Investigating the Roles of Coat Protein and Triple Gene Block Proteins of Potato Mop-Top Virus Using a Heterologous Expression System
Abstract
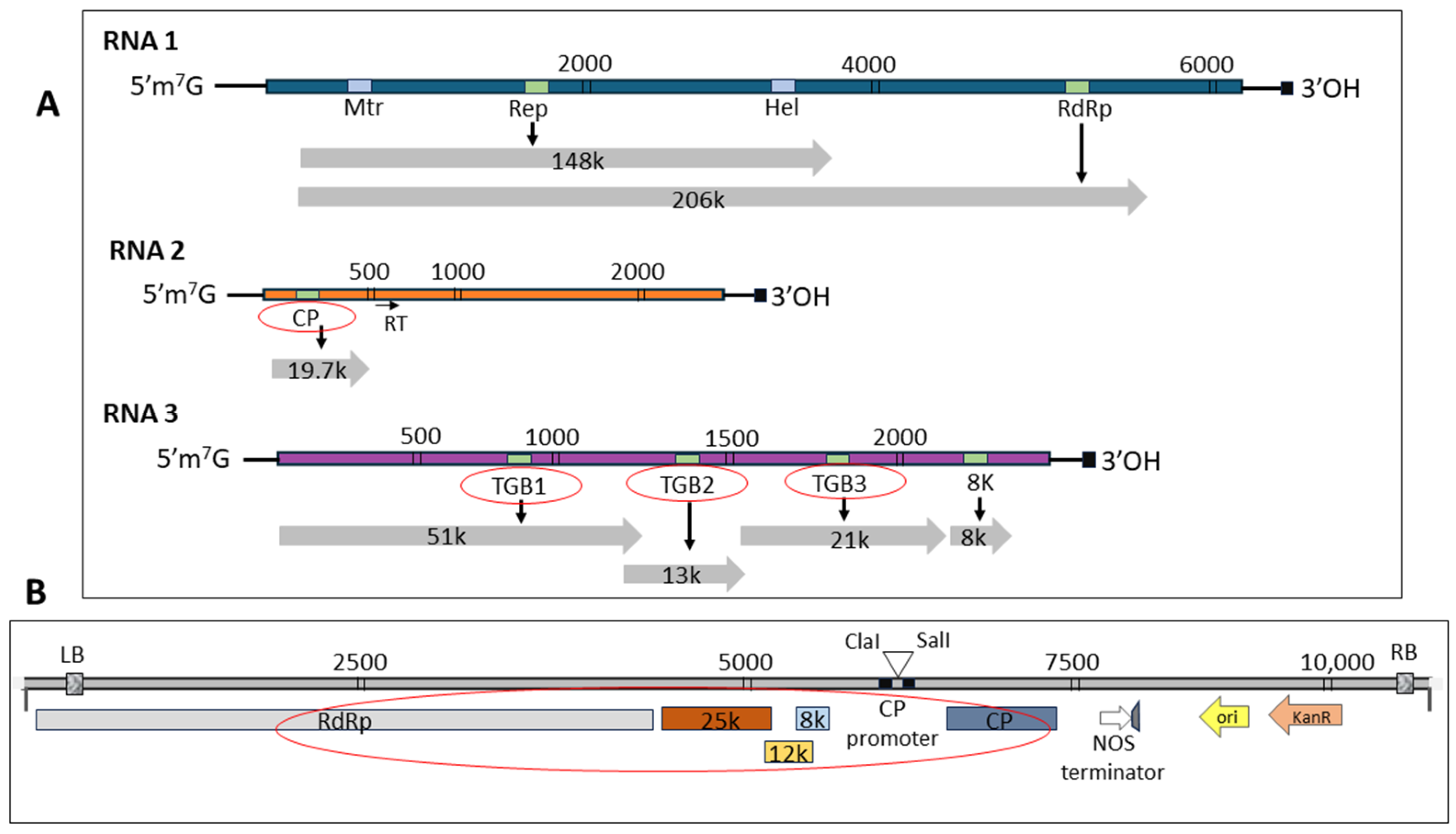
1. Introduction
2. Results
2.1. Effect of Expression of Individual TGBs and CP on Host Response
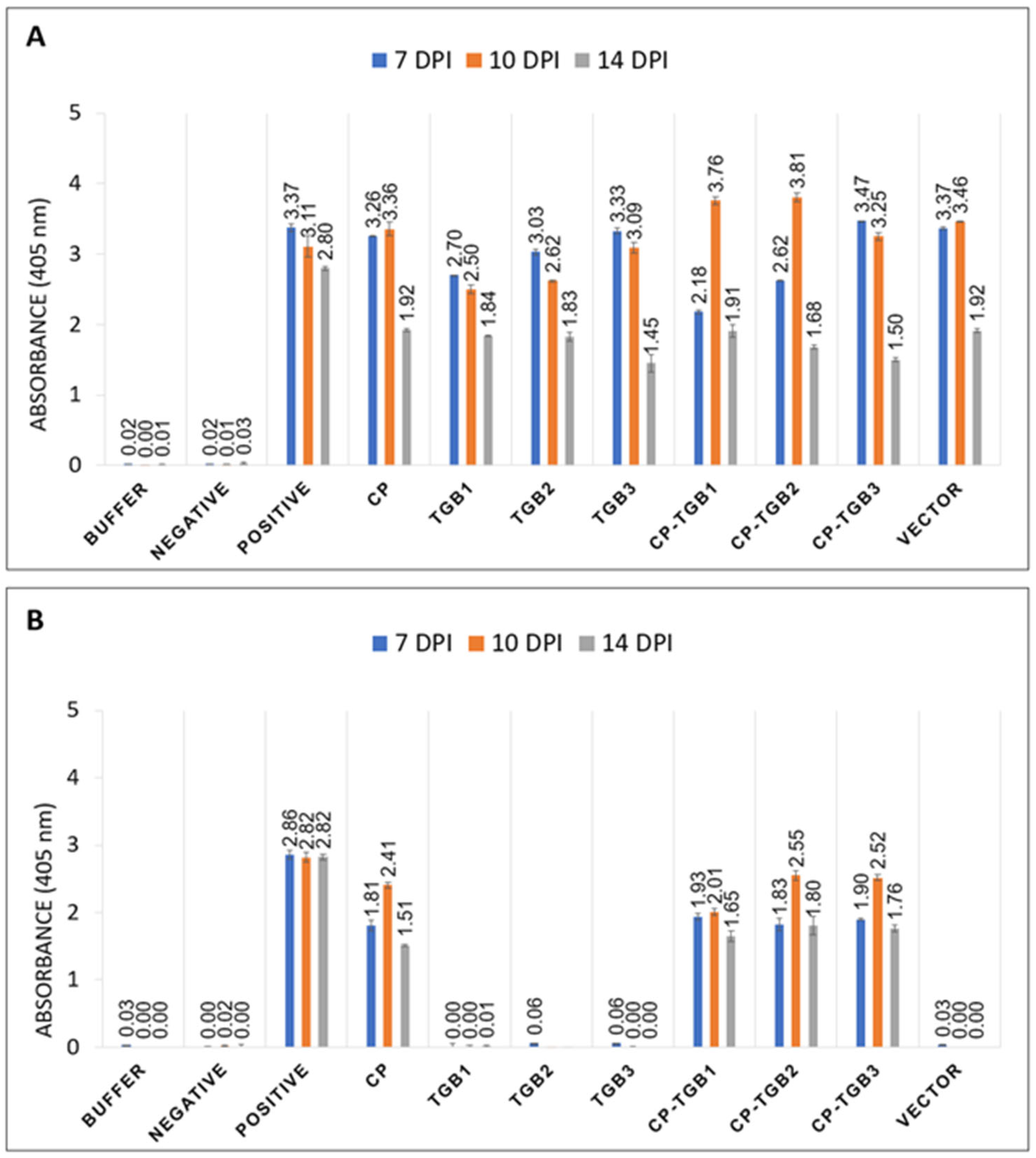
2.2. Combined Effect of Expression of CP with Each TGB ORF on Infiltrated Plants
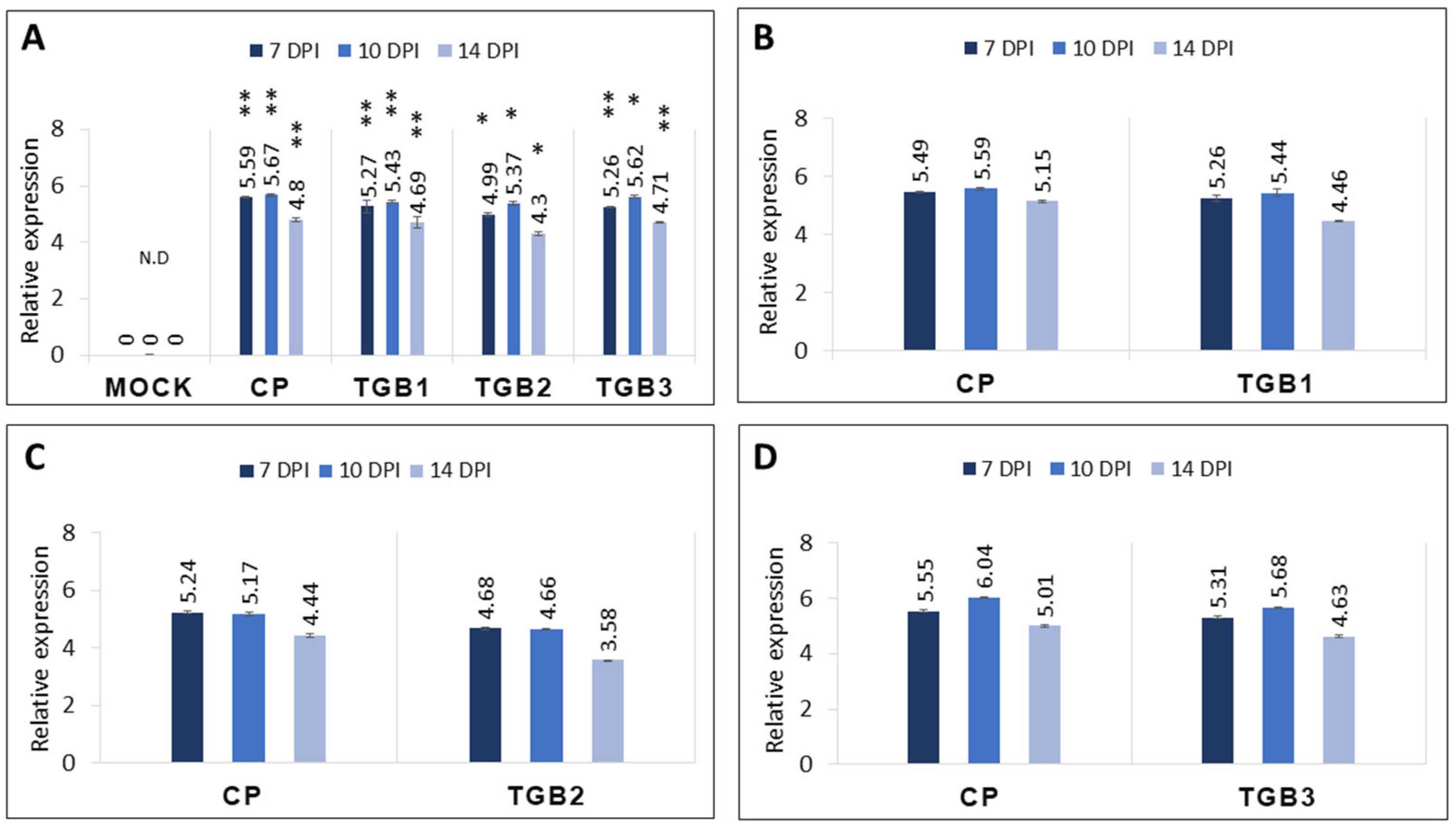
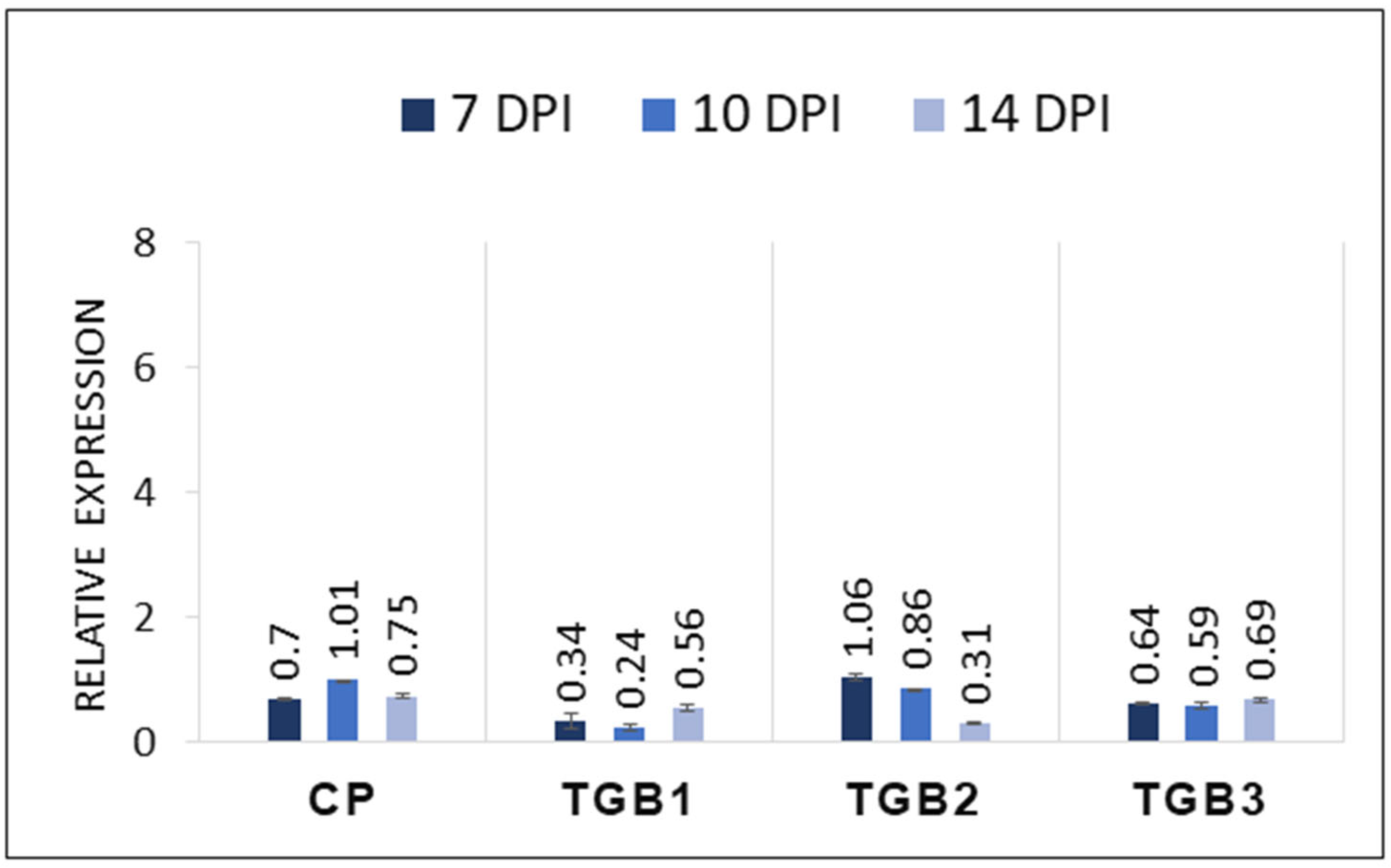
2.3. Transcript Levels of CP and TGB Genes in Inoculated Plants
2.4. Serological Testing of CP in Infected Plant Tissue
2.5. Sequence Comparison of PVX and PMTV Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Gene Amplification
4.2. Quantitative Reverse Transcriptase PCR
4.3. Double Antibody Sandwich Enzyme-Linked Immunosorbent Assay (DAS-ELISA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beals, K.A. Potatoes, Nutrition and Health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Taylor, R.J.; Pasche, J.S.; Gudmestad, N.C. Susceptibility of Eight Potato Cultivars to Tuber Infection by Phytophthora Erythroseptica and Pythium Ultimum and Its Relationship to Mefenoxam-mediated Control of Pink Rot and Leak. Ann. Appl. Biol. 2008, 152, 189–199. [Google Scholar] [CrossRef]
- Wang, H.; Trusch, F.; Turnbull, D.; Aguilera-Galvez, C.; Breen, S.; Naqvi, S.; Jones, J.D.; Hein, I.; Tian, Z.; Vleeshouwers, V. Evolutionarily Distinct Resistance Proteins Detect a Pathogen Effector through Its Association with Different Host Targets. New Phytol. 2021, 232, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Mora-Romero, G.A.; Félix-Gastélum, R.; Bomberger, R.A.; Romero-Urías, C.; Tanaka, K. Common Potato Disease Symptoms: Ambiguity of Symptom-Based Identification of Causal Pathogens and Value of on-Site Molecular Diagnostics. J. Gen. Plant Pathol. 2022, 88, 89–104. [Google Scholar] [CrossRef]
