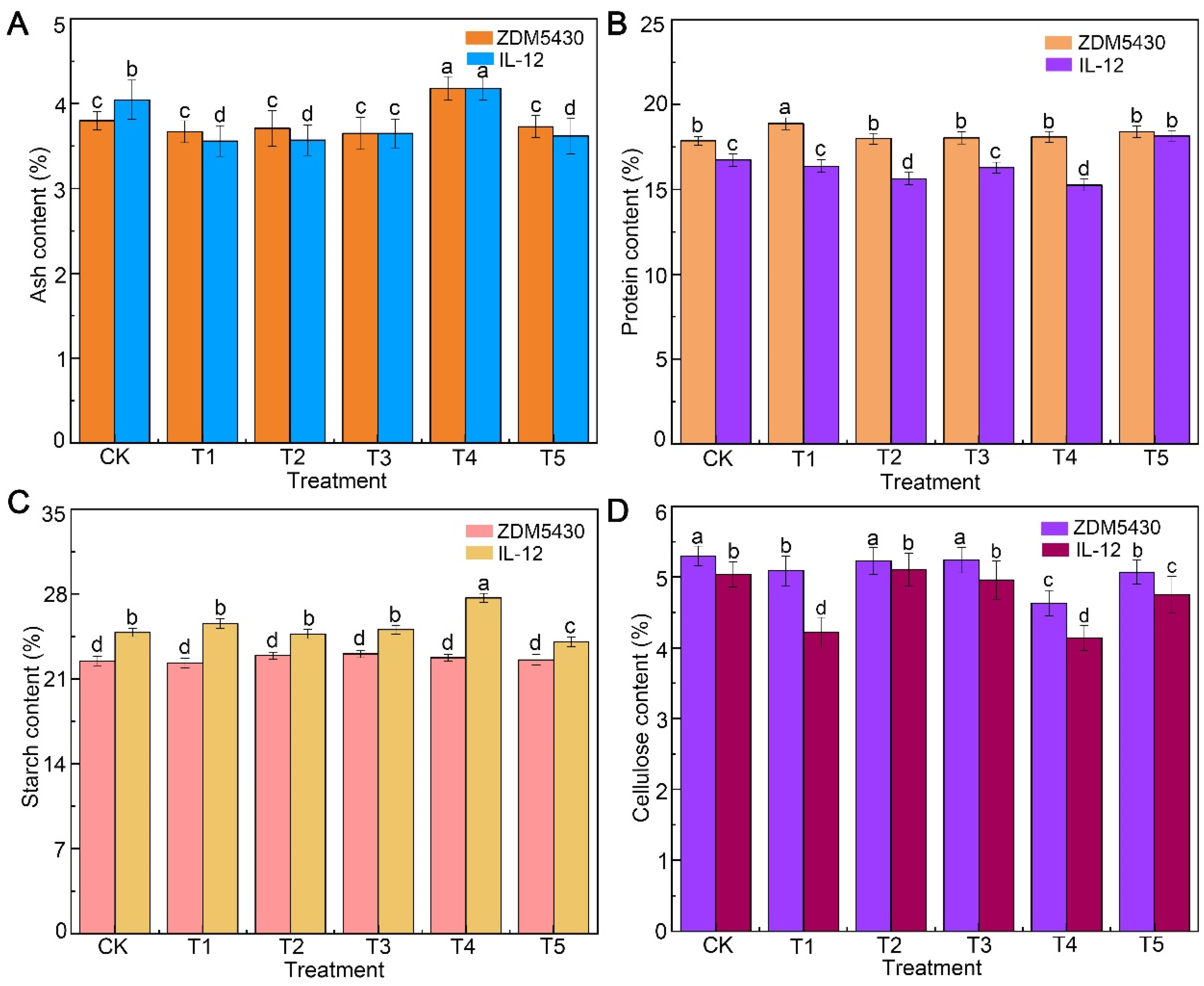
2.1. Effect of Drought Stress on Agronomic and Quality Traits of Barley
2.2. Effect of Different Drought Stress Levels on Grain Morphology and Starch Structure Features
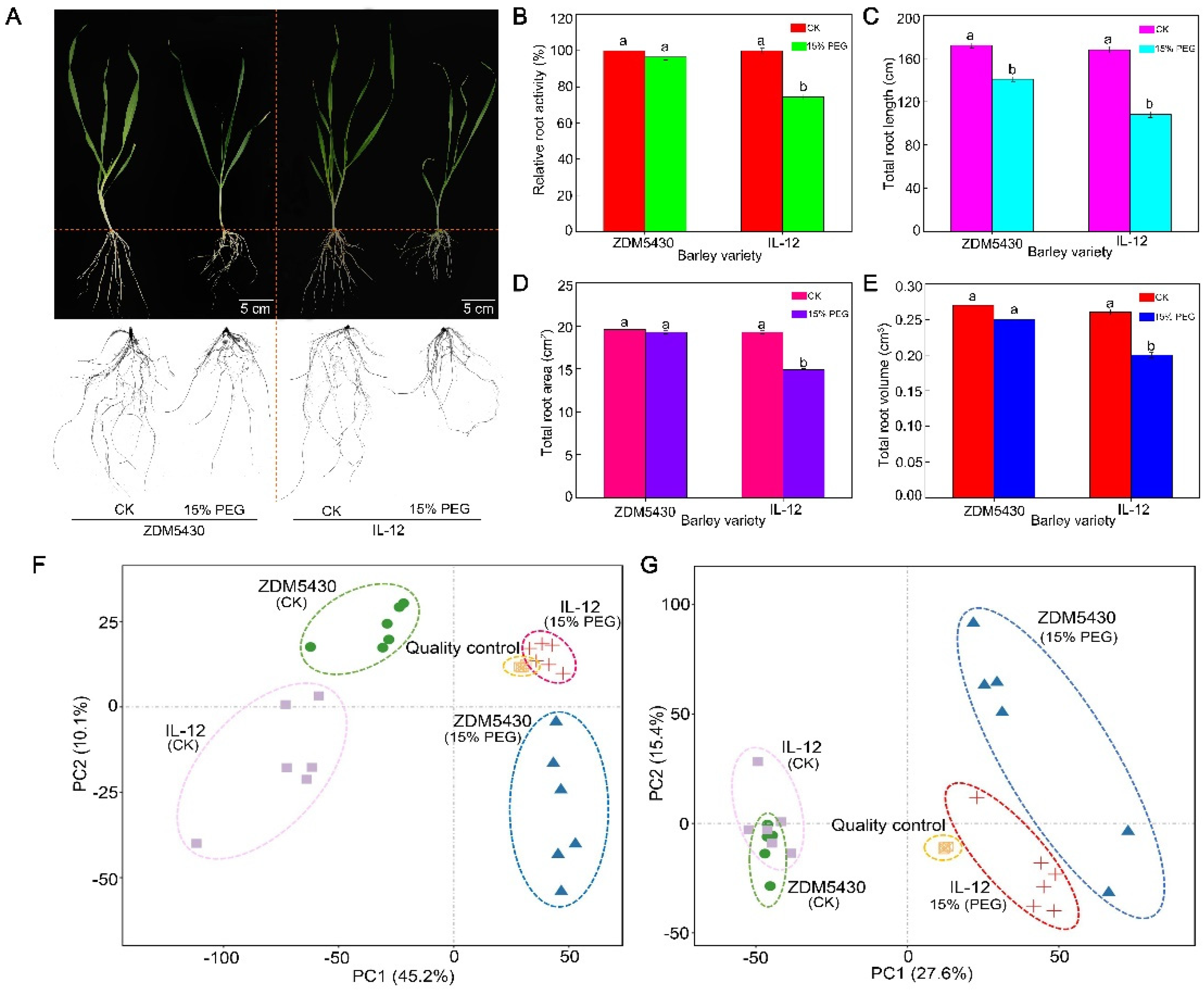
2.3. Phenotypic and Physiological Responses and Metabolic Profiles of Two Barley Genotypes under Drought Stress
2.4. Qualitative and Quantitative Metabolites
2.5. Different Metabolite Identification between Varieties
2.6. Analysis of Differential Metabolites between Genotypes
2.7. Comprehensive Metabolic Networks Analysis under Drought Stress
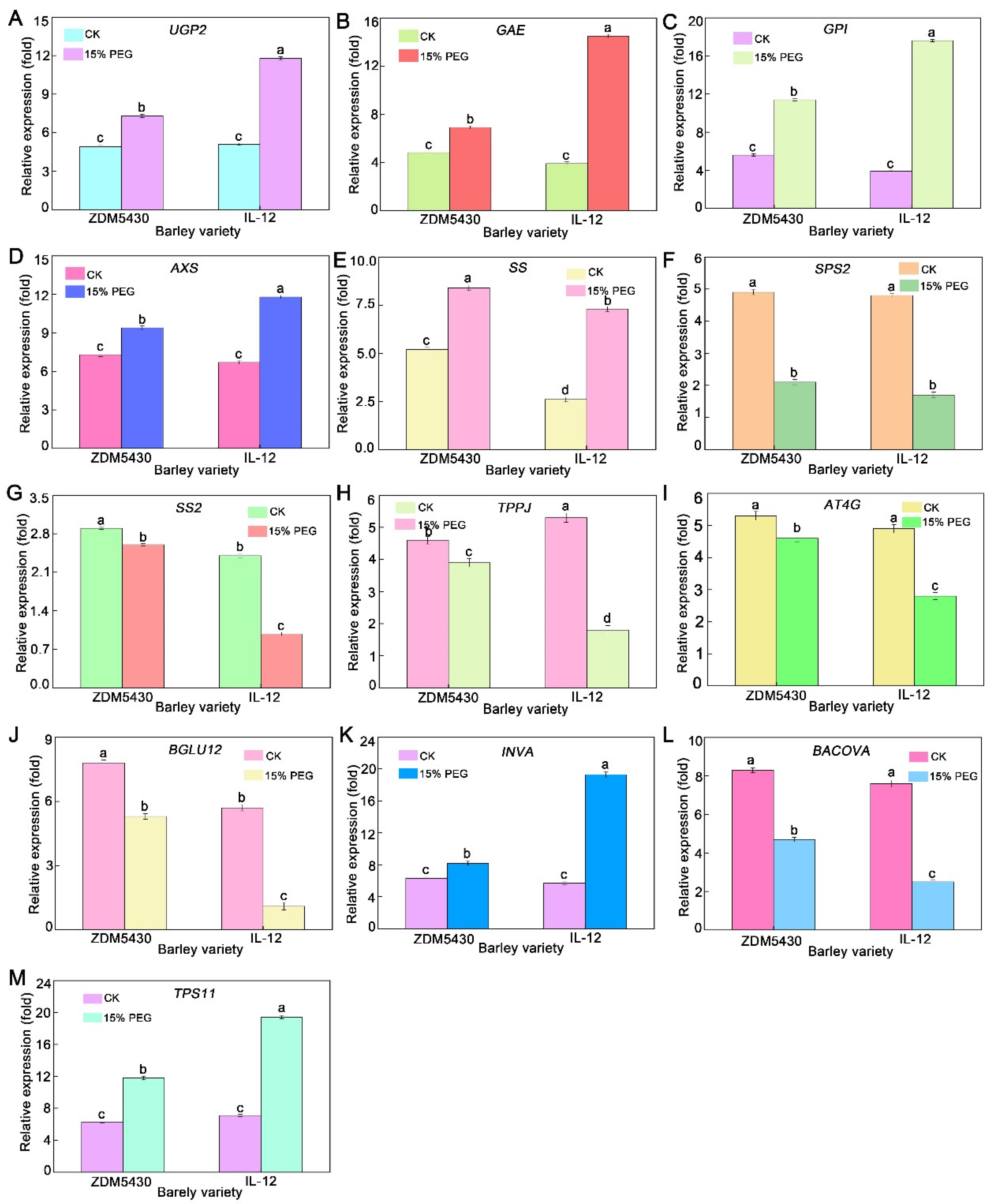
2.8. qRT-PCR Analysis Elucidated That Drought Treatment Orchestrated the Expression of Genes Involved in Metabolic Networks
3. Discussion
3.1. Growth of Barley under Different Drought Conditions
3.2. Root Growth of ZDM5430 and IL-12 under Drought Stress
3.3. Amino Acids Change in Response to Drought Stress
3.4. Proline and Phenylalanine Changes in Response to Drought Stress
3.5. qRT-PCR Analysis Elucidated That Drought Treatment Orchestrated the Expression of Genes Involved in Starch and Sucrose Metabolism
4. Material and Methods
4.1. Plant Growth and Drought Treatments
4.2. Agronomic and Quality Traits Analysis
4.3. Observation of Seed Morphology and Starch Granules
4.4. Root Morphology and Root Viability Assay
4.5. Metabolite Extraction
4.6. Metabolite Detection
4.7. Metabolite Identification
4.8. Statistical Analysis
4.9. Real-Time Quantitative RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, X.; Liu, S.; Qin, Y.; Xing, R.G.; Li, K.C.; Yu, C.L.; Chen, X.L.; Li, P.C. Metabonomics analysis of drought resistance of wheat seedlings induced by beta-aminobutyric acid-modified chitooligosaccharide derivative. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2021, 272, 118437. [Google Scholar]
