Four New Furofuran Lignans from Phryma leptostachya Inhibit the Accumulation of Molting Hormones in Armyworm
Abstract
:1. Introduction
2. Results and Discussion
2.1. Elucidation of the Structures of the Isolated Compounds
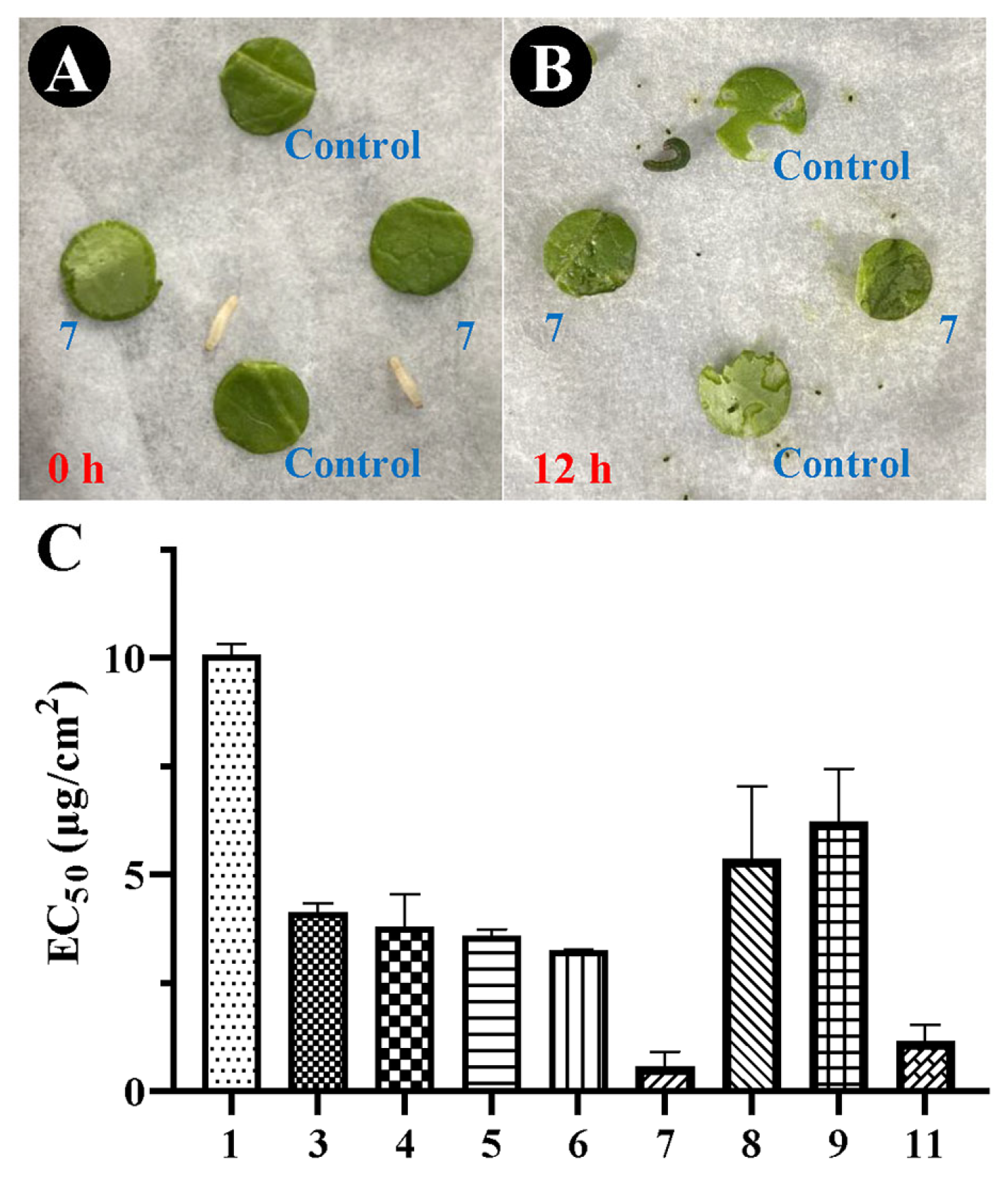
2.2. Antifeedant Activity
2.3. Growth Inhibition Activity
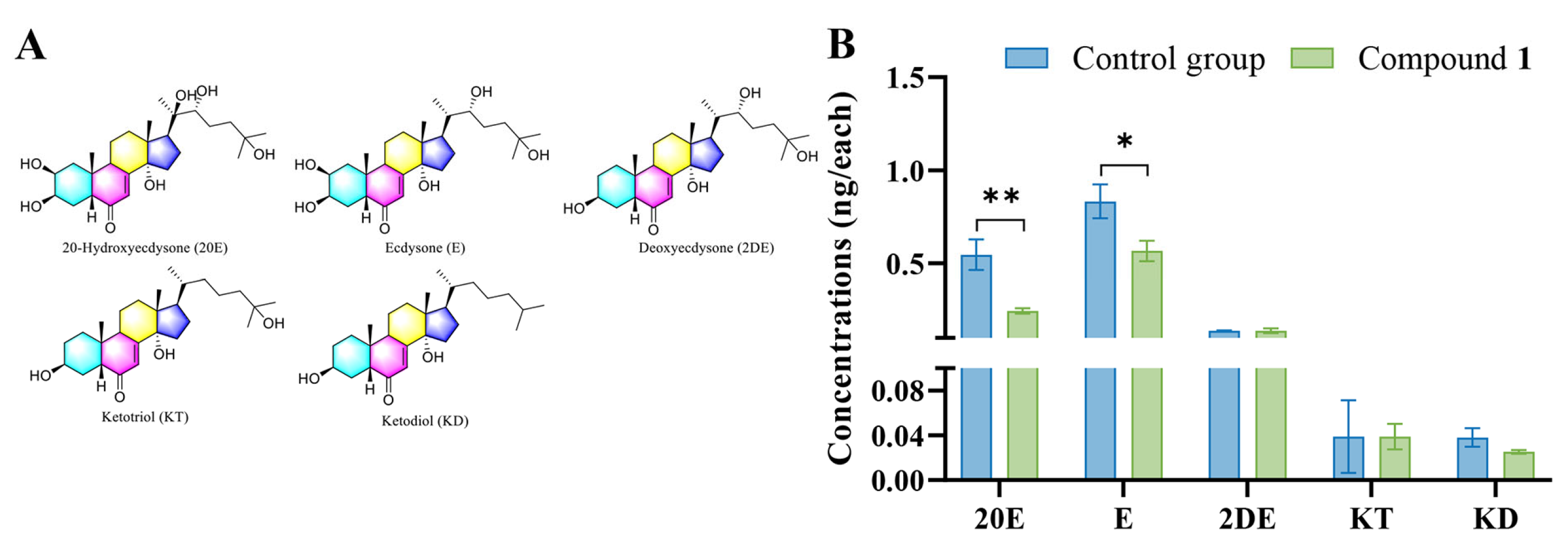
2.4. Detection of Hormones in M. separata Larvae
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antifeedant Assay
3.5. Growth Inhibition Assay
3.6. Determination of Nutritional Indexes
3.7. Quantitative Analysis of Hormones through UPLC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malook, S.U.; Xu, Y.X.; Qi, J.F.; Li, J.; Wang, L.; Wu, J.Q. Mythimna separata herbivory primes maize resistance in systemic leaves. J. Exp. Bot. 2021, 72, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Y.; Liu, Y.Q.; Zhang, L.; Cheng, Y.X.; Stanley, D.; Jiang, X.F. The clock gene, period, influences migratory flight and reproduction of the oriental armyworm, Mythimna separata (Walker). Insect Sci. 2023, 30, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.F.; Zhang, L.; Yang, H.X.; Sappington, T.W.; Cheng, Y.X.; Luo, L.Z. Biocontrol of the oriental armyworm, Mythimna separata, by the tachinid fly Exorista civilis is synergized by Cry1Ab protoxin. Sci. Rep. 2016, 6, 26873. [Google Scholar] [CrossRef]
- Xu, C.; Ji, J.; Zhu, X.; Huangfu, N.; Xue, H.; Wang, L.; Zhang, K.; Li, D.; Niu, L.; Chen, R.; et al. Chromosome level genome assembly of oriental armyworm Mythimna separata. Sci. Data 2023, 10, 597. [Google Scholar] [CrossRef]
- Li, X.R.; Li, Y.; Wang, W.; He, N.; Tan, X.L.; Yang, X.Q. LC50 of lambda-cyhalothrin stimulates reproduction on the moth Mythimna separata (Walker). Pestic. Biochem. Phys. 2019, 153, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ren, Y.; Zhao, J.; Liu, Y.; Xu, B.; Yang, M.; Zhao, W.; Zheng, X.; Wang, J.; Deng, L. Research on the bioactivity of plant essential oils on armyworm Mythimna separata (Walker) larvae. Front. Chem. 2022, 10, 936873. [Google Scholar] [CrossRef]