- Sandgren, M.; Savenkov, E.I.; Valkonen, J.P.T. The Readthrough Region of Potato Mop-Top Virus (PMTV) Coat Protein Encoding RNA, the Second Largest RNA of PMTV Genome, Undergoes Structural Changes in Naturally Infected and Experimentally Inoculated Plants. Arch. Virol. 2001, 146, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.L.; Nicolaisen, M. Identification of Two Nucleotide Sequence Sub-Groups within Potato Mop-Top Virus. Arch. Virol. 2003, 148, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C.; Harrison, B.D. The Behaviour of Potato Mop-top Virus in Soil, and Evidence for Its Transmission by Spongospora subterranea (Wallr.) Lagerh. Ann. App Biol. 1969, 63, 1–17. [Google Scholar] [CrossRef]
- Arif, M.; Torrance, L.; Reavy, B. Acquisition and Transmission of Potato Mop-to Furovirus by a Culture of Spongospora subterranea f. Sp. Subterranea derived from a Single Cystosorus. Ann. Appl. Biol. 1995, 126, 493–503. [Google Scholar]
- Arif, M.; Reavy, B.; Torrance, L. Read-Through Protein Gene of Potato Mop-Top Furovirus Is Associated with Acquisition and Transmission of the Virus by Spongospora subterranea f. Sp. Subterranea. Pak. J. Bot. 1999, 31, 125–136. [Google Scholar]
- Pandey, B.; Mallik, I.; Gudmestad, N.C. Development and Application of a Real-Time Reverse-Transcription PCR and Droplet Digital PCR Assays for the Direct Detection of Potato Mop Top Virus in Soil. Phytopathology 2020, 110, 58–67. [Google Scholar] [CrossRef]
- Valkonen, J.P. Viruses: Economical Losses and Biotechnological Potential. In Potato Biology and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 619–641. [Google Scholar]
- Swisher Grimm, K.D.; Quick, R.A.; Cimrhakl, L.; Brown, C.; Pavek, M.J. Detection of Potato Mop-Top Virus in Potato Seed Lots Entering Washington State. Am. J. Potato Res. 2022, 99, 390–394. [Google Scholar] [CrossRef]
