Oleanolic Acid Dimers with Potential Application in Medicine—Design, Synthesis, Physico-Chemical Characteristics, Cytotoxic and Antioxidant Activity
Abstract
:1. Introduction
2. Results
2.1. Synthesis of OADs
2.2. Structure–Activity Relationship Analysis
2.3. Cytotoxic Activity of the Obtained OADs 2a–2n
2.3.1. In Vitro Assay
2.3.2. Selectivity Index
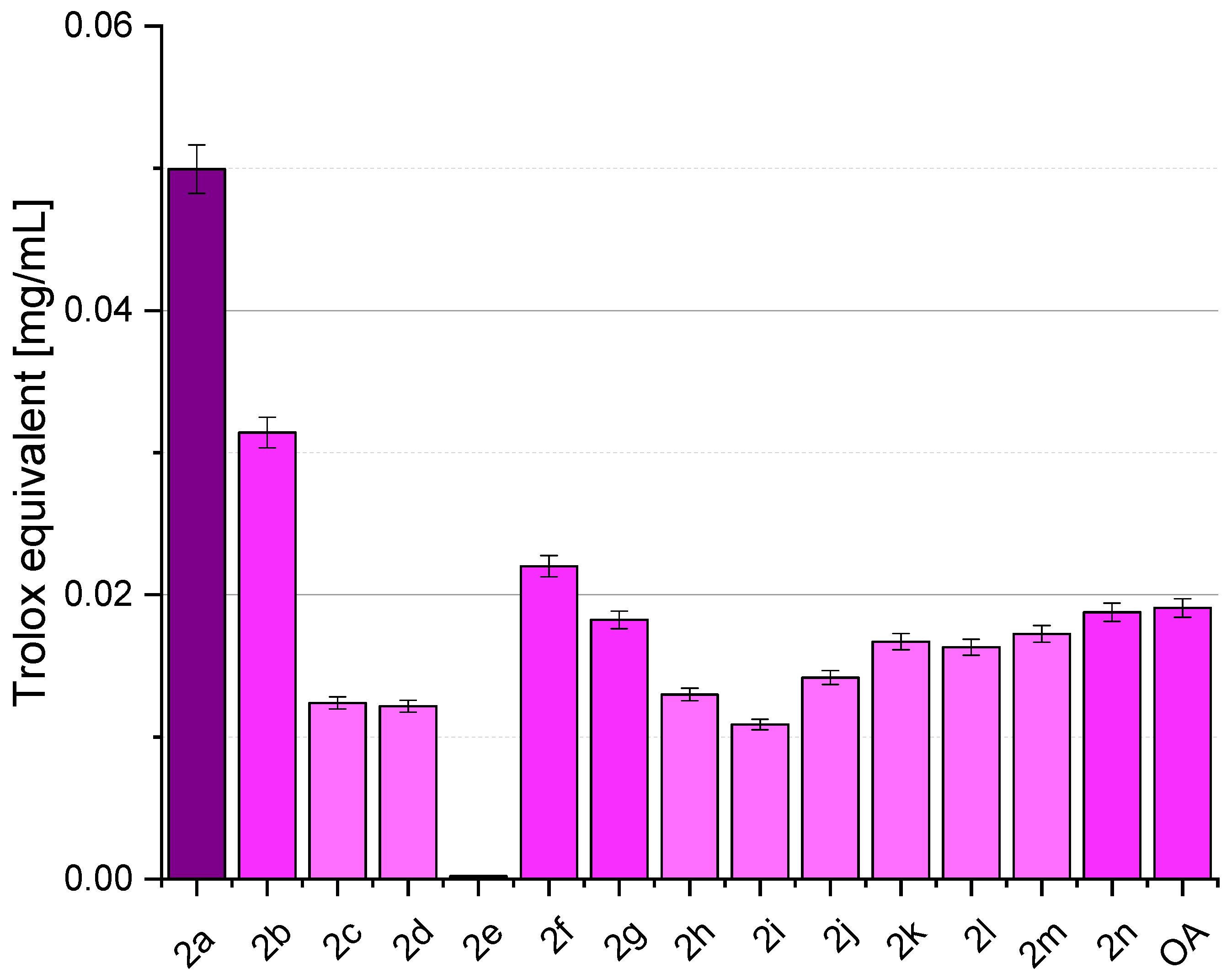
2.4. Antioxidant Activity
3. Discussion
3.1. Synthesis
3.1.1. Synthesis of Cytotoxic Agents 2a–2n
3.1.2. The Dependency between Linker Length and Melting Point
3.1.3. The Polarity of OADs 2a–2n Compared to Polarity of Oleanolic Acid (1)
3.1.4. Spectral Characterisation
- Spectral Characterisation of Dimer 2a (With One-Carbon Linker)
- Summary of IR Spectral Data of OADs (2a–2n)
- Summary of 1H NMR Spectral Data of OADs (2a–2n)
- Summary of 13C NMR Spectral Data of OADs (2a–2n)
3.2. SAR Analysis
3.3. Cytotoxic Activity
3.3.1. MTT Assay
3.3.2. Selectivity Index
3.4. Antioxidant Activity
4. Materials and Methods
4.1. General Information
4.2. Preparation of OADs
General Method for Dimerisation of Oleanolic Acid (1)
4.3. Polarity of OADs (2a–2n)
4.4. SAR Study
4.5. MTT Assay
4.6. Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirmala, M.J.; Samundeeswari, A.; Sankar, P.D. Natural plant resources in anti-cancer therapy—A review. Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Roaa, M.H.; Shoker, A. The Importance of the Major groups of Plants Secondary Metabolism Phenols, Alkaloids, and Terpenes. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 354–358. [Google Scholar] [CrossRef]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.F. A review on the presence of oleanolic acid in natural products. Nat. Proda Med. 2009, 2, 77–290. [Google Scholar]
- Min, B.S. Anticomplementary activity of oleanane-type triterpenes from the roots of Aceriphyllum rossii. Arch. Pharm. Res. 2012, 35, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.S.; Campos, B.L.S.; Laurenti, M.D.; Lago, J.H.G.; dos Santos Grecco, S.; Corbett, C.E.P.; Passero, L.F.D. Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mic. Parasit. Res. 2014, 113, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Balanehru, S.; Nagarajan, B. Protective effect of oleanolic acid and ursolic acid against lipid peroxidation. Biochem. Int. 1991, 24, 981–990. [Google Scholar]
- Kuo, R.Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1–7) upregulation. Biomed. Pharmacother. 2018, 97, 1694–1700. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef]
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2016, 31, 1085–1096. [Google Scholar] [CrossRef]