- Tong, D.; Zhang, L.; Wu, N.; Xie, D.; Fang, G.; Coates, B.S.; Sappington, T.W.; Liu, Y.; Cheng, Y.; Xia, J.; et al. The oriental armyworm genome yields insights into the long-distance migration of noctuid moths. Cell Rep. 2022, 41, 111834. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Castroverde, C.D.M.; Huang, S.; Li, C.; Hilleary, R.; Seroka, A.; Sohrabi, R.; Medina-Yerena, D.; Huot, B.; Wang, J.; et al. Increasing the resilience of plant immunity to a warming climate. Nature 2022, 607, 339–344. [Google Scholar] [CrossRef]
- Zhang, J.M.; Liu, J.Y.; Li, H.D.; Hua, J.; Luo, S.H. Esterification with a long-chain fatty acid elevates the exposure toxicity of tigliane diterpenoids from Euphorbia fischeriana roots against nematodes. J. Agric. Food Chem. 2023, 71, 12730–12740. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From fighting critters to saving lives: Polyphenols in plant defense and human health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Pei, Y.; Cao, W.; Yu, W.; Peng, C.; Xu, W.; Zuo, Y.; Wu, W.; Hu, Z. Identification and functional characterization of the dirigent gene family in Phryma leptostachya and the contribution of PlDIR1 in lignan biosynthesis. BMC Plant Biol. 2023, 23, 291. [Google Scholar] [CrossRef]
- Li, Y.; Wei, J.; Fang, J.; Lv, W.; Ji, Y.; Aioub, A.A.; Zhang, J.; Hu, Z. Insecticidal activity of four lignans isolated from Phryma leptostachya. Molecules 2019, 24, 1976. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.H.; Zuo, Y.Y.; Fang, J.M.; Sun, A.Q.; Aioub, A.A.A.; Hu, Z.N. Transcriptome analysis of Aedes albopictus (diptera: Culicidae) larvae exposed with a sublethal dose of haedoxan A. J. Med. Entomol. 2021, 58, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Qie, X.T.; Sun, A.Q.; Hao, H.H.; Lv, B.; Wu, W.J.; Hu, Z.N. A potential lignan botanical insecticide from Phryma leptostachya against Aedes aegypti: Laboratory selection, metabolic mechanism, and resistance risk assessment. J. Pest. Sci. 2022, 95, 397–408. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Xiao, S.; Li, D.; Tang, Z.; Wei, H.; Zhang, Y.; Yang, J.; Zhao, C.; Liu, Y.; Wang, W. Supplementary UV-B radiation effects on photosynthetic characteristics and important secondary metabolites in Eucommia ulmoides leaves. Int. J. Mol. Sci. 2023, 24, 8168. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C.; Kim, C.S.; Lee, H.J.; Choi, W.S.; Ahn, Y.J. Larvicidal activity of lignans identified in Phryma leptostachya var. asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar] [PubMed]