- Cornic, G.; Briantais, J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 1991, 183, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowski, L.A.; Edwards, G.E.; Blackwell, R.D.; Givan, L.C.V. Enzymology of the reduction of hydroxypyruvate and glyoxylate in a mutant of barley lacking peroxisomal hydroxypyruvate reductase. Plant Physiol. 1990, 94, 819–825. [Google Scholar] [CrossRef]
- Li, W.X.; Oono, Y.; Zhu, J.; He, X.J.; Wu, J.M.; Lida, K.; Lu, X.Y.; Cui, X.P.; Jin, H.L.; Zhu, J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Biehler, K.; Fock, H.P. Evidence for the Contribution of the Mehler-Peroxidase Reaction in Dissipating Excess Electrons in Drought-Stressed Wheat. Plant Physiol. 1996, 112, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Reply to Leuzinger et al.: Drought-induced tree mortality temperature sensitivity requires pressing forward with best available science. Proc. Natl. Acad. Sci. USA 2009, 106, 38. [Google Scholar] [CrossRef]
- Kosová, K.; Urban, M.O.; Vítámvás, P.; Práil, I.T. Drought stress response in common wheat, durum wheat, and barley: Transcriptomics, proteomics, metabolomics, physiology, and breeding for an enhanced drought tolerance. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; pp. 319–324. [Google Scholar]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and Metabolomic Studies Disclose Key Metabolism Pathways Contributing to Well-maintained Photosynthesis under the Drought and the Consequent Drought-Tolerance in Rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef]
- Naimat, U.; Meral, Y.Z.; Neslihan, Ö.G.; Hikmet, B. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar]
- Chmielewska, K.; Rodziewicz, P.; Swarcewicz, B.; Sawikowska, A.; Krajewski, P.; Marczak, U.; Ciesioka, D.; Kuczyńska, A.; Mikoajczak, K.; Ogrodowicz, P. Analysis of drought-induced proteomic and metabolomic changes in barley (Hordeum vulgare L.) leaves and roots unravels some aspects of biochemical mechanisms involved in drought tolerance. Front. Plant Sci. 2016, 7, 1108. [Google Scholar] [CrossRef]