- Petronellia, A.; Pannitterib, G.; Testaa, U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef]
- Yan, S.L.; Huang, C.Y.; Wua, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Li, J.; Guo, W.J.; Yang, Q.Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J. Gastroenterol. 2002, 8, 493–495. [Google Scholar] [CrossRef]
- Linwei, W.U.; Qiang, P.U.; Xiaozhen, C.; Kaize, H.E. Inhibiting effect of oleanolic acid on ovarian carcinomas IGROV1 and breast cancer cell line MDAMB-231. Chin. J. App. Environ. Biol. 2010, 2, 202–204. [Google Scholar]
- Lucio, K.A.; da Graça Rocha, G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattas, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Zaprutko, L.; Ruszkowski, P.; Hładoń, B. Anti-cancer effect of A-ring or/and C-ring modified oleanolic acid derivatives on KB, MCF-7 and HeLa cell lines. Org. Biomol. Chem. 2012, 10, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Hładoń, T.; Zaprutko, L. Oleanolic acid A-lactams inhibit the growth of HeLa, KB, MCF-7 and Hep-G2 cancer cell lines at micromolar concentrations. Anticancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Atamanyuk, D.; Lesyk, R.; Zaprutko, Z. Hybrids of Oleanolic Acid with Norbornene-2,3-dicarboximide-N-carboxylic Acids as Potential Anticancer Agents. Acta Pol. Pharm. Drug Res. 2017, 74, 827–835. [Google Scholar]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jarosz, T.; Krukiewicz, K. Enhancing anticancer activity through the combination of bioreducing agents and triterpenes. Future Med. Chem. 2018, 10, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Ruszkowski, P. Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies. Pharmaceutics 2024, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.L.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe 579 tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Oxidized LDL and atherogenesis. Ann. N. Y. Acad. Sci. 1999, 874, 134–137. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant Activity of Maslinic Acid, a Triterpene Derivative Obtained from Olea europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [CrossRef]
- Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules 2021, 26, 3435. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathogen. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Kalra, N.; Singh, M.; Shukla, Y. Protective effects of lupeol and mango extract against androgen induced oxidative stress in Swiss albino mice. Asian J. Androl. 