- Shao, Y.; Hu, L.H.; Sim, K.Y.; Goh, S.H. Lignanoids and diterpenoids from Callicarpa furfuraceae. Helv. Chim. Acta 2006, 89, 64–72. [Google Scholar] [CrossRef]
- Kang, M.H.; Katsuzaki, H.; Osawa, T. Inhibition of 2,2′-azobis(2,4-dimethylvaleronitrile)-induced lipid peroxidation by sesaminols. Lipids 1998, 33, 1031–1036. [Google Scholar] [CrossRef]
- Ishibashi, F.; Hayashita, M.; Okazaki, M.; Shuto, Y. Improved procedure for the enantiometric synthesis of 1-hydroxy/acetoxy-2,6-diaryl-3,7-dioxabicyclo[3.3.0]octane lignans: Total syntheses of (+)-paulownin, (+)-phrymarin I and (+)-phrymarin II. Biosci. Biotechol. Biochem. 2001, 65, 29–34. [Google Scholar] [CrossRef]
- Okazaki, M.; Ishibashi, F.; Shuto, Y.; Taniguchi, E. Total synthesis of (+)-phrymarolin I from (+)-malic acid. Biosci. Biotechol. Biochem. 1997, 61, 660–663. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhu, H.C.; Zhao, D.; Deng, J. Lignans from Phryma leptostachya L. Helv. Chim. Acta 2012, 95, 333–338. [Google Scholar] [CrossRef]
- Milmanda, G.L.F. Smells like trouble: β-ocimene primes plant defenses through chromatin remodeling. Plant Physiol. 2022, 189, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hua, J.; Wang, Y.Y.; Guo, X.Y.; Luo, S.H. Caterpillars detoxify diterpenoid from Nepeta stewartiana by the molting hormone gene CYP306A1. J. Agric. Food Chem. 2023, 71, 10670–10682. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I.; Rybczynski, R.; Warren, J.T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 2002, 47, 883–916. [Google Scholar] [CrossRef]
- Keshan, B.; Thounaojam, B.; Kh, S.D. Insulin and 20-hydroxyecdysone action in Bombyx mori: Glycogen content and expression pattern of insulin and ecdysone receptors in fat body. Gen. Comp. Endocrinol. 2017, 241, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Liu, Y.; Xiao, C.J.; Jing, S.X.; Luo, S.H.; Li, S.H. Chemical profile and defensive function of the latex of Euphorbia peplus. Phytochemistry 2017, 136, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, S.; Hua, J.; Li, D.; Ling, Y.; Luo, Q.; Li, S. Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum. New Phytol. 2020, 229, 1740–1754. [Google Scholar] [CrossRef]