- Okada, T.; Afendi, F.M.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of medicinal plants: The importance of multivariate analysis of analytical chemistry data. Curr. Comput.-Aid. Drug 2010, 6, 179–196. [Google Scholar] [CrossRef]
- Seiler, C.; Harshavardhan, V.T.; Reddy, P.S.; Hensel, G.; Kumlehn, J.; Eschen-Lippold, L.; Rajesh, K.; Korzun, V.; Wobus, U.; Lee, J.; et al. Abscisic acid flux alterations result in differential abscisic acid signaling responses and impact assimilation efficiency in barley under terminal drought stress. Plant Physiol. 2014, 164, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Physical, A. The International Barley Genome Sequencing Consortium (IBSC). Nature 2012, 491, 711–716. [Google Scholar] [CrossRef]
- Farahani, S.M.; Chaichi, M.R.; Mazaheri, D.; Afshari, R.T.; Savaghebi, G. Barley grain mineral analysis as affected by different fertilizing systems and by drought stress. J. Agric. Sci. Technol. 2011, 13, 315–326. [Google Scholar]
- Zhao, M.; Ren, Y.; Wei, W.; Yang, J.; Zhong, Q.; Li, Z. Metabolite analysis of Jerusalem Artichoke (Helianthus tuberosus L.) seedlings in response to polyethylene glycol-simulated drought stress. Int. J. Mol. Sci. 2021, 22, 3294. [Google Scholar] [CrossRef] [PubMed]
- Akhondi, M.; Safarnejad, A.; Lahouti, M. Effect of drought stress on proline accumulation and mineral nutrients changes in Alfalfa (Medicago sativa L.). J. Sci. Technol. Agric. Nat. Resour. 2006, 10, 165–175. [Google Scholar]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z.; Ahuja, L.R.; Yu, Q. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; American Society of Agronomy: Madison, WI, USA, 2008. [Google Scholar]
- Coles, G.D.; Hartunian-Sowa, S.M.; Jamieson, P.D.; Hay, A.J.; Atwell, W.A.; Fulcher, R.G. Environmentally-induced variation in starch and non-starch polysaccharide content in wheat. J. Cereal Sci. 1997, 26, 47–54. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Pipolo, A.E.; Sinclair, T.R.; Camara, G.M.S. Effects of temperature on oil and protein concentration in soybean seeds cultured in vitro. Ann. Appl. Biol. 2015, 144, 71–76. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Xu, F.; Li, X.; Liu, H. Analysis of physiological and metabolite response of celosia argentea to copper stress. Plant Biol. 2020, 2, 391–399. [Google Scholar] [CrossRef]
- Villate, A.; Nicolas, M.S.; Gallastegi, M.; Aulas, P.A.; Aizpurua-Olaizola, O. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2021, 303, 110789. [Google Scholar] [CrossRef]
- Rabara, R.C.; Prateek, T.; Rushton, P.J. Comparative metabolome profile between tobacco and soybean grown under water-stressed conditions. Biomed. Res. Int. 2017, 2017, 3065251. [Google Scholar] [CrossRef] [PubMed]
- Sicher, R.C.; Timlin, D.; Bailey, B. Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J. Plant Physiol. 2012, 169, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Sureshkumar, S.; Shewade, D.G. Metabolomics: A tool ahead for understanding molecular mechanisms of drugs and diseases. Indian J. Clin. Biochem. 2015, 30, 247–254. [Google Scholar] [CrossRef]
- Guo, X.Y.; Xin, Z.Y.; Yang, T.G.; Ma, X.L.; Zhang, Y.; Wang, Z.Q.; Ren, Y.Z.; Lin, T.B. Metabolomics response for drought stress tolerance in Chinese wheat genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Vian, A.; Stankovic, B.; Davies, E. Signalomics: Diversity and methods of analysis of systemic signals in plants. In PlantOmics: The Omics of Plant Science; Springer: New Delhi, India, 2015; pp. 459–489. [Google Scholar]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Envion. 2018, 42, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Sun, C.; Zuo, Y.; Li, G.Y.; Li, G.B.; Ren, L.Y.; Chen, G.L. Integrating transcriptomics and metabolomics to characterise the response of Astragalus membranaceus Bge. var. mongolicus (Bge.) to progressive drought stress. BMC Genom. 2016, 17, 188. [Google Scholar] [CrossRef]
