Systemic Pharmacotherapeutic Treatment of the ACTA1-MCM/FLExDUX4 Preclinical Mouse Model of FSHD
Abstract
1. Introduction
2. Results
2.1. Berberine Treatment Has Minimal Effect on Muscle Mass and Other Muscle Functions but Improves Forelimb Muscle Strength
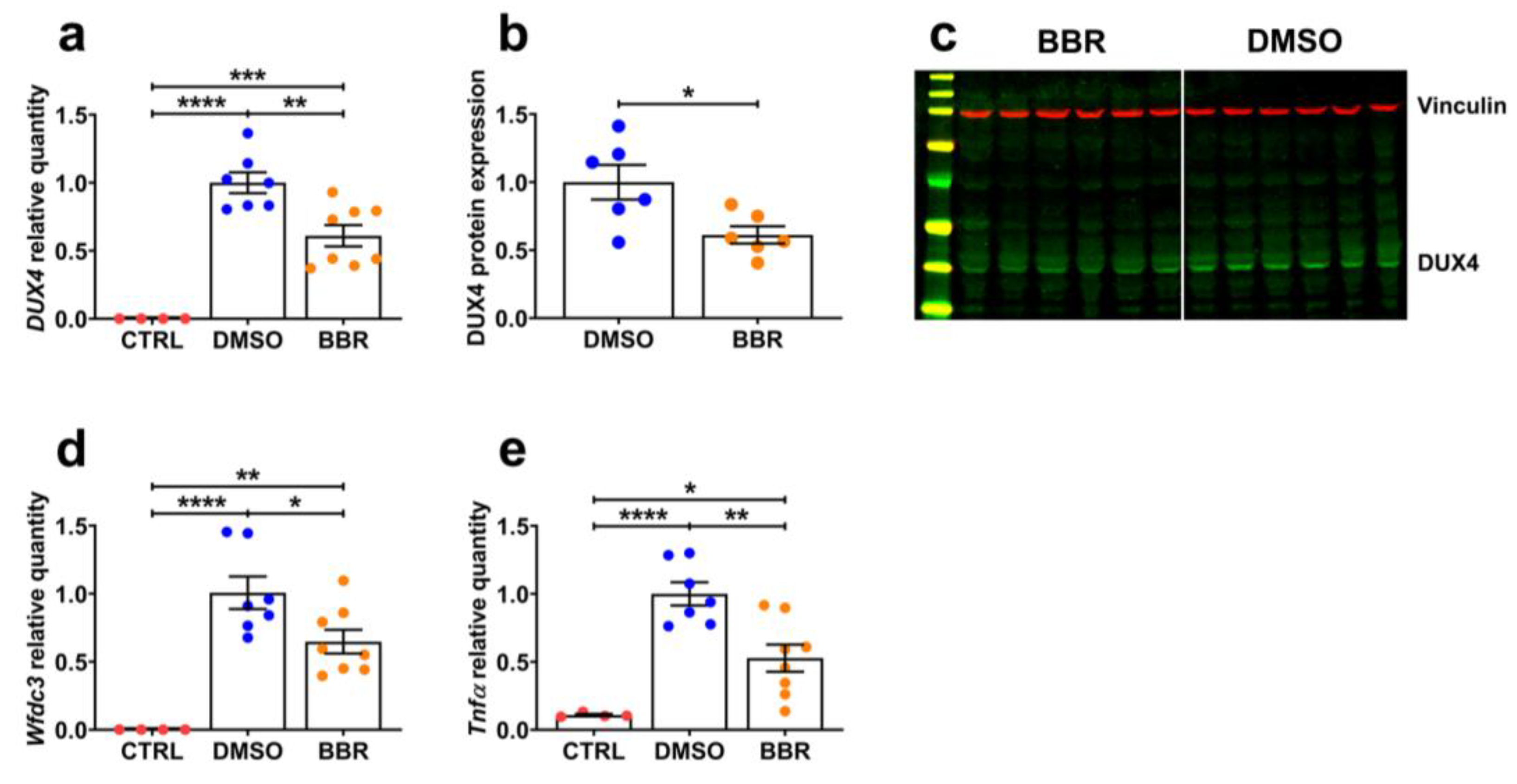
2.2. BBR Treatment Efficiently Inhibits mRNA and Protein Expression of DUX4 and Its Downstream Targets
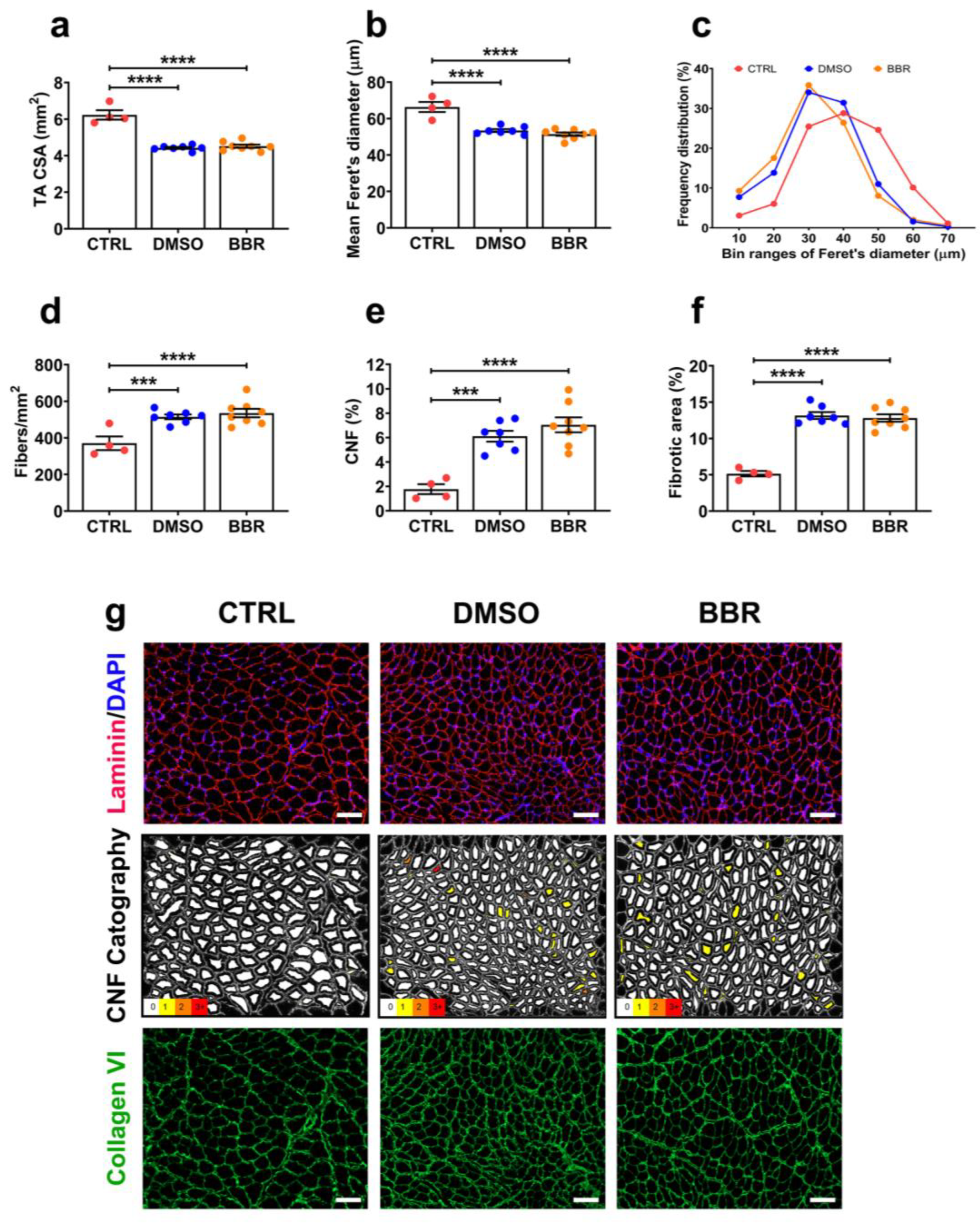
2.3. Insufficient Improvement in TA Muscle Histopathology following BBR Administration
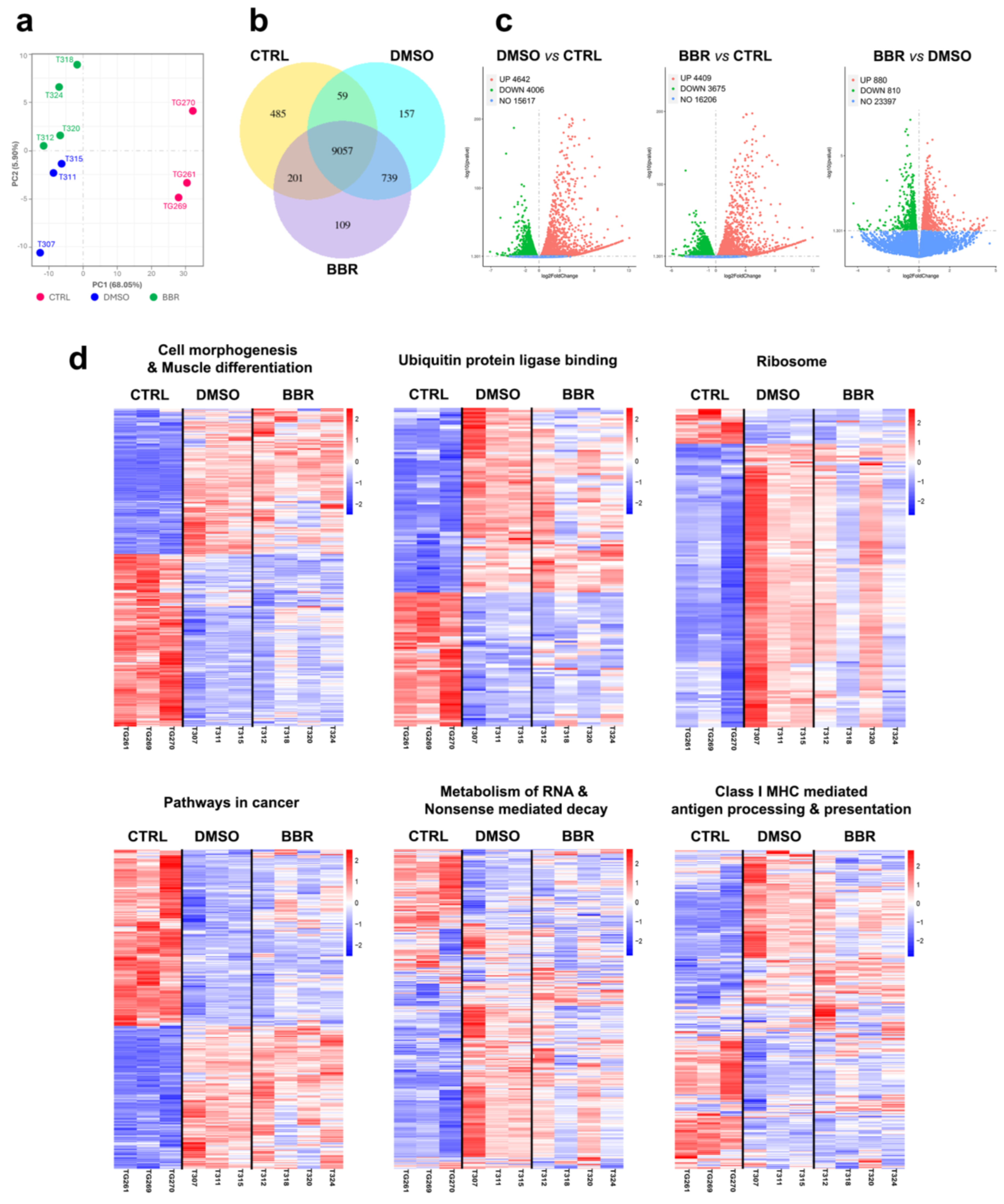
2.4. BBR Treatment Positively Modifies Important DUX4-Related Cellular Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. Functional Tests
4.4. Post-Mortem Tissue Processing
4.5. RNA Extraction, cDNA Synthesis, and RT-qPCR Quantification for DUX4 and Relevant Genes
4.6. Protein Extraction, Protein Assay, and DUX4 Immunoblotting
4.7. Immunohistochemistry
4.8. Histological Analyses
4.9. RNA-seq Analyses
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Maarel, S.M.; Miller, D.G.; Tawil, R.; Filippova, G.N.; Tapscott, S.J. Facioscapulohumeral Muscular Dystrophy: Consequences of Chromatin Relaxation. Curr. Opin. Neurol. 2012, 25, 614–620. [Google Scholar] [CrossRef] [PubMed]
- van der Maarel, S.M.; Tawil, R.; Tapscott, S.J. Facioscapulohumeral Muscular Dystrophy and DUX4: Breaking the Silence. Trends Mol. Med. 2011, 17, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Van Overveld, P.G.M.; Lemmers, R.J.F.L.; Sandkuijl, L.A.; Enthoven, L.; Winokur, S.T.; Bakels, F.; Padberg, G.W.; Van Ommen, G.J.B.; Frants, R.R.; Van Der Maarel, S.M. Hypomethylation of D4Z4 in 4q-Linked and Non-4q-Linked Facioscapulohumeral Muscular Dystrophy. Nat. Genet. 2003, 35, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, R.J.L.F.; Tawil, R.; Petek, L.M.; Balog, J.; Block, G.J.; Santen, G.W.E.; Amell, A.M.; van der Vliet, P.J.; Almomani, R.; Straasheijm, K.R.; et al. Digenic Inheritance of an SMCHD1 Mutation and an FSHD-Permissive D4Z4 Allele Causes Facioscapulohumeral Muscular Dystrophy Type 2. Nat. Genet. 