- Falloon, R.; Kirkwood, I.; Delmiglio, C.; Bleach, C.; Monk, J.; Clelland, S. Potato Mop-Top Virus: Knowledge Review, and Evaluation of the Biosecurity Response to ‘Incursion’ of This Virus in New Zealand. N. Z. Plant Prot. 2024, 77, 25–35. [Google Scholar] [CrossRef]
- Sandgren, M. Potato Mop-Top Virus (PMTV): Distribution in Sweden, Development of Symptoms during Storage and Cultivar Trials in Field and Glasshouse. Potato Res. 1995, 38, 379–389. [Google Scholar] [CrossRef]
- Savenkov, E.I.; Sandgren, M.; Valkonen, J.P. Complete Sequence of RNA 1 and the Presence of tRNA-like Structures in All RNAs of Potato Mop-Top Virus, Genus Pomovirus. J. Gen. Virol. 1999, 80, 2779–2784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santala, J.; Samuilova, O.; Hannukkala, A.; Latvala, S.; Kortemaa, H.; Beuch, U.; Kvarnheden, A.; Persson, P.; Topp, K.; Ørstad, K. Detection, Distribution and Control of Potato Mop-top Virus, a Soil-borne Virus, in Northern Europe. Ann. Appl. Biol. 2010, 157, 163–178. [Google Scholar] [CrossRef]
- Savenkov, E.I.; Germundsson, A.; Zamyatnin, A.A., Jr.; Sandgren, M.; Valkonen, J.P. Potato Mop-Top Virus: The Coat Protein-Encoding RNA and the Gene for Cysteine-Rich Protein Are Dispensable for Systemic Virus Movement in Nicotiana Benthamiana. J. Gen. Virol. 2003, 84, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Samuilova, O.; Santala, J.; Valkonen, J.P. Tyrosine Phosphorylation of the Triple Gene Block Protein 3 Regulates Cell-to-Cell Movement and Protein Interactions of Potato Mop-Top Virus. J. Virol. 2013, 87, 4313–4321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cerovska, N.; Moravec, T.; Rosecka, P.; Filigarova, M.; Pecenkova, T. Nucleotide Sequences of Coat Protein Coding Regions of Six Potato Mop-Top Virus Isolates. Acta Virol. 2003, 47, 37–40. [Google Scholar]
- Čeřovská, N.; Pečenková, T.; Filigarová, M.; Dědič, P. Sequence Analysis of the Czech Potato Mop-Top Virus (PMTV) Isolate Korneta-Nemilkov. Folia Microbiol. 2007, 52, 61–64. [Google Scholar] [CrossRef]