- Yang, L.M.; Fountain, J.C.; Ji, P.S.; Ni, X.Z.; Chen, S.X.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xie, Y.; Fan, S.; Wang, Z.S.; Wang, F.H.; Zhang, B.; Li, H.S.; Song, J.; Kong, L.G. Comparative analysis of root transcriptome profiles between drought-tolerant and susceptible wheat genotypes in response to water stress. Plant Sci. 2018, 272, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Law, S.R. Finding the metabolomic signature of drought resistance in poplar. Plant Physiol. 2020, 168, 529–530. [Google Scholar] [CrossRef]
- Zhu, J.K. Over expression of a delta-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water and salt stress in transgenic rice. Trends Plant Sci. 2000, 6, 66–72. [Google Scholar] [CrossRef]
- Moschen, S.; Di, R.; Julio, A.; Higgins, J.; Tohge, T.; Watanabe, M.; González, S.; Rivarola, M.; García-García, F.; Dopazo, J.; et al. Integration of transcriptomic and metabolic data reveals hub transcription factors involved in drought stress response in sunflower (Helianthus annuus L.). Plant Mol. Biol. 2017, 94, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Matsuda, F.; Kasai, K. Mutation of a rice gene encoding a phenylalanine biosynthetic enzyme results in accumulation of phenylalanine and tryptophan. Plant Cell 2008, 20, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Barchet, G.L.H.; Rebecca, D.; Guy, R.D.; Schroeder, W.R.; Soolanayakanahally, R.Y.; Campbell, M.M.; Mansfield, S.D. Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol. 2013, 11, 1203. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [PubMed]
- Swarcewicz, B.; Sawikowska, A.; Marczak, Ł.; Łuczak, M.; Ciesiołka, D.; Krystkowiak, K.; Kuczyńska, A.; Piślewska-Bednarek, M.; Krajewski, P.; Stobiecki, M. Effect of drought stress on metabolite contents in barley recombinant inbred line population revealed by untargeted GC-MS profiling. Acta Physiol. Plant 2017, 39, 158–159. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1937, 347, 39. [Google Scholar]