2012, 44, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, M.L.; Lemmers, R.J.L.F.; Balog, J.; Wohlgemuth, M.; Auranen, M.; Mitsuhashi, S.; van der Vliet, P.J.; Straasheijm, K.R.; van den Akker, R.F.P.; Kriek, M.; et al. Mutations in DNMT3B Modify Epigenetic Repression of the D4Z4 Repeat and the Penetrance of Facioscapulohumeral Dystrophy. Am. J. Hum. Genet. 2016, 98, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Snider, L.; Balog, J.; Lemmers, R.J.L.F.; Van Der Maarel, S.M.; Tawil, R.; Tapscott, S.J. DUX4-Induced Gene Expression Is the Major Molecular Signature in FSHD Skeletal Muscle. Hum. Mol. Genet. 2014, 23, 5342–5352. [Google Scholar] [CrossRef] [PubMed]
- Kowaljow, V.; Marcowycz, A.; Ansseau, E.; Conde, C.B.; Sauvage, S.; Mattéotti, C.; Arias, C.; Corona, E.D.; Nuñez, N.G.; Leo, O.; et al. The DUX4 Gene at the FSHD1A Locus Encodes a Pro-Apoptotic Protein. Neuromuscul. Disord. 2007, 17, 611–623. [Google Scholar] [CrossRef]
- Wallace, L.M.; Garwick, S.E.; Mei, W.; Belayew, A.; Coppee, F.; Ladner, K.J.; Guttridge, D.; Yang, J.; Harper, S.Q. DUX4, a Candidate Gene for Facioscapulohumeral Muscular Dystrophy, Causes P53-Dependent Myopathy in Vivo. Ann. Neurol. 2011, 69, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Tassin, A.; Laoudj-Chenivesse, D.; Vanderplanck, C.; Barro, M.; Charron, S.; Ansseau, E.; Chen, Y.W.; Mercier, J.; Coppée, F.; Belayew, A. DUX4 Expression in FSHD Muscle Cells: How Could Such a Rare Protein Cause a Myopathy? J. Cell. Mol. Med. 2013, 17, 76–89. [Google Scholar] [CrossRef]
- Daxinger, L.; Tapscott, S.J.; van der Maarel, S.M. Genetic and Epigenetic Contributors to FSHD. Curr. Opin. Genet. Dev. 2015, 33, 56–61. [Google Scholar] [CrossRef]
- Lemmers, R.J.L.F.; Goeman, J.J.; van der Vliet, P.J.; van Nieuwenhuizen, M.P.; Balog, J.; Vos-Versteeg, M.; Camano, P.; Ramos Arroyo, M.A.; Jerico, I.; Rogers, M.T.; et al. Inter-Individual Differences in CpG Methylation at D4Z4 Correlate with Clinical Variability in FSHD1 and FSHD2. Hum. Mol. Genet. 