- Zou, C.S.; Lv, C.H.; Wang, Y.J.; Cao, C.W.; Zhang, G.C. Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pestic. Biochem. Phys. 2017, 142, 123–132. [Google Scholar] [CrossRef]
- Hikiba, J.; Ogihara, M.H.; Iga, M.; Saito, K.; Fujimoto, Y.; Suzuki, M.; Kataoka, H. Simultaneous quantification of individual intermediate steroids in silkworm ecdysone biosynthesis by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring. J. Chromatogr. B 2013, 915, 52–56. [Google Scholar] [CrossRef]
- Hua, J.; Liu, Y.C.; Luo, S.H.; Liu, Y.; Xiao, C.J.; Li, X.N.; Li, S.H. Immunostimulatory 6/6/6/6 tetracyclic triterpenoid saponins with the methyl-30 incorporated cyclization from the root of Colquhounia elegans. Org. Lett. 2021, 23, 7462–7466. [Google Scholar] [CrossRef] [PubMed]



| Position | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| 1 | - | - | - | - |
| 2 | - | 6.61 (1H, d, 2.4) | - | 6.58 (1H, d, 2.3) |
| 3 | - | - | - | - |
| 4 | - | - | - | - |
| 5 | 6.33 (1H, s) | 6.73 (1H, d, 8.4) | 6.51 (1H, d, 8.4) | 6.61 (1H, dd, 2.2, 8.4) |
| 6 | - | 6.52 (1H, dd, 2.4, 8.4) | 6.68 (1H, d, 8.4) | 6.45 (1H, dd, 2.3, 8.4) |
| 7 | 5.11 (1H, s) | 5.67 (1H, s) | 5.30 (1H, s) | 5.33 (1H, s) |
| 8 | - | - | - | - |
| 9a | 4.07 (1H, d, 11.5) | 4.44 (1H, d, 11.0) | 4.25 (1H, d, 9.6) | 4.06 (1H, d, 9.7) |
| 9b | 3.48 (1H, d, 11.5) | 3.63 (1H, overlap) | 3.68 (1H, dd, 1.5, 9.6) | 3.78 (1H, overlap) |
| 10 | 5.79 (2H, m) | 5.95 (2H, overlap) | 5.99 (2H, s) | 5.81 (2H, overlap) |
| 1′ | - | - | - | - |
| 2′ | 6.95 (1H, s) | - | 7.09 (1H, s) | - |
| 3′ | - | - | - | - |
| 4′ | - | - | - | - |
| 5′ | 6.55 (1H, s) | 6.45 (1H, s) | 6.70 (1H, s) | 6.30 (1H, dd, 8.3,14.2) |
| 6′ | - | - | - | 6.75 (1H, d, 8.1) |
| 7′ | 4.69 (1H, d, 6.1) | 4.96 (1H, d, 8.1) | 4.86 (1H, d, 6.1) | 5.22 (1H, s) |
| 8′ | 2.34 (1H, dd, 3.9, 9.5) | 3.46 (1H, m) | 2.51 (1H, m) | 2.99 (1H, m) |
| 9′a | 4.44 (1H, dd, 7.6, 8.8) | 4.14 (1H, dd, 6.2,9.2) | 4.43 (1H, dd, 7.4, 9.1) | 3.78 (1H, overlap) |
| 9′b | 3.81 (1H, d,2.4) | 3.67 (1H, overlap) | 4.03 (1H, dd, 2.4, 9.1) | 3.40 (1H, m) |
| 10′ | 5.81 (2H, m) | 5.95 (2H, overlap) | 5.96 (2H, d, 2.5) | 5.81 (2H, overlap) |
| 6′-OMe | 3.68 (3H, s) | 3.78 (3H, s) | 3.83 (3H, s) | |
| 2-OMe | 3.82 (3H, s) | 3.96 (3H, s) | ||
| 6-OMe | 3.65 (3H, s) | |||
| 2′-OMe | 3.96 (3H, s) | |||
| 8-OAC | 2.16 (3H, s) |