- Latvala-Kilby, S.; Aura, J.M.; Pupola, N.; Hannukkala, A.; Valkonen, J.P. Detection of Potato Mop-Top Virus in Potato Tubers and Sprouts: Combinations of RNA2 and RNA3 Variants and Incidence of Symptomless Infections. Phytopathology 2009, 99, 519–531. [Google Scholar] [CrossRef]
- Budziszewska, M.; Wieczorek, P.; Nowaczyk, K.; Borodynko, N.; Pospieszny, H.; Obrepalska-Steplowska, A. First Report of Potato Mop-Top Virus on Potato in Poland. Plant Dis. 2010, 94, 920. [Google Scholar] [CrossRef] [PubMed]
- Crosslin, J.M. First Report of Potato Mop-Top Virus on Potatoes in Washington State. Plant Dis. 2011, 95, 1483. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Crosslin, J.M. Detection of Potato Mop Top Virus (Furovirus) on Potato in Southeast Idaho. Plant Dis. 2013, 97, 149. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.V.; Raikhy, G.; Brown, C.R.; Whitworth, J.L.; Pappu, H.R. Complete Genomic Characterization of a Potato Mop-Top Virus Isolate from the United States. Arch. Virol. 2014, 159, 3427–3433. [Google Scholar] [CrossRef]
- Zamyatnin, A.A., Jr.; Solovyev, A.G.; Savenkov, E.I.; Germundsson, A.; Sandgren, M.; Valkonen, J.P.; Morozov, S.Y. Transient Coexpression of Individual Genes Encoded by the Triple Gene Block of Potato Mop-Top Virus Reveals Requirements for TGBp1 Trafficking. Mol. Plant-Microbe Interact. 2004, 17, 921–930. [Google Scholar] [CrossRef]
- Cowan, G.H.; Roberts, A.G.; Chapman, S.N.; Ziegler, A.; Savenkov, E.I.; Torrance, L. The Potato Mop-Top Virus TGB2 Protein and Viral RNA Associate with Chloroplasts and Viral Infection Induces Inclusions in the Plastids. Front. Plant Sci. 2012, 3, 290. [Google Scholar] [CrossRef]
- Haupt, S.; Cowan, G.H.; Ziegler, A.; Roberts, A.G.; Oparka, K.J.; Torrance, L. Two Plant-Viral Movement Proteins Traffic in the Endocytic Recycling Pathway. Plant Cell 2005, 17, 164–181. [Google Scholar] [CrossRef]