2015, 24, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.E.; Lyle, R.; Clark, L.N.; Valleley, E.M.; Wright, T.J.; Wijmenga, C.; Van Deutekom, J.C.; Francis, F.; Sharpe, P.T.; Hofker, M.; et al. Analysis of the Tandem Repeat Locus D4Z4 Associated with Facioscapulohumeral Muscular Dystropothhy. Hum. Mol. Genet. 1994, 3, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Thijssen, P.E.; de Greef, J.C.; Shah, B.; van Engelen, B.G.M.; Yokomori, K.; Tapscott, S.J.; Tawil, R.; van der Maarel, S.M. Correlation Analysis of Clinical Parameters with Epigenetic Modifications in the DUX4 Promoter in FSHD. Epigenetics 2012, 7, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Tsumagari, K.; Qi, L.; Jackson, K.; Shao, C.; Lacey, M.; Sowden, J.; Tawil, R.; Vedanarayanan, V.; Ehrlich, M. Epigenetics of a Tandem DNA Repeat: Chromatin DNaseI Sensitivity and Opposite Methylation Changes in Cancers. Nucleic Acids Res. 2008, 36, 2196–2207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guilbaud, G.; Murat, P.; Recolin, B.; Campbell, B.C.; Maiter, A.; Sale, J.E.; Balasubramanian, S. Local Epigenetic Reprogramming Induced by G-Quadruplex Ligands. Nat. Chem. 2017, 9, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, Topology and Structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.J.; Hurley, L.H. Direct Evidence for a G-Quadruplex in a Promoter Region and Its Targeting with a Small Molecule to Repress c-MYC Transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef] [PubMed]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-Throughput Sequencing of DNA G-Quadruplex Structures in the Human Genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef]
- Rhodes, D.; Lipps, H.J. G-Quadruplexes and Their Regulatory Roles in Biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef]
- Ciszewski, L.; Lu-Nguyen, N.; Slater, A.; Brennan, A.; Williams, H.E.L.; Dickson, G.; Searle, M.S.; Popplewell, L. G-Quadruplex Ligands Mediate Downregulation of DUX4 Expression. Nucleic Acids Res. 2020, 48, 4179–4194. [Google Scholar] [CrossRef]