2008, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.G.; Su, C.H.; Yang, L.D.; Liu, J.; Chen, Z.F. Synthesis of oleanolic acid dimers linked at C-28 and evaluation of anti-tumor activity. Eur. J. Med. Chem. 2015, 89, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Sasikuma, K.; Dubey, V.; Ghosh, A.R. Oleanolic acid from black raisins, Vitis vinifera with antioxidant and antiproliferative potentials on HCT 116 colon cancer cell line. Braz. J. Pharm. Sci. 2020, 56, e17158. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinform. Appl. Note 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Sukandar, E.R.; Kaennakam, S.; Raab, P.; Nöst, X.; Rassamee, K.; Bauer, R.; Siripong, P.; Ersam, T.; Tip-pyang, S.; Chavasiri, W. Cytotoxic and Anti-Inflammatory Activities of Dihydroisocoumarin and Xanthone Derivatives from Garcinia picrorhiza. Molecules 2021, 26, 6626. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Calderon, P.B.; Muccioli, G.G. Synthesis and cytotoxic activity on human cancer cells of novel isoquinolinequinone-amino acid derivatives. Molecules 2016, 21, 1199. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for Reducing or Preventing the Generation of Oxidative Stress. Oxid. Med. Cell. Longev. 2011, 2011, e194586. [Google Scholar] [CrossRef]
- Kamaljeet; Singh, S.; Gupta, G.D.; Aran, K.R. Emerging role of antioxidants in Alzheimer’s disease: Insight into physiological, pathological mechanisms and management. Pharm. Sci. Adv. 2024, 2, 100021–100030. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef] [PubMed]





| Activity | Pa Factor (and Pi Factor) of Compounds 1 and 2a–2n | |||||||
|---|---|---|---|---|---|---|---|---|
| OA (1) | 2a | 2b | 2c | 2d | 2e | 2f | 2g | |
| Apoptosis agonist | 0.901 (0.004) | 0.905 (0.004) | 0.880 (0.005) | 0.880 (0.005) | 0.867 (0.005) | 0.873 (0.005) | 0.873 (0.005) | 0.865 (0.005) |
| Caspase 3 stimulant | 0.984 (0.002) | 0.962 (0.003) | 0.978 (0.002) | 0.978 (0.002) | 0.965 (0.002) | 0.961 (0.003) | 0.961 (0.003) | 0.965 (0.002) |
| Chemopreventive | 0.937 (0.002) | 0.914 (0.002) | 0.895 (0.002) | 0.895 (0.002) | 0.886 (0.003) | 0.927 (0.002) | 0.927 (0.002) | 0.887 (0.003) |
| Insulin promoter | 0.869 (0.004) | 0.962 (0.002) | 0.978 (0.001) | 0.978 (0.001) | 0.974 (0.001) | 0.972 (0.001) | 0.972 (0.001) | 0.973 (0.001) |
| Lipid metabolism regulator | <0.700 | <0.700 | <0.700 | <0.700 | 0.902 (0.004) | 0.906 (0.004) | 0.906 (0.004) | 0.918 (0.003) |
| Lipid peroxidase inhibitor | 0.810 (0.003) | 0.852 (0.003) | 0.902 (0.004) | 0.902 (0.004) | 0.902 (0.004) | 0.702 (0.005) | 0.702 (0.005) | 0.782 (0.004) |
| Membrane integrity antagonist | 0.928 (0.002) | 0.884 (0.003) | 0.930 (0.002) | 0.930 (0.002) | 0.934 (0.001) | 0.904 (0.003) | 0.904 (0.003) | 0.937 (0.001) |