- Uemura, M. Comparison of plasma membrane proteomic changes of arabidopsis suspension-cultured cells (T87 Line) after cold and ABA treatmentn in association with freezing tolerance development. Plant Cell Physiol. 2012, 53, 543–544. [Google Scholar]
- Sanchez-Arcos, C.; Kai, M.; Svato, A.; Gershenzon, J.; Kunert, G. Untargeted metabolomics approach reveals differences in host plant chemistry before and after infestation with different pea aphid host races. Front. Plant Sci. 2019, 28, 188. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.; Grando, S.; Backes, G.; Jahoor, A.; Sabbagh, A.; Ceccarelli, S. QTLs for agronomic traits in the Mediterranean environment identified in recombinant inbred lines of the cross ‘Arta’ × H. spontaneum 41-1. Theor. Appl. Genet. Theor. Angew. Genet. 2003, 107, 1215–1225. [Google Scholar] [CrossRef]
- Borner, A.; Schumann, E.; Furste, A.; Coster, H.; Leithold, B.; Roder, M.; Weber, W. Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 2002, 105, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Sangwongchai, W.; Krusong, K.; Thitisaksakul, M. Salt tolerance at vegetative stage is partially associated with changes in grain quality and starch physicochemical properties of rice exposed to salinity stress at reproductive stage. J. Sci. Food Agric. 2021, 102, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Peng, Z.; Jun, P.; Yong, Z.; Jun, X.; Xu, Y. Melatonin enhances astaxanthin accumulation in the green microalga Haematococcus pluvialis by mechanisms possibly related to abiotic stress tolerance. Algal Res. 2018, 33, 256–265. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D.L. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Cultivars | Treatment | Length (cm) | Width (cm) | Width-to-Length Ratio | Volume (cm3) |
|---|---|---|---|---|---|
| ZDM5430 | CK | 1.41 ± 0.01 a | 0.73 ± 0.02 b | 0.52 ± 0.03 d | 0.21 ± 0.01 ab |
| T1 | 1.32 ± 0.02 b | 0.65 ± 0.01 h | 0.49 ± 0.03 e | 0.17 ± 0.02 cd | |
| T2 | 1.31 ± 0.02 bc | 0.72 ± 0.02 c | 0.55 ± 0.02 c | 0.19 ± 0.01 bc | |