- Jones, T.; Jones, P.L. A Cre-Inducible DUX4 Transgenic Mouse Model for Investigating Facioscapulohumeral Muscular Dystrophy. PLoS ONE 2018, 13, e0192657. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.I.; Chew, G.-L.; Barraza-Flores, P.; Schreier, S.; Ramirez, M.; Wuebbles, R.D.; Burkin, D.J.; Bradley, R.K.; Jones, P.L. Transgenic Mice Expressing Tunable Levels of DUX4 Develop Characteristic Facioscapulohumeral Muscular Dystrophy-like Pathophysiology Ranging in Severity. Skelet. Muscle 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.Y.; Al-Kharsan, M.; Garwick-Coppens, S.E.; Chermahini, G.A.; Harper, M.A.; Palo, A.; Boudreau, R.L.; Harper, S.Q. Human MiRNA MiR-675 Inhibits DUX4 Expression and May Be Exploited as a Potential Treatment for Facioscapulohumeral Muscular Dystrophy. Nat. Commun. 2021, 12, 7128. [Google Scholar] [CrossRef] [PubMed]
- Himeda, C.L.; Jones, T.I.; Jones, P.L. Targeted Epigenetic Repression by CRISPR/DSaCas9 Suppresses Pathogenic DUX4-Fl Expression in FSHD. Mol. Ther. Methods Clin. Dev. 2021, 20, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Bittel, A.; Maruyama, R.; Echigoya, Y.; Nguyen, Q.; Huang, Y.; Dzierlega, K.; Zhang, A.; Chen, Y.-W.; Yokota, T. DUX4 Transcript Knockdown with Antisense 2′-O-Methoxyethyl Gapmers for the Treatment of Facioscapulohumeral Muscular Dystrophy. Mol. Ther. 2021, 29, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Lu-Nguyen, N.; Malerba, A.; Herath, S.; Dickson, G.; Popplewell, L. Systemic Antisense Therapeutics Inhibiting DUX4 Expression Ameliorates FSHD-like Pathology in an FSHD Mouse Model. Hum. Mol. Genet. 2021, 30, 1398–1412. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Henderson, D.; Tawil, R.N.; Zammit, P.S. Skeletal Muscle Regeneration in Facioscapulohumeral Muscular Dystrophy Is Correlated with Pathological Severity. Hum. Mol. Genet. 2020, 29, 2746–2760. [Google Scholar] [CrossRef]
- McGeachie, J.K.; Grounds, M.D.; Partridge, T.A.; Morgan, J.E. Age-Related Changes in Replication of Myogenic Cells in Mdx Mice: Quantitative Autoradiographic Studies. J. Neurol. Sci. 1993, 119, 169–179. [Google Scholar] [CrossRef]