| Oxidoreductase inhibitor | 0.904 (0.002) | 0.866 (0.003) | 0.897 (0.002) | 0.897 (0.002) | 0.897 (0.002) | 0.906 (0.002) | 0.906 (0.002) | 0.900 (0.002) |
| Transcription factor NF kappa B stimulant | 0.954 (0.001) | 0.930 (0.001) | 0.935 (0.001) | 0.935 (0.001) | 0.930 (0.001) | 0.930 (0.001) | 0.930 (0.001) | 0.927 (0.001) |
| Transcription factor stimulant | 0.954 (0.001) | 0.934 (0.001) | 0.935 (0.001) | 0.935 (0.001) | 0.930 (0.001) | 0.930 (0.001) | 0.930 (0.001) | 0.927 (0.001) |
| Activity | Pa Factor (and Pi Factor) of Compounds 1 and 2a–2n | ||||||
|---|---|---|---|---|---|---|---|
| 2h | 2i | 2j | 2k | 2l | 2m | 2n | |
| Apoptosis agonist | 0.865 (0.005) | 0.865 (0.005) | 0.865 (0.005) | 0.865 (0.005) | 0.865 (0.005) | 0.865 (0.005) | 0.865 (0.005) |
| Caspase 3 stimulant | 0.965 (0.002) | 0.965 (0.002) | 0.965 (0.002) | 0.965 (0.002) | 0.965 (0.002) | 0.965 (0.002) | 0.965 (0.002) |
| Chemopreventive | 0.887 (0.003) | 0.887 (0.003) | 0.887 (0.003) | 0.887 (0.003) | 0.887 (0.003) | 0.887 (0.003) | 0.887 (0.003) |
| Insulin promotor | 0.973 (0.001) | 0.973 (0.001) | 0.973 (0.001) | 0.973 (0.001) | 0.973 (0.001) | 0.973 (0.001) | 0.973 (0.001) |
| Lipid metabolism regulator | 0.918 (0.003) | 0.918 (0.003) | 0.918 (0.003) | 0.918 (0.003) | 0.918 (0.003) | 0.918 (0.003) | 0.918 (0.003) |
| Lipid peroxidase inhibitor | 0.782 (0.004) | 0.782 (0.004) | 0.782 (0.004) | 0.782 (0.004) | 0.782 (0.004) | 0.782 (0.004) | 0.782 (0.004) |
| Membrane integrity antagonist | 0.937 (0.001) | 0.937 (0.001) | 0.937 (0.001) | 0.937 (0.001) | 0.937 (0.001) | 0.937 (0.001) | 0.937 (0.001) |
| Oxidoreductase inhibitor | 0.900 (0.002) | 0.900 (0.002) | 0.900 (0.002) | 0.900 (0.002) | 0.900 (0.002) | 0.900 (0.002) | 0.900 (0.002) |
| Transcription factor NF kappa B stimulant | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) |
| Transcription factor stimulant | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) | 0.927 (0.001) |

| Number of C Atoms in a Linker | Comp. No. | IC50 (SD), μM | ||||
|---|---|---|---|---|---|---|
| SKBR-3 | SKOV-3 | PC-3 | U-87 | HDF | ||
| 0 | 1 (OA) | 19.62 (0.02) | 18.81 (0.09) | 18.63 (0.05) | 18.15 (0.01) | 24.87 (0.04) |
| 1 | 2a | 3.96 (0.09) | 3.89 (0.01) | 3.38 (0.02) | 3.32 (0.08) | 6.56 (0.08) |
| 2 sat. | 2b | 6.67 (0.11) | 6.49 (0.01) | 6.43 (0.03) | 6.59 (0.01) | 11.20 (0.03) |
| 3 sat. | 2c | 7.64 (0.19) | 8.22 (0.01) | 7.40 (0.09) | 7.46 (0.02) | 18.94 (0.06) |
| 4 sat. | 2d | 1.12 (0.03) | 1.56 (0.01) | 1.64 (0.01) | 1.20 (0.11) | 2.95 (0.01) |
| 4 unsat. cis | 2e | 2.61 (0.01) | 2.09 (0.03) | 2.27 (0.01) | 2.74 (0.09) | 2.68 (0.01) |