| T3 | 1.21 ± 0.01 f | 0.68 ± 0.01 e | 0.56 ± 0.02 bc | 0.16 ± 0.01 d | |
| T4 | 1.21 ± 0.03 f | 0.70 ± 0.02 d | 0.58 ± 0.01 ab | 0.17 ± 0.02 cd | |
| T5 | 1.09 ± 0.01 ij | 0.63 ± 0.01 i | 0.58 ± 0.02 ab | 0.14 ± 0.01 e | |
| IL-12 | CK | 1.30 ± 0.01 c | 0.74 ± 0.01 a | 0.57 ± 0.01 b | 0.22 ± 0.01 a |
| T1 | 1.24 ± 0.02 d | 0.73 ± 0.02 b | 0.59 ± 0.01 a | 0.21 ± 0.02 a | |
| T2 | 1.23 ± 0.01 de | 0.70 ± 0.01 d | 0.57 ± 0.02 b | 0.20 ± 0.01 b | |
| T3 | 1.18 ± 0.01 h | 0.67 ± 0.02 f | 0.57 ± 0.03 b | 0.18 ± 0.01 c | |
| T4 | 1.20 ± 0.01 fg | 0.66 ± 0.01 g | 0.55 ± 0.02 c | 0.18 ± 0.02 c | |
| T5 | 1.10 ± 0.02 i | 0.60 ± 0.02 j | 0.55 ± 0.01 c | 0.15 ± 0.02 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yao, L.; Hao, J.; Li, C.; Li, B.; Meng, Y.; Ma, X.; Si, E.; Yang, K.; Zhang, H.; et al. Growth Properties and Metabolomic Analysis Provide Insight into Drought Tolerance in Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2024, 25, 7224. https://doi.org/10.3390/ijms25137224
Wang J, Yao L, Hao J, Li C, Li B, Meng Y, Ma X, Si E, Yang K, Zhang H, et al. Growth Properties and Metabolomic Analysis Provide Insight into Drought Tolerance in Barley (Hordeum vulgare L.). International Journal of Molecular Sciences. 2024; 25(13):7224. https://doi.org/10.3390/ijms25137224
Chicago/Turabian StyleWang, Juncheng, Lirong Yao, Jing Hao, Chengdao Li, Baochun Li, Yaxiong Meng, Xiaole Ma, Erjing Si, Ke Yang, Hong Zhang, and et al. 2024. "Growth Properties and Metabolomic Analysis Provide Insight into Drought Tolerance in Barley (Hordeum vulgare L.)" International Journal of Molecular Sciences 25, no. 13: 7224. https://doi.org/10.3390/ijms25137224
APA StyleWang, J., Yao, L., Hao, J., Li, C., Li, B., Meng, Y., Ma, X., Si, E., Yang, K., Zhang, H., Shang, X., & Wang, H. (2024). Growth Properties and Metabolomic Analysis Provide Insight into Drought Tolerance in Barley (Hordeum vulgare L.). International Journal of Molecular Sciences, 25(13), 7224. https://doi.org/10.3390/ijms25137224