- Lassche, S.; Küsters, B.; Heerschap, A.; Schyns, M.V.P.; Ottenheijm, C.A.C.; Voermans, N.C.; van Engelen, B.G.M. Correlation Between Quantitative MRI and Muscle Histopathology in Muscle Biopsies from Healthy Controls and Patients with IBM, FSHD and OPMD. J. Neuromuscul. Dis. 2020, 7, 495–504. [Google Scholar] [CrossRef]
- Boldrin, L.; Ross, J.A.; Whitmore, C.; Doreste, B.; Beaver, C.; Eddaoudi, A.; Pearce, D.J.; Morgan, J.E. The Effect of Calorie Restriction on Mouse Skeletal Muscle Is Sex, Strain and Time-Dependent. Sci. Rep. 2017, 7, 5160. [Google Scholar] [CrossRef]
- Bouwman, L.F.; den Hamer, B.; van den Heuvel, A.; Franken, M.; Jackson, M.; Dwyer, C.A.; Tapscott, S.J.; Rigo, F.; van der Maarel, S.M.; de Greef, J.C. Systemic Delivery of a DUX4-Targeting Antisense Oligonucleotide to Treat Facioscapulohumeral Muscular Dystrophy. Mol. Ther. Nucleic Acids 2021, 26, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Homma, S.; Beermann, M.L.; Boyce, F.M.; Miller, J.B. Expression of FSHD-Related DUX4-FL Alters Proteostasis and Induces TDP-43 Aggregation. Ann. Clin. Transl. Neurol. 2015, 2, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.E.; Dyle, M.C.; Albanese, R.; Matheny, T.; Sudheendran, K.; Cortázar, M.A.; Forman, T.; Fu, R.; Gillen, A.E.; Caruthers, M.H.; et al. Compromised Nonsense-Mediated RNA Decay Results in Truncated RNA-Binding Protein Production upon DUX4 Expression. Cell Rep. 2023, 42, 112642. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.N.; Yao, Z.; Snider, L.; Fong, A.P.; Cech, J.N.; Young, J.M.; vanderMaarel, S.M.; Ruzzo, W.L.; Gentleman, R.C.; Tawil, R.; et al. DUX4 Activates Germline Genes, Retroelements, and Immune Mediators: Implications for Facioscapulohumeral Dystrophy. Dev. Cell 2012, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Snider, L.; Jagannathan, S.; Tawil, R.; van der Maarel, S.M.; Tapscott, S.J.; Bradley, R.K. A Feedback Loop between Nonsense-Mediated Decay and the Retrogene DUX4 in Facioscapulohumeral Muscular Dystrophy. Elife 2015, 4, e04996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, L.L.; Xiao, G.F.; Luo, Q.Z.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Berberine Increases Doxorubicin Sensitivity by Suppressing STAT3 in Lung Cancer. Am. J. Chin. Med. 2015, 43, 1487–1502. [Google Scholar] [CrossRef]