| 4 unsat. trans | 2f | 5.09 (0.06) | 4.79 (0.08) | 5.11 (0.01) | 4.91 (0.05) | 2.78 (0.04) |
| 5 sat. | 2g | 5.21 (0.15) | 5.16 (0.11) | 5.80 (0.04) | 5.32 (0.06) | 3.37 (0.03) |
| 6 sat. | 2h | 6.02 (0.05) | 5.39 (0.02) | 5.34 (0.07) | 5.87 (0.09) | 17.31 (0.08) |
| 7 sat. | 2i | 3.46 (0.09) | 3.68 (0.04) | 3.16 (0.08) | 3.06 (0.03) | 5.39 (0.07) |
| 8 sat. | 2j | 9.99 (0.04) | 10.27 (0.05) | 9.81 (0.02) | 10.68 (0.04) | 15.96 (0.09) |
| 9 sat. | 2k | 6.93 (0.03) | 6.89 (0.01) | 6.93 (0.05) | 7.34 (0.09) | 16.82 (0.09) |
| 10 sat. | 2l | 18.71 (0.11) | 18.37 (0.08) | 18.37 (0.07) | 18.71 (0.03) | 36.27 (0.07) |
| 11 sat. | 2m | 3.93 (0.09) | 4.58 (0.01) | 3.91 (0.06) | 4.25 (0.04) | 7.43 (0.16) |
| 12 sat. | 2n | 6.79 (0.25) | 7.41 (0.12) | 6.96 (0.04) | 6.51 (0.01) | 7.30 (0.61) |

| Number of C Atoms in a Linker | Comp. No. | SI | |||
|---|---|---|---|---|---|
| SKBR-3 | SKOV-3 | PC-3 | U-87 | ||
| 0 | 1 (OA) | 1.27 | 1.32 | 1.33 | 1.37 |
| 1 | 2a | 1.66 | 1.69 | 1.94 | 1.97 |
| 2 sat. | 2b | 1.68 | 1.72 | 1.74 | 1.70 |
| 3 sat. | 2c | 2.48 | 2.30 | 2.56 | 2.54 |
| 4 sat. | 2d | 2.63 | 1.89 | 1.80 | 2.46 |
| 4 unsat. cis | 2e | 1.03 | 1.28 | 1.18 | 0.98 |
| 4 unsat. trans | 2f | 0.54 | 0.58 | 0.54 | 0.57 |
| 5 sat. | 2g | 0.65 | 0.65 | 0.58 | 0.63 |
| 6 sat. | 2h | 2.87 | 3.21 | 3.24 | 2.95 |
| 7 sat. | 2i | 1.57 | 1.46 | 1.70 | 1.76 |
| 8 sat. | 2j | 1.60 | 1.55 | 1.63 | 1.49 |
| 9 sat. | 2k | 2.43 | 2.44 | 2.43 | 2.29 |
| 10 sat. | 2l | 1.94 | 1.97 | 1.97 | 1.94 |
| 11 sat. | 2m | 1.89 | 1.62 | 1.90 | 1.75 |
| 12 sat. | 2n | 1.07 | 0.98 | 1.05 | 1.12 |

| Number of C Atoms in a Linker | Comp. No. | Rf Value in C6H6:AcOEt (v:v) | ||||||
|---|---|---|---|---|---|---|---|---|
| AcOEt | 1:1 | 2:1 | 4:1 | 9:1 | 15:1 | 25:1 | ||
| 0 | 1 (OA) | 0.86 | 0.77 | 0.62 | 0.29 | 0.16 | --- | --- |
| 1 | 2a | 085 | 0.74 | 0.55 | 0.22 | 0.13 | --- | --- |
| 2 sat. | 2b | 0.85 | 0.69 | 0.51 | 0.18 | 0.08 | --- | --- |
| 3 sat | 2c | 0.85 | 0.71 | 0.52 | 0.18 | 0.10 | --- | --- |
| 4 sat. | 2d | 0.86 | 0.71 | 0.52 | 0.19 | 0.09 | --- | --- |
| 4 unsat. cis | 2e | 0.86 | 0.71 | 0.53 | 0.19 | 0.09 | --- | --- |
| 4 unsat. trans | 2f | 0.87 | 0.73 | 0.53 | 0.21 | 0.10 | --- | --- |
| 5 sat. | 2g | 0.87 | 0.73 | 0.54 | 0.22 | 0.11 | --- | --- |
| 6 sat. | 2h | 0.87 | 0.74 | 0.55 | 0.26 | 0.11 | --- | --- |
| 7 sat. | 2i | 0.86 | 0.76 | 0.55 | 0.24 | 0.10 | --- | --- |
| 8 sat. | 2j | 0.91 | 0.81 | 0.58 | 0.21 | 0.08 | --- | --- |
| 9 sat. | 2k | 0.89 | 0.77 | 0.60 | 0.28 | 0.13 | --- | --- |
| 10 sat. | 2l | 0.89 | 0.78 | 0.63 | 0.30 | 0.15 | --- | --- |