| Position | 7 | 8 | 9 | 10 |
|---|---|---|---|---|
| 1 | 132.2, s | 153.1, s | 144.7, s | 153.0, s |
| 2 | 139.2, s | 101.5, d | 137.2, s | 101.1, d |
| 3 | 132.4, s | 144.0, s | 139.0, s | 143.6, s |
| 4 | 145.5, s | 149.0, s | 145.8, s | 148.9, s |
| 5 | 91.1, d | 108.6, d | 102.5, d | 108.6, d |
| 6 | 149.0, s | 110.9, d | 113.4, d | 110.3, d |
| 7 | 105.7, d | 104.3, d | 105.4, d | 106.2, d |
| 8 | 93.4, s | 97.7, s | 93.1, s | 90.3, s |
| 9 | 78.5, t | 76.3, t | 78.5, t | 78.0, t |
| 10 | 102.0, t | 102.2, t | 102.4, t | 102.2, t |
| 1′ | 124.0, s | 112.2, s | 123.9, s | 122.1, s |
| 2′ | 106.7, d | 144.1, s | 106.7, d | 138.4, s |
| 3′ | 142.0, s | 132.5, s | 142.0, s | 135.3, s |
| 4′ | 148.0, s | 150.6, s | 148.0, s | 148.8, s |
| 5′ | 95.2, d | 90.8, d | 95.2, d | 100.8, d |
| 6′ | 152.1, s | 155.2, s | 152.1, s | 120.2, d |
| 7′ | 85.0, d | 81.1, d | 85.0, d | 80.5, d |
| 8′ | 59.5, d | 53.9, d | 59.4, d | 52.5, d |
| 9′ | 71.8, t | 68.8, t | 71.5, t | 67.8, t |
| 10′ | 102.0, t | 102.1, t | 102.0, t | 102.0, t |
| 6′-OMe | 57.4, q | 57.2, q | 56.6, q | |
| 2-OMe | 60.4, q | 60.5, q | ||
| 6-OMe | 56.7, q | |||
| 2′-OMe | 60.4, q | |||
| 8-OAC | 21.1, q | |||
| 171.3, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cong, Q.; Sun, Y.; Hua, J.; Luo, S. Four New Furofuran Lignans from Phryma leptostachya Inhibit the Accumulation of Molting Hormones in Armyworm. Int. J. Mol. Sci. 2024, 25, 7081. https://doi.org/10.3390/ijms25137081
Zhang J, Cong Q, Sun Y, Hua J, Luo S. Four New Furofuran Lignans from Phryma leptostachya Inhibit the Accumulation of Molting Hormones in Armyworm. International Journal of Molecular Sciences. 2024; 25(13):7081. https://doi.org/10.3390/ijms25137081
Chicago/Turabian StyleZhang, Jiaming, Qi Cong, Yuyao Sun, Juan Hua, and Shihong Luo. 2024. "Four New Furofuran Lignans from Phryma leptostachya Inhibit the Accumulation of Molting Hormones in Armyworm" International Journal of Molecular Sciences 25, no. 13: 7081. https://doi.org/10.3390/ijms25137081
APA StyleZhang, J., Cong, Q., Sun, Y., Hua, J., & Luo, S. (2024). Four New Furofuran Lignans from Phryma leptostachya Inhibit the Accumulation of Molting Hormones in Armyworm. International Journal of Molecular Sciences, 25(13), 7081. https://doi.org/10.3390/ijms25137081