- Pahuja, I.; Negi, K.; Kumari, A.; Agarwal, M.; Mukhopadhyay, S.; Mathew, B.; Chaturvedi, S.; Maras, J.S.; Bhaskar, A.; Dwivedi, V.P. Berberine Governs NOTCH3/AKT Signaling to Enrich Lung-Resident Memory T Cells during Tuberculosis. PLoS Pathog. 2023, 19, e1011165. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Zhou, J.Y.; Mekala, N.; Welchko, R.; Rosca, M.G.; Li, L. Berberine Hydrochloride Protects against Cytokine-Induced Inflammation through Multiple Pathways in Undifferentiated C2c12 Myoblast Cells. Can. J. Physiol. Pharmacol. 2019, 97, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yuan, Y.; Zhang, H.; Meng, X.; Jin, L.; Yang, J.; Wang, W.; Ning, G.; Zhang, Y.; Zhang, Z. Berberine Attenuates the Abnormal Ectopic Lipid Deposition in Skeletal Muscle. Free Radic. Biol. Med. 2020, 159, 66–75. [Google Scholar] [CrossRef]
- Wang, L.; Deng, L.; Lin, N.; Shi, Y.; Chen, J.; Zhou, Y.; Chen, D.; Liu, S.; Li, C. Berberine Inhibits Proliferation and Apoptosis of Vascular Smooth Muscle Cells Induced by Mechanical Stretch via the PDI/ERS and MAPK Pathways. Life Sci. 2020, 259, 118253. [Google Scholar] [CrossRef]
- He, X.F.; Zhang, L.; Zhang, C.H.; Zhao, C.R.; Li, H.; Zhang, L.F.; Tian, G.F.; Guo, M.F.; Dai, Z.; Sui, F.G. Berberine Alleviates Oxidative Stress in Rats with Osteoporosis through Receptor Activator of NF-KB/Receptor Activator of NF-KB Ligand/Osteoprotegerin (RANK/RANKL/OPG) Pathway. Bosn. J. Basic Med. Sci. 2017, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, H.; Zhu, L.; Yang, H.; Ye, P.; Liu, P.; Gu, Y.; Chen, S. Berberine Attenuates Hypoxia-Induced Pulmonary Arterial Hypertension via Bone Morphogenetic Protein and Transforming Growth Factor-β Signaling. J. Cell. Physiol. 2019, 234, 17482–17493. [Google Scholar] [CrossRef] [PubMed]