| 11 sat. | 2m | 0.90 | 0.81 | 0.64 | 0.32 | 0.15 | --- | --- |
| 12 sat. | 2n | 0.87 | 0.82 | 0.64 | 0.33 | 0.17 | --- | --- |

| Number of C Atoms in a Linker | Comp. No. | Wavenumber Value, ν [cm−1] | ||
|---|---|---|---|---|
| -OH | C=O | C-O- | ||
| 0 | 1 (OA) | 3446 * | 1687 * | 1452 * |
| 1 | 2a | 3556.01–3205.29 | 1748.60 | 1467.60 |
| 2 sat. | 2b | 3553.84–3203.87 | 1736.21 | 1466.29 |
| 3 sat. | 2c | 3553.84–3203.87 | 1728.25 | 1462.19 |
| 4 sat. | 2d | 3556.00–3201.82 | 1728.25 | 1462.19 |
| 4 unsat. cis | 2e | 3551.81–3207.99 | 1726.31 | 1462.47 |
| 4 unsat. trans | 2f | 3550.46–3208.67 | 1726.32 | 1462.54 |
| 5 sat. | 2g | 3554.18–3203.87 | 1726.32 | 1462.19 |
| 6 sat. | 2h | 3557.89–3208.67 | 1726.32 | 1462.54 |
| 7 sat. | 2i | 3557.89–3208.67 | 1726.21 | 1463.83 |
| 8 sat. | 2j | 3557.89–3204.95 | 1728.25 | 1462.54 |
| 9 sat. | 2k | 3553.84–3201.24 | 1726.16 | 1462.19 |
| 10 sat. | 2l | 3554.18–3201.24 | 1726.32 | 1462.25 |
| 11 sat. | 2m | 3554.02–3202.91 | 1726.34 | 1462.89 |
| 12 sat. | 2n | 3553.93–3202.75 | 1726.77 | 1463.01 |

| Number of C Atoms in a Linker | Comp. No. | Chemical Shift, δ [ppm] (Multiplicity, J [Hz]) | |||
|---|---|---|---|---|---|
| C12-H | linker | C3-Hα | C18-Hβ | ||
| 0 | 1 (OA) | 5.27 (t, n.d.) * | --- | 3.18 (dd, 11.0, 5.0) * | 2.85 (dd, 14.0, 4.0) * |
| 1 | 2a | 5.29 (t, 3.7) | 5.74 (s) | 3.21 (dd, 10.4, 5.0) | 2.83 (dd, 13.7, 4.8) |
| 2 sat. | 2b | 5.29 (t, 3.5) | 4.30–4.10 (m) | 3.21 (dd, 10.3, 4.9) | 2.86 (dd, 13.6, 3.7) |
| 3 sat. | 2c | 5.28 (t, 3.3) | 4.09 (t, 6.2) | 3.21 (dd, 11.1, 4.7) | 2.85 (dd, 13.7, 3.9) |
| 4 sat. | 2d | 5.28 (t, 3.7) | 4.30–4.08 (m) | 3.21 (dd, 10.4, 4.8) | 2.86 (dd, 13.7, 4.8) |
| 4 unsat. cis | 2e | 5.28 (t, 3.5) | 4.63 (ddd, 17.8, 13.0, 4.3) | 3.21 (dd, 11.1, 4.8) | 2.85 (dd, 13.8, 4.0) |
| 4 unsat. trans | 2f | 5.30 (t, 3.4) | 4.45 (t, 13.9) | 3.16 (dd, 11.5, 3.9) | 2.77 (dd, 14.1, 4.1) |
| 5 sat. | 2g | 5.29 (t, 3.6) | 4.07 (t, 6.5) | 3.21 (dd, 11.2, 4.6) | 2.86 (dd, 13.6, 4.1) |
| 6 sat. | 2h | 5.28 (t, 3.6) | 4.01 (t, 6.5) | 3.21 (dd, 10.5, 5.0) | 2.87 (dd, 13.7, 3.9) |
| 7 sat. | 2i | 5.29 (t, 3.5) | 4.10–3.94 (m) | 3.22 (dd, 11.3, 4.3) | 2.87 (dd, 14.0, 4.7) |
| 8 sat. | 2j | 5.29 (t, 3.7) | 4.02 (td, 6.5, 2.0) | 3.25 (dd, 10.8, 4.6) | 2.89 (dd, 13.7, 3.8) |
| 9 sat. | 2k | 5.29 (t, 3.2) | 4.07–3.95 (m) | 3.22 (dd, 10.9, 4.6) | 2.87 (dd, 13.9, 3.7) |
| 10 sat. | 2l | 5.30 (t, 3.4) | 4.10–3.93 (m) | 3.22 (dd, 11.2, 4.0) | 2.88 (dd, 13.8, 4.1) |
| 11 sat. | 2m | 5.28 (t, 3.4) | 4.00 (td, 6.4, 3.1) | 3.21 (dd, 11.0, 4.6) | 2.87 (dd, 13.8, 4.1) |