- Tilsner, J.; Cowan, G.H.; Roberts, A.G.; Chapman, S.N.; Ziegler, A.; Savenkov, E.; Torrance, L. Plasmodesmal Targeting and Intercellular Movement of Potato Mop-Top Pomovirus Is Mediated by a Membrane Anchored Tyrosine-Based Motif on the Lumenal Side of the Endoplasmic Reticulum and the C-Terminal Transmembrane Domain in the TGB3 Movement Protein. Virology 2010, 402, 41–51. [Google Scholar] [CrossRef]
- Lukhovitskaya, N.I.; Yelina, N.E.; Zamyatnin, A.A., Jr.; Schepetilnikov, M.V.; Solovyev, A.G.; Sandgren, M.; Morozov, S.Y.; Valkonen, J.P.T.; Savenkov, E.I. Expression, Localization and Effects on Virulence of the Cysteine-Rich 8 kDa Protein of Potato Mop-Top Virus. J. Gen. Virol. 2005, 86, 2879–2889. [Google Scholar] [CrossRef]
- Gil, J.F.; Adams, I.; Boonham, N.; Nielsen, S.L.; Nicolaisen, M. Molecular and Biological Characterization of Potato Mop-top Virus (PMTV, Pomovirus) Isolates from the Potato-growing Regions of Colombia. Plant Pathol. 2016, 65, 1210–1220. [Google Scholar] [CrossRef]
- Browning, I.; Craigie, J.; Darling, M.; Darling, D.; Holmes, R. Studies on the Detection, Transmission to Progeny and Symptom Expression of Potato Mop Top Virus in Potato. In Proceedings of the Virology Section Meeting of EAPR, Třešť, Czech Republic, 7–13 October 2001; pp. 7–13. [Google Scholar]
- Montero-Astúa, M.; Vasquéz, V.; Turechek, W.W.; Merz, U.; Rivera, C. Incidence, Distribution, and Association of Spongospora subterranea and Potato Mop-Top Virus in Costa Rica. Plant Dis. 2008, 92, 1171–1176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Needleman, S.B.; Wunsch, C.D. A General Method Applicable to the Search for Similarities in the Amino Acid Sequence of Two Proteins. J. Mol. Biol. 1970, 48, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.F.; Waterman, M.S. Identification of Common Molecular Subsequences. J. Mol. Biol. 1981, 147, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Takken, F.L.W.; Luderer, R.; Gabriels, S.H.E.J.; Westerink, N.; Lu, R.; de Wit, P.J.G.M.; Joosten, M.H.A.J. A Functional Cloning Strategy, Based on a Binary PVX-Expression Vector, to Isolate HR-Inducing cDNAs of Plant Pathogens. Plant J. 2000, 24, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, S.F.; Cameron, A.M.; McCreath, M. Foliar Symptoms Caused by Potato Mop-Top Virus on Potato Plants during Vegetative Propagation in Scotland and Their Association with Tuber Yield, Spraing and Tuber Infection. Potato Res. 2010, 53, 83–93. [Google Scholar] [CrossRef]
- Carnegie, S.F.; Davey, T.; Saddler, G.S. Effect of Temperature on the Transmission of Potato Mop-top Virus from Seed Tuber and by Its Vector, Spongospora subterranea. Plant Pathol. 2010, 59, 22–30. [Google Scholar] [CrossRef]
- Davey, T.; Carnegie, S.F.; Saddler, G.S.; Mitchell, W.J. The Importance of the Infected Seed Tuber and Soil Inoculum in Transmitting Potato Mop-Top Virus to Potato Plants. Plant Pathol. 2014, 63, 88–97. [Google Scholar] [CrossRef]
- Lucas, W.J. Plant Viral Movement Proteins: Agents for Cell-to-Cell Trafficking of Viral Genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Bragg, J.N.; Ganesan, U.; Lawrence, D.M.; Yu, J.; Isogai, M.; Hammond, J.; Jackson, A.O. Triple Gene Block Protein Interactions Involved in Movement of Barley Stripe Mosaic Virus. J. Virol. 2008, 82, 4991–5006. [Google Scholar] [CrossRef]
- Torrance, L.; Lukhovitskaya, N.I.; Schepetilnikov, M.V.; Cowan, G.H.; Ziegler, A.; Savenkov, E.I. Unusual Long-Distance Movement Strategies of Potato Mop-Top Virus RNAs in Nicotiana Benthamiana. Mol. Plant-Microbe Interact. 2009, 22, 381–390. [Google Scholar] [CrossRef]