- Lek, A.; Zhang, Y.; Woodman, K.G.; Huang, S.; DeSimone, A.M.; Cohen, J.; Ho, V.; Conner, J.; Mead, L.; Kodani, A.; et al. Applying Genome-Wide CRISPR-Cas9 Screens for Therapeutic Discovery in Facioscapulohumeral Muscular Dystrophy. Sci. Transl. Med. 2020, 12, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, T.; Ogasawara, A.; Ishikawa, K.; Kurita, T.; Yoshida, K.; Harada, S.; Nonaka, T.; Inoue, Y.; Uchida, K.; Tateoka, T.; et al. A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice. Biomedicines 2023, 11, 2339. [Google Scholar] [CrossRef]
- Lu-Nguyen, N.; Dickson, G.; Malerba, A.; Popplewell, L. Long-Term Systemic Treatment of a Mouse Model Displaying Chronic FSHD-like Pathology with Antisense Therapeutics That Inhibit DUX4 Expression. Biomedicines 2022, 10, 1623. [Google Scholar] [CrossRef]
- Nunes, A.M.; Ramirez, M.; Jones, T.I.; Jones, P.L. Identification of Candidate MiRNA Biomarkers for Facioscapulohumeral Muscular Dystrophy Using DUX4-Based Mouse Models. Dis. Model. Mech. 2021, 14, dmm049016. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Zhang, A.; Bittel, A.J.; Chen, Y.W. Molecular and Phenotypic Changes in FLExDUX4 Mice. J. Pers. Med. 2023, 13, 1040. [Google Scholar] [CrossRef]
- Tonini, M.M.O.; Passos-Bueno, M.R.; Cerqueira, A.; Matioli, S.R.; Pavanello, R.; Zatz, M. Asymptomatic Carriers and Gender Differences in Facioscapulohumeral Muscular Dystrophy (FSHD). Neuromuscul. Disord. 2004, 14, 33–38. [Google Scholar] [CrossRef]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute Toxicity of Berberine and Its Correlation with the Blood Concentration in Mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the Study of Berberine and Its Derivatives: A Focus on Anti-Inflammatory and Anti-Tumor Effects in the Digestive System. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Franceschin, M.; Rossetti, L.; D’Ambrosio, A.; Schirripa, S.; Bianco, A.; Ortaggi, G.; Savino, M.; Schultes, C.; Neidle, S. Natural and Synthetic G-Quadruplex Interactive Berberine Derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Yagiz, Y.; Wang, G.; Gu, L. Emulsification by TPGS or Quillaja Extract Increased Absorption and Affected Metabolism of Berberine in Humans. Curr. Dev. Nutr. 2021, 5, 381. [Google Scholar] [CrossRef]