| 12 sat. | 2n | 5.28 (t, 3.5) | 4.06–3.94 (m) | 3.21 (dd, 11.0, 4.6 | 2.87 (dd, 14.3, 3.7) |

| Number of C Atoms in a Linker | Comp. No. | Chemical Shift, δ [ppm] | |||||
|---|---|---|---|---|---|---|---|
| C-28 | C-13 | C-12 | linker | C-3 | C-17 | ||
| 0 | OA | 180.4 * | 143.79 * | 122.25 * | --- | 78.31 * | 45.85 * |
| 1 | 2a | 176.30 | 143.29 | 122.58 | 79.32 | 78.95 | 46.71 |
| 2 sat. | 2b | 177.44 | 143.51 | 122.46 | 62.15 | 78.92 | 46.63 |
| 3 sat. | 2c | 177.34 | 143.61 | 122.20 | 60.76 | 78.87 | 46.65 |
| 4 sat. | 2d | 177.62 | 143.74 | 122.36 | 63.65 | 78.91 | 46.66 |
| 4 unsat. cis | 2e | 177.29 | 143.58 | 122.38 | 59.76 | 78.93 | 46.61 |
| 4 unsat. trans | 2f | 177.33 | 143.67 | 122.39 | 63.72 | 78.89 | 46.76 |
| 5 sat. | 2g | 177.64 | 143.70 | 122.29 | 63.94 | 78.89 | 46.59 |
| 6 sat. | 2h | 177.69 | 144.01 | 122.29 | 64.15 | 78.82 | 46.63 |
| 7 sat. | 2i | 177.64 | 143.57 | 122.21 | 64.05 | 78.97 | 46.67 |
| 8 sat. | 2j | 177.77 | 143.86 | 122.34 | 64.23 | 79.01 | 46.68 |
| 9 sat. | 2k | 177.76 | 143.82 | 122.32 | 64.24 | 78.96 | 46.66 |
| 10 sat. | 2l | 177.77 | 143.83 | 122.32 | 64.25 | 78.99 | 46.66 |
| 11 sat. | 2m | 177.74 | 143.77 | 122.24 | 64.21 | 78.91 | 46.62 |
| 12 sat. | 2n | 177.72 | 143.75 | 122.25 | 64.20 | 78.89 | 46.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, A.; Zalewski, P.; Sip, S.; Ruszkowski, P.; Bednarczyk-Cwynar, B. Oleanolic Acid Dimers with Potential Application in Medicine—Design, Synthesis, Physico-Chemical Characteristics, Cytotoxic and Antioxidant Activity. Int. J. Mol. Sci. 2024, 25, 6989. https://doi.org/10.3390/ijms25136989
Günther A, Zalewski P, Sip S, Ruszkowski P, Bednarczyk-Cwynar B. Oleanolic Acid Dimers with Potential Application in Medicine—Design, Synthesis, Physico-Chemical Characteristics, Cytotoxic and Antioxidant Activity. International Journal of Molecular Sciences. 2024; 25(13):6989. https://doi.org/10.3390/ijms25136989
Chicago/Turabian StyleGünther, Andrzej, Przemysław Zalewski, Szymon Sip, Piotr Ruszkowski, and Barbara Bednarczyk-Cwynar. 2024. "Oleanolic Acid Dimers with Potential Application in Medicine—Design, Synthesis, Physico-Chemical Characteristics, Cytotoxic and Antioxidant Activity" International Journal of Molecular Sciences 25, no. 13: 6989. https://doi.org/10.3390/ijms25136989
APA StyleGünther, A., Zalewski, P., Sip, S., Ruszkowski, P., & Bednarczyk-Cwynar, B. (2024). Oleanolic Acid Dimers with Potential Application in Medicine—Design, Synthesis, Physico-Chemical Characteristics, Cytotoxic and Antioxidant Activity. International Journal of Molecular Sciences, 25(13), 6989. https://doi.org/10.3390/ijms25136989