- Verchot-Lubicz, J.; Torrance, L.; Solovyev, A.G.; Morozov, S.Y.; Jackson, A.O.; Gilmer, D. Varied Movement Strategies Employed by Triple Gene Block–Encoding Viruses. Mol. Plant-Microbe Interact. 2010, 23, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Wright, K.; Crutzen, F.; Cowan, G.; Lukhovitskaya, N.; Bragard, C.; Savenkov, E. Unusual Features of Pomoviral RNA Movement. Front. Microbiol. 2011, 2, 259. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Zafar, M.M.; Razzaq, A.; Ijaz, A.; Anvar, Z.; Topçu, H.; Elhindi, K.M.; Saeed, A.; Fatima, U.; Jiang, X. Using computational modeling to design antiviral strategies and understand plant-virus interactions. Turk. J. Agric. For. 2024, 48, 417–429. [Google Scholar] [CrossRef]
- Lu, R.; Malcuit, I.; Moffett, P.; Ruiz, M.T.; Peart, J.; Wu, A.-J.; Rathjen, J.P.; Bendahmane, A.; Day, L.; Baulcombe, D.C. High Throughput Virus-Induced Gene Silencing Implicates Heat Shock Protein 90 in Plant Disease Resistance. EMBO J. 2003, 22, 5690–5699. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Lynch-Holm, V.; Pappu, H.R.; Tanaka, K. Starch Plays a Key Role in Sporosorus Formation by the Powdery Scab Pathogen Spongospora subterranea. Phytopathology 2024, 114, 568–579. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Constructs | Symptoms | qRT-PCR | ELISA |
|---|---|---|---|
| PMTV-CP | Modest symptoms—curling, crumpling, necrosis | Positive | Positive |
| PMTV-TGB1 | Mild symptoms—curling and crumpling | Positive | Negative for CP |
| PMTV-TGB2 | Mild symptoms—curling and crumpling | Positive | Negative for CP |
| PMTV-TGB3 | Higher mild to modest symptoms—curling, crumpling, necrosis | Positive | Negative for CP |
| PMTV- CP-TGB1 | Higher mild to modest symptoms—curling, crumpling, necrosis | Positive (Both) | Positive for CP |
| PMTV- CP-TGB2 | Higher mild to modest symptoms—curling, crumpling, necrosis | Positive (Both) | Positive for CP |
| PMTV-CP-TGB3 | Modest to severe symptoms—curling, crumpling, necrosis | Positive (Both) | Positive for CP |
| Vector only | Light green chlorosis area | Negative | Negative |
| Primers | Sequences a | |
|---|---|---|