- Lu-Nguyen, N.; Ferry, A.; Schnell, F.J.; Hanson, G.J.; Popplewell, L.; Dickson, G.; Malerba, A. Functional Muscle Recovery Following Dystrophin and Myostatin Exon Splice Modulation in Aged Mdx Mice. Hum. Mol. Genet. 2019, 28, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Wells, D.J. Histopathological Evaluation of Skeletal Muscle with Specific Reference to Mouse Models of Muscular Dystrophy. Curr. Protoc. Mouse Biol. 2016, 6, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Mayeuf-Louchart, A.; Hardy, D.; Thorel, Q.; Roux, P.; Gueniot, L.; Briand, D.; Mazeraud, A.; Bouglé, A.; Shorte, S.L.; Staels, B.; et al. MuscleJ: A High-Content Analysis Method to Study Skeletal Muscle with a New Fiji Tool. Skelet. Muscle 2018, 8, 25. [Google Scholar] [CrossRef]
- Mohd Razali, N.; Bee Wah, Y. Power Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling Tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu-Nguyen, N.; Snowden, S.; Popplewell, L.; Malerba, A. Systemic Pharmacotherapeutic Treatment of the ACTA1-MCM/FLExDUX4 Preclinical Mouse Model of FSHD. Int. J. Mol. Sci. 2024, 25, 6994. https://doi.org/10.3390/ijms25136994
Lu-Nguyen N, Snowden S, Popplewell L, Malerba A. Systemic Pharmacotherapeutic Treatment of the ACTA1-MCM/FLExDUX4 Preclinical Mouse Model of FSHD. International Journal of Molecular Sciences. 2024; 25(13):6994. https://doi.org/10.3390/ijms25136994
Chicago/Turabian StyleLu-Nguyen, Ngoc, Stuart Snowden, Linda Popplewell, and Alberto Malerba. 2024. "Systemic Pharmacotherapeutic Treatment of the ACTA1-MCM/FLExDUX4 Preclinical Mouse Model of FSHD" International Journal of Molecular Sciences 25, no. 13: 6994. https://doi.org/10.3390/ijms25136994
APA StyleLu-Nguyen, N., Snowden, S., Popplewell, L., & Malerba, A. (2024). Systemic Pharmacotherapeutic Treatment of the ACTA1-MCM/FLExDUX4 Preclinical Mouse Model of FSHD. International Journal of Molecular Sciences, 25(13), 6994. https://doi.org/10.3390/ijms25136994