| PMTV- TGB1 | F = ATCGATATGGAAAGTGGATTCAACGGAAG R = GTCGACTTATTCCGGACCATACCTGTCTG | For cloning and sequencing |
| PMTV- TGB2 | F = ATCGATATGATGGTCCGGAATAACGAAATTGG R = GTCGACTTAGCTTCCATATGACCTGCAGCAG | |
| PMTV- TGB3 | F = ATCGATATGGATCCTCCAGTAATAATACAT R = GTCGACTTAATAACGAGCTAACATAGCACCC | |
| PMTV- CP | F = ATCGATATGGCTGAAAACAGAGGTGAGCG R = GTCGACCTATGCACCAGCCCAGCGTAAC | |
| PMTV- TGB1 | F = CCGCAGGCTCAAGCGTGAGAAT R = GGGCTTACCAGTAGATTTGAATCCAC | For detection propose b |
| PMTV- TGB2 | F = TAAATTTGCTAACGGTGGCCAG R = AGCAGTCGCCTCTACATGT | |
| PMTV- TGB3 | F = TTTCAGGAATTTCCTTATGGGAATATTCCTT R = ACGAGCTAACATAGCACCCAC | |
| PMTV- CP | F = AAAACAGAGGTGAGCGAAGAGCA R = AGACACCTGGCTCAACACGCTAGTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, H.; Kotapati, K.V.; Tanaka, K.; Pappu, H.R. Investigating the Roles of Coat Protein and Triple Gene Block Proteins of Potato Mop-Top Virus Using a Heterologous Expression System. Int. J. Mol. Sci. 2024, 25, 6990. https://doi.org/10.3390/ijms25136990
Kamal H, Kotapati KV, Tanaka K, Pappu HR. Investigating the Roles of Coat Protein and Triple Gene Block Proteins of Potato Mop-Top Virus Using a Heterologous Expression System. International Journal of Molecular Sciences. 2024; 25(13):6990. https://doi.org/10.3390/ijms25136990
Chicago/Turabian StyleKamal, Hira, Kasi Viswanath Kotapati, Kiwamu Tanaka, and Hanu R. Pappu. 2024. "Investigating the Roles of Coat Protein and Triple Gene Block Proteins of Potato Mop-Top Virus Using a Heterologous Expression System" International Journal of Molecular Sciences 25, no. 13: 6990. https://doi.org/10.3390/ijms25136990
APA StyleKamal, H., Kotapati, K. V., Tanaka, K., & Pappu, H. R. (2024). Investigating the Roles of Coat Protein and Triple Gene Block Proteins of Potato Mop-Top Virus Using a Heterologous Expression System. International Journal of Molecular Sciences, 25(13), 6990. https://doi.org/10.3390/ijms25